Process Biochemistry 34 (1999) 417 – 420
Bioconversion with whole cell penicillin acylase in aqueous
two-phase systems
Li-Chun Liao
a,b, Chester S. Ho
b, Wen-Teng Wu
a,*
aDepartment of Chemical Engineering, National Tsing Hua Uni6ersity, Hsinchu, Taiwan bInstitute of Biological Science and Technology, National Chiao Tung Uni6ersity, Hsinchu, Taiwan
Received 23 February 1998; received in revised form 3 July 1998; accepted 11 July 1998
Abstract
The deacylation of Pen G was carried out by using recombinant E. coli in an aqueous two-phase system consisting of polyethylene glycol and potassium phosphate solution, which partitions the cells to the bottom phase and the products to the top phase. Bioconversion and product separation were carried out in the same reactor. Repeated batch conversion was employed ten times and enzymic activity showed only a slight decline. When pure enzyme was used for bioconversion in an aqueous two-phase system, the decline was fast and bioconversion using whole cell penicillin acylase was better than that obtained using the pure acylase. © 1999 Elsevier Science Ireland Ltd. All rights reserved.
Keywords:Penicillin acylase; Whole cell; Aqueous two-phase system; E. coli; 6-APA; Repeated batch
1. Introduction
Enzymic hydrolysis of penicillin G is commonly em-ployed in the industrial production of 6-aminopenicil-lanic acid (6-APA) for semisynthetic penicillins. Since the enzyme is expensive, reuse of enzyme is always required. There are three main approaches for repeated use of penicillin acylase. One is immobilization of the enzyme to a solid support [1 – 3], the second is retention of the enzyme in a membrane reactor [4,5], and the third is enzymic conversion in an aqueous two-phase system (ATPS) [6].
An ATPS is formed by adding two solutions of water-soluble polymers together or mixing a solution of water-soluble polymer with another salt solution. Since an ATPS has low interficial tension between two phases, mass transfer can be easily carried out when the system is stirred. Furthermore, the partition character-istics of biocatalyst and product in an ATPS can be employed as an extractive bioconversion [7]. Andersson et al. made a review of bioconversions in aqueous two-phase systems [8].
In this study, an approach using whole cell penicillin acylase in an ATPS is developed. Most applications using whole cell are based on immobilization [9 – 11]. Rodriguez et al. [12] developed a two-phase dispersion system consisting of a suspension of cells and agar in oil. The proposed ATPS is composed of polyethylene glycol (PEG) and phosphate salt. The cells are parti-tioned to the bottom phase and the product to the top phase. Using whole cell in the ATPS has some advan-tages such as improving the partition of the biocatalyst to one phase and eliminating the enzyme purification step. Moreover, for repeated batch enzymic hydrolysis of penicillin G, little enzyme is lost when the top phase is removed. The operation of deacylation is prolonged by repeated use of the immobilized whole cell penicillin acylase.
2. Materials and methods 2.1. Materials
The potassium salt of penicillin G (Glaxo, UK) was used for conversion studies. The potassium salt of penicillin G, 6-APA and phenylacetic acid (PAA) were * Corresponding author.
0032-9592/99/$ - see front matter © 1999 Elsevier Science Ireland Ltd. All rights reserved. PII: S 0 0 3 2 - 9 5 9 2 ( 9 8 ) 0 0 0 9 9 - 5
L.-C. Liao et al./Process Biochemistry34 (1999) 417 – 420
418
purchased from Sigma. Polyethylene glycol was PEG 6000 with the molecular weight between 5000 and 7000 (Merck, Germany). All chemicals were of reagent grade.
2.2. Microorganism and medium
A genetically engineered strain, E. coli ATCC 9637 (PGL5) [13] for producing penicillin acylase was used in this study. The microorganisms, which were provided by Union Chemical Laboratories (Hsinchu, Taiwan), had the property that penicillin acylase production was repressed by glucose and was not induced by pheny-lacetic acid addition. The microorganisms were main-tained by monthly transfer on LB agar plates and stored at 4°C. Choramphenicol 30mg ml− 1was added
to the media of the agar plates to maintain a selection pressure. The composition of the medium was as fol-lows: sorbitol 40 g l− 1, yeast extract 5 g l− 1, KH
2PO4
5 g l− 1, Na
2HPO4 4 g l− 1, (NH4)2SO47 g l− 1, NH4Cl
1·2 g l− 1, MgSO
4·7H2O 2·4 g l− 1, CaCl2 0·02 g l− 1,
and trace metal solution 10 ml l− 1. The pH value of the
medium was 7.
2.3. The aqueous two-phase system
An ATPS consists of two separated aqueous phases. The principle of separation in the ATPS is the non-uni-form distribution of substances between the two phases [14]. The partition coefficient K is defined as:
K = CT/CB
where CT is the concentration of the substance in the top phase and CB is the concentration of the same substance in the bottom phase.
Another important parameter of the ATPS is the phase volume ratio, R, which is defined as:
R = VT/VB
where VTis the volume in the top phase and VBis the
volume in the bottom phase.
The ATPS in the present study was composed of PEG 6000 (15% w/w) and potassium phosphate solu-tion (0·55 molarity). For determinasolu-tion of the partisolu-tions of Pen G, 6-APA, PAA, and cells, a 15-ml test tube was used. A vortex mixer was applied to mixing. After mixing of the ATPS, a batch-scale centrifuge was em-ployed for separation at 1500 rpm for 2 min. Experi-ments on the bioconversion in the ATPS were carried out in a 500 ml flask with 15 g of the PEG 6000, 18 g of cells, and 0·55 M of the potassium phosphate solu-tion. The working volume was 100 ml and incubation involved shaking at 150 rpm at 32°C for 60 min. After stopping the shaking, for 15 min, the system became two phases. Samples were carefully taken from both top and bottom phases with a pipette without dis-turbing the interface.
2.4. Assay
The concentration of Pen G was determined by using HPLC with an Interstil 7 ODS-3 4·6 × 250 nm (Verco-pak, Taiwan). The solvent system for the column was 20% acetonitrile, 0.1% triethylamine, 0.1% H3PO4 and 50 mM KH2PO4 at a flow rate of 1.0 ml min− 1. The
detection was done by absorption at 220 nm with a UV detector. The concentrations of 6-APA and PAA are determined by using HPLC with an Interstil C4 5 mm
column (GL Sciences, Japan). The solvent system for the column was 10 mol m− 3 of (NH
4)2HPO4 and
methanol (4:1 v/v) at a flow rate of 0.6 ml min− 1. The
detection was done by absorption at 225 nm. Cell concentration was measured using a spectrophotometer at 600 nm and was correlated to the wet weight with a standard curve. Enzyme activity was determined ac-cording to the method proposed by Kornfeld [15].
3. Results and discussion
For bioconversion in an aqueous two-phase system, partition and stability of the enzymatic reaction are two important characteristic factors. The effects of composi-tion of the ATPS and operating condicomposi-tions on the partition and enzymic reaction are given below. 3.1. Partition of cells in the ATPS
Partition of cells to the bottom phase requires that the partition coefficient K should be very small. The experimental data show that for the ATPS the partition coefficient was less than 0·01 and the phase volume ratio was 4 at pH 7·8 and 32°C.
3.2. Partition of penicillin G, 6-APA and PAA
By applying an ATPS for bioconversion, the enzyme and products should be partitioned to different phases, especially for a system with product inhibition [16,17]. The partition coefficients of Pen G, 6-APA, and PAA in the ATPS under conditions of pH 7·8 and 32°C gave
KpenG= 64, K6-APA= 7·3 and KPAA= 8·55. Hence, Pen
G, 6-APA, and PAA were partitioned to the top phase. Although the partition coefficient of Pen G was high, most of the Pen G were deacylated to 6-APA during the period of bioconversion.
3.3. Effect of Pen G
Pen G had the effect of inhibiting, the activity of penicillin acylase [18,19]. A high concentration of Pen G in the ATPS decreased the conversion rate. Fig. 1 shows the relative activity with respect to the concen-tration of Pen G. The relative activity of 100%
peni-L.-C. Liao et al./Process Biochemistry34 (1999) 417 – 420 419
Fig. 1. Relative activity of whole cell penicillin acylase with respect to the penicillin G concentration at pH 7·8 and 32°C.
Fig. 3. Relative activity of whole cell penicillin acylase with respect to temperature at pH 7·8.
cillin acylase was equal to 32 IU g− 1, wet weight. The
concentration of Pen G up to 4% (w/w) did not have the effect of inhibition. The designated concentration of penicillin G for the reaction was 4% (w/w).
3.4. Effect of phosphate solution
Potassium phosphate in the ATPS had a substantial effect on the activity of penicillin acylase [20]. Fig. 2 shows the relative activity of whole cell penicillin acy-lase with respect to the concentration of potassium phosphate solution. Highest relative activity was ob-tained around 0·55 molarity.
3.5. Effect of temperature
Four different temperatures were employed to inves-tigate the temperature effect on the bioconversion. Fig. 3 shows the relative activity with respect to tempera-ture. A temperature above 32°C had almost the same
relative activity of bioconversion. Since at higher tem-peratures the production of penicillin acylase in the cells gave an inactive form [21], the designated tempera-ture for reaction was 32°C.
3.6. Repeated batch con6ersion
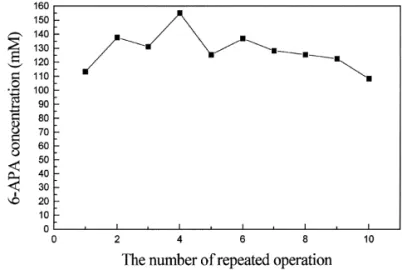
Repeated batch operation was performed by remov-ing the top phase and replacremov-ing it with a new top phase which had the same initial feed composition of the top phase of the ATPS with fresh penicillin G (4% w/w). Each batch operation took 60 min. Fig. 4 shows the experimental results of repeated operation. Penicillin G hydrolysis remained above 96% for ten repeated opera-tions without adding fresh cells. The phase volume ratio of the ATPS was not significantly changed. The higher values of 6-APA concentration during the sec-ond to fourth repeated operations might be due to some 6-APA from the initial operation remaining in the bottom phase. The experimental results indicated that
Fig. 4. Repeated batch conversion of penicillin G (4% w/w) to 6-APA (concentration in the top phase) with whole cell penicillin acylase at 32°C.
Fig. 2. Relative activity of whole cell penicillin acylase with respect to the concentration of the potassium phosphate solution at pH 7·8 and 32°C.
L.-C. Liao et al./Process Biochemistry34 (1999) 417 – 420
420
using a biocatalyst consisting of whole cells in the ATPS gave a better stable system. In a similar experi-ment [6] which was carried out using pure enzyme for deacylation of penicillin in the ATPS, fresh enzyme had to be added even for four repeated operations. Using the whole cell acylase significantly improves performance.
4. Conclusion
A whole cell penicillin acylase in an ATPS has been developed for bioconversion of penicillin G to 6-APA. Cells were partitioned to the bottom phase while the products, 6-APA and PAA, were partitioned to the top phase. Since the concentrations of penicillin G, potas-sium phosphate, and 6-APA could degrade the activity of penicillin acylase, an ATPS was employed to resolve the problem.
Using whole cell penicillin acylase for deacylation provided several significant advantages including:
1. Enzyme purification was not required.
2. Whole cell penicillin acylase could be re-used ten times before the enzymic activity began to decline. In the present study, experiments were carried on a small scale. Although scale-up could be applied easily, details of the operation require further investigation.
References
[1] Nagashima M, Azuma M, Nogushi S, Inuzuka K, Samejima H. Continuous ethanol fermentation using immobilized yeast cells. Biotechnol Bioeng 1984;26:992 – 7.
[2] Carleysmith SW, Lilly MD. Deacylation of benzylpenicillin by immobilized penicillin acylase in a continuous four-stage stirred-tank reactor. Biotechnol Bioeng 1979;21:1057 – 73.
[3] Park JM, Choli CY, Seong BL, Han MH. The production of 6-aminopenicillanic acid by a multistage tubular reactor packed with immobilized penicillin acylase. Biotechnol Bioeng 1982;24:1623 – 37.
[4] Azhar A, Hamdy MK. Alcohol fermentation of sweet potato: membrane reactor in enzymatic hydrolysis. Biotechnol Bioeng
1981;23:1297 – 307.
[5] Hong J, Tsao GT, Wankat PC. Membrane reactor for enzymatic hydrolysis of cellobiose. Biotechnol Bioeng 1981;23:1501 – 16. [6] Andersson E, Mattiasson B, Hahn-Hagerdal B. Enzymatic
con-version in aqueous two-phase systems: deacylation of ben-zylpenicillin to 6-aminopenicillanic acid with penicillin acylase. Enzyme Microb Technol 1984;6:301 – 6.
[7] Mattiasson B. Bioconversion in aqueous two-phase systems: an alternative to conversional immobilization. Method Enzymol 1988;137:657 – 67.
[8] Andersson E, Hahn-Hagerdal B. Bioconversions in aqueous two-phase systems. Enzyme Microb Technol 1990;12:242 – 54. [9] Klein J, Wagner F. Immobilization of whole microbial cells for
production of 6-aminopenicillanic acid. Enzyme Eng 1984;5:335 – 45.
[10] Castillo E, Rodriguez M, Casas L, Quintero R, Lopez-Munguia A. Design of two immobilized cell catalysts by entrapment on gelatin: internal diffusion aspects. Enzyme Microb Technol 1991;13:127 – 33.
[11] Vojtisek V, Jirku V. Immobilized cells. Folia Microbiol 1983;28:309 – 40.
[12] Rodriguez ME, Quintero R, Lopez-Munguia A. Design and kinetic characterization of a whole cell penicillin acylase biocata-lyst using E. coli. Process Biochem 1994;29:213 – 8.
[13] Liu Y-T, Ji Chou D-D, Chao C-C, Liao H-Y, Han S-H. Improvement of penicillin G acylase production by using pac gene clone. Processings of the National Science Council ROC Part B: Life Sciences 1994;18:95 – 100.
[14] Albersson PA. Partition of cell particles and macromolecules, 3rd ed. New York: John Wiley and Sons, 1986.
[15] Kornfeld JM. A new colorimetric method for the determination of 6-aminopenicillanic acid. Anal Biochem 1978;86:118 – 26. [16] Stredansky M, Svorc R, Sturdik E, Dercova K. Repeated
batch-amylase production in aqueous two-phase system with Bacillus strains. J Biotechnol 1993;27:181 – 91.
[17] Chen J-P, Wang C-H. Lactose hydrolysis byb-galactosidase in aqueous two-phase systems. J Ferment Bioeng 1991;71:168 – 75. [18] Shewale JG, Sivarman H. Penicillin acylase: enzyme production and its application in the manufacture of 6-APA. Process Biochem 1989;24(4):146 – 54.
[19] Vandamme EJ. Biotechnology of industrial antibiotics. New York and Basel: Marcel Dekker, 1984.
[20] Andersson E, Hahn-Hagerdal B. Enzyme action in polymer and salt solutions. II Activity of penicillin acylase in poly(ethylene glycol) and potassium phosphate solutions in relation to water activity. Biochim Biophys Acta 1987;912:325 – 8.
[21] Oh SJ, Kim YC, Park YW, Min SY, Kim IS, King HS. Complete nucleotide sequence of penicillin G acylase gene and the flanking regions, and its expression in Escherichia coli. Gene 1987;56:87 – 97.
. .