國立臺灣大學生命科學院生化科技系 碩士論文
Department of Biochemical Science and Technology College of Life Science
National Taiwan University Master thesis
Thioredoxin 與化療藥物治療後產生癌細胞抗藥性之關聯性 The Possible Role of Thioredoxin in causing Drug
Resistance following Chemotherapy
胡景涵 Ching-Han Hu
指導教授:莊榮輝 博士 闕壯卿 博士
Advisor: Rong-Huay Juang, Ph.D.
Chuang-Chin Chiueh, Ph.D.
中華民國 102 年 6 月
June, 2013
誌謝
從一開始進入這個所到現在,兩年的時光就這樣匆匆的過去了。由於大學時是 念護理系,因此為了要轉進入研究的領域,花費了一年的時間終於讓我考上了,
抱著好奇及期待的心來到莊榮輝老師的實驗室。碩一的時候由於什麼都還不懂,
任何實驗也都沒接觸過,在學長界帶領下學了很多實驗技巧,也在修課中學了很 多知識,但是當時對於自己的實驗方向卻是一片茫然,直到碩一下有去北醫和闕 壯卿老師討論後,才漸漸有了眉目。
在碩一下及碩二上的時間,對我來說是一段很辛苦的過程,在試著用不同方法 做實驗時一開始總是失敗再失敗,有時真的感覺快被實驗失敗的挫折打倒。感謝 莊榮輝老師與闕壯卿老師,在每次的討論中都了我寶貴的建議及提供我思考實驗 方向的建議;感謝承佑學長及惠玉姊在我什麼都不懂時,帶我學會了最基礎的實 驗,並與我討論;感謝玉米學姐及蛋姊總是在我實驗失敗的時候,幫我查看實驗 條件,尋找可能讓實驗失敗的原因,每次都讓我在失敗中感覺燃起了一線希望;
感謝明孝學長教會我很多實驗技術及小撇步,常常要幫在我實驗出現問題時出手 救急,也常常一起實驗做到很晚,因此在實驗室天南地北的聊天;感謝于嬍總是 在一些小地方幫我的忙,也會提醒健忘的我很多事情;另外還要感謝張麗冠老師,
提供螢光顯微鏡,使我的實驗能夠進行。
總而言之,真的很感謝及520的大家,讓我度過了充實快樂的兩年碩士生活。
感謝莊老師及闕老師這兩年來的指導, 在老師們的提點下也讓我漸漸學會邏輯 發現問題,並且解決問題。也感謝各位前來幫我口試的老師,並給予我意見及指 導。
List of Contents
List of Contents………... I Abstract……….………...II 中文摘要………..………...V List of Tables………...VII List of Figures……….VIII Abbreviations……….IX Text………...X
I
Abstract
The possible Role of Thioredoxin
in Causing Drug Resistance following Chemotherapy
Ching-Han Hu
Department of Biochemical Science and Technology College of Life Science, National Taiwan University
Backgrounds
Neuroblastoma is a kind of children cancer that has a relatively high mortality, recurrence and metastasis; drug resistance is frequently seen after the treatment with chemotherapy (Marvis, 2010). According to the theory of cancer stem cell (CSC), the reason why cancer usually recurs and metastasizes is due to the CSC exists in the tumor which can even survive and grow into a new tumor after chemotherapy (Jordan,
Guzman, and Noble, 2007).
After a new discovery of our team (Andoh, Chock, and Chiueh, 2002), a CD-133 positive and Trx-expressed CSC-like cells (N cells) were isolated from the pre-conditioned human SH-SY5Y cells (unpublished observations of Chiueh et al). N cells produced a solid tumor but not by its wild type control cells (D cells) in nude mice.
D cells are associated with low Trx expression and also sensitive to oxidative injury and related apoptotic cell death.
II
Objective
To understand whether Trx expression affects the relationship between the preconditioned N cells and resistance to chemotherapy; to investigate the redox cycling role of Trx in mediating chemo-resistance
Methods
Trx expression in control D cells and pre-conditioned N cells were measured by the Western blotting. Etoposide (0 to 83 μM)-induced apoptotic changes and cell viability in D and N cells were assayed by Hoechst 33258 nuclear staining and Trypan blue exclusion assay, respectively. The redox cycling of Trx was blocked by a Trx reductase inhibitor 1-Chloro-2,4-dinitrobenzene (DNCB (50 μM) in N cells. Cell morphology in control D cells after etoposide treatment (0 to 83 μM) for 24 hours was imaged by using fluorescence microscopy.
Results
First, this experiment was to compare the Trx expression of the pre-conditioned N cells with the control D cells. The result indicates that the Trx expression of N cells was 3 times greater than D cells. Etoposide-induced apoptosis in the pre-conditioned N cells were less than that in the control D cells by 27-fold. Next, after the
co-treatment of N cells with 50 μM DNCB to block the redox cycling of Trx, the apoptosis cell percentage of DNCB-treated N cells was close to the control D cells.
The present results show that the elevation of the Trx expression improved not only the survival rate of N cells, but also induced chemo-resistance reflected by a reduction in the apoptotic cell death caused by etoposide. In addition, following the etoposide (83
III
μM) treatment, the phenotype of the D cells was changed to N cell morphology with a concurrent increase in Trx levels. This phenomenon infers that the normal cancer cell may be mutated and altered into CSC-like cells under the stimulation of chemopherapy.
Conclusions
The expression of Trx can prevent cancer cells such as N cells from apoptotic cell death caused by etoposide. The Trx expression and the cell morphology were altered after the treatment of etoposide in the control D cells. The redox cycling of Trx in human neuroblastoma cancer cells may play a critical role in the development of chemo-resistance.
Key words
cancer stem cell, chemotherapy, drug resistance, etoposide, metastasis, thioredoxin
IV
Abstract in Chinese
Thioredoxin 與化學藥物治療後產生癌細胞抗藥性之關聯性 胡景涵
國立臺灣大學生命科學院生化科技系
研究背景
神經纖維母細胞乃常見好發於兒童之惡性腫瘤,死亡率極高,且經治療後,仍 常見復發及轉移之現象 (Marvis, 2010)。根據癌症幹細胞之理論,認為癌症之所以 常見復發及轉移之現象,是由於腫瘤裡面存在著癌症幹細胞,此類細胞在經由化 療藥物處理下可以繼續存活 (Jordan, Guzman, and Noble, 2007)。
而根據本研究團隊於 2002 年所做之實驗發現 (Andoh , Chock , and Chiueh, 2001),經由 pre-conditioned 之 SH-SY5Y cells (N cells) 會表現 CD-133 細胞表面抗 原及 Trx,相較於 wild type control cells (D cells) N cells 可形成一顆腫瘤及轉移。 D cells 之 Trx 表現量較低並且對於外界氧化壓力較敏感而較容易造成細胞凋亡之現 象。
研究目的
希望了解 Thioredoxin 表現量是否影響癌症幹細胞與化療藥物抗藥性之間的關
係,並了解 Trx 之 redox cycling 與化療藥物抗藥性之關係。
研究方法
希望藉由 Western blotting 來了解在 control D cell 及 pre-conditioned N cell 其 Thioredoxin 表現量的差異。並藉由 Hoechst 33258 細胞核染劑及 Trypan blue exclusion assay 來了解在加入 etoposide (0 to 83 μM) 後,兩株細胞細胞凋亡與存活 率之差異。藉由螢光顯微鏡觀察觀察 control D cell 在加入 etoposide (0 to 83 μM) 作用 24 小時後,細胞型態變化情形。使用 DNCB (50 μM) 做為 Trx 之 redox cycling 的抑制劑。
V
結果
此次實驗首先比較 pre-conditioned N cells 與 control D cells 之 Trx 表現量之差 異,結果顯示 N cells 之 thioredoxin 表現量確實較 D cells 高 3 倍。接著進行 etoposide 加藥實驗,經實驗計算發現,pre-conditioned N cells 要達到與 control D cells 相同 之 a 細胞凋亡比率,需 27 倍的藥物劑量。再者加入DNCB (50 μM)抑制 Trx 之 redox cycling,可發現經 DNCB 處理之 N cells 其細胞凋亡之比率與 control D cells 相近。
此結果顯示 thioredoxin 表現量差異的確會影響 N cells 之存活率,並且影響 etoposide 所產生之細胞凋亡導致細胞對化療藥物產生抗藥性之現象。另外在進行加藥實驗 時發現,D cells 在高劑量化療藥物 (83 μM etoposide) 作用時,經由顯微鏡觀察,
發現其細胞型態改變且與 N cell 之型態相似,並且 Trx 表現量上升。因此推測可 能在化療藥物作用下,normal cancer cell 會經由刺激而突變或是篩選成與癌症幹細 胞相似之細胞。
結論
Trx 表現量確實會避免癌症細胞對化療藥物 etoposide 所導致之細胞凋亡產生抗藥 性。並且 control D cells 在 etoposide 作用之下,Trx 表現量及細胞型態皆有改變。
Trx 之 redox cycling 在化療藥物產生抗藥性中扮演重要的角色。
關鍵字
癌症幹細胞、化療、抗藥性、 etoposide、轉移、 thioredoxin
VI
List of Tables
Table 1. Cancer stem cell markers……….10
VII
List of Figures
Figure 1. After mutation, normal stem cell may turn into cancer stem cell, which can probably transform to a tumor itself………..7 Figure 2.Cancer stem cell and chemo-resistance………...…..9 Figure 3.The expression of Trx in N cell and D cell by Western blotting……….18 Figure 4.Effcts of Etoposide on apoptotic changes in nuclear staining by Hoechst
33258 fluorescent dye in D and N cells………...20 Figure 5.Apoptotic cell death of D cells and N cells evoked by Etoposide
(0 to 83 μM). For methods, please see the legend of Figure 2………....21 Figure 6. Comparison by Trypan blue exclusion method in D cells and N cells
following Etoposide treatment (0 to 83 μM)………...23 Figure 7. Effect of Etoposide on Cell viability, reflected by cell mitochondrial activity
was assayed by MTT method in D and N cells………...24 Figure 8. Effect of endogenous Trx redox cycling on the concentration-dependent
apoptosis caused by Etoposide in N cells………26 Figure 9.Statistical analysis of the effects of DNCB on the effect of Etoposide in N
cells………..27 Figure 10.The expression of Trx in N cell with DNCB (0, 5, 10 and 50 μM) added by
Western blotting...………...29 Figure 11. The expression of Trx in D cell with Etoposide treatment (0 to 83 μM) by
Western blotting………. 31 Figure 12. The phenol type under white light and Hoehst 33258 nuclear staining of cell
nuclear following etoposide treatment (0 or 83 μM) for 24 hours in N and D cells……….32 Figure 13. Trx Pathway Blocks Etoposid-induced DNA Breaks………..38
VIII
Abbreviations
CSC cancer stem cell Trx thioredoxin
TrxR thioredoxin reductase
DNCB 1-Chloro-2,4-dinitrobenzene SH-SY5Y neuroblastoma
IX
Text
Chapter 1. Preface………1
1.1 Rationale………...….1
1.2 Working Hypothesis………..2
1.3 Research Goal………....…2
Chapter 2. Literature Review……….…….3
2.1 Neuroblastoma………..………3
2.2 Etoposide and Topoisomerase II……….…………..4
2.3 Cancer Stem Cell………..5
2.3.1 Cancer Stem Cell Theory……….………5
2.3.2 Cancer Stem Cell Markers and Chemo-resistance……...…….……..8
2.4 Thioredoxin (Trx)………...11
2.4.1 Trx System……….………...11
2.4.2 Inhibition of Trx System……….………..12
Chapter 3. Materials and Methods……….13
3.1 Materials……….13
3.2 Cell Cultures………...14
3.3 Nuclear Staining of Apoptotic Cells by Hoechst 33258……….14
3.4 Trypan Blue Exclusion Assay……….…15
3.5 Western Blotting……….……15
3.6 MTT Assay……….…16
Chapter 4. Preliminary Results………..………...17
4.1 Trx expression and N cells……….17
4.2 Effects of etoposide………19
4.2.1 Apoptotic cell changes…………...………...19
4.2.2 Comparison of cell viability in D and N cells following etoposide treatment………....22
4.3 The effect of the inhibitor of redox cycling of Trx on etoposide-induced apoptosis in N cells……….25
4.3.1 The apoptosis cell death may increase in DNCB treated N cells....25
4.3.2 Effect of DNCB on Trx levels………,,,… 28
X
4.4
The changes of etoposide-treated D cells……….……..30 4.4.1 Trx level in etoposide-treated D cells………..30 4.4.2 D cell morphology has changed after etoposide treated…………..30 Chapter 5. Discussion………..………...33
5.1 Role of Trx on etoposide-treated N cells………..33 5.2 D cell may promote it cell survival by increased Trx expression……….36 Chapter 6. References………..39
XI
1
Chapter 1: Preface
Rationale
The mortality of neuroblastoma is extremely high; this cancer attacks in younger children, recurrence and metastasis are often happened (Marvis, 2010). To search for an effective method for curing these kinds of cancer is the research goal of many biomedical researchers. The actual mechanism of tumorigenesis is not currently known whether drug-resistant cancer stem cells play a key role in metastasis and / or recurrence of cancers reminds to be studied (Huntly and Gilliand, 2005).
According to the theory of cancer stem cell (CSC), CSCs are usually survive and growing into a new tumor after chemotherapy and metastasis to other organs (Jordan, Guzman, and Noble, 2007). Based on a previous report before (Andoh, Chock, and Chiueh, 2001), thioredoxin (Trx) expression is higher than normal cells in some
preconditioned SH-SY5Y cancer cells which are less sensitive by 30-fold than the wild type neuroblastoma cells (D cells) to cytotoxic insults evoked by oxidative stress and neurotoxin as well. Recently, CD-133 positive CSCs were isolated from the
preconditioned SH-SY-5Y cells and purified by cell sorting procedure to reach a 90%
homogeneity (unpublished observations of Chiueh et al). These CD-133 positive and Trx-enriched CSCs (N cells) produced tumor in vivo in nude mice which was
2
aggressive in metastasis to other internal organs (Chiueh et al., in preparation). In theory, these Trx-enriched cancer stem cells may be highly resistant to most of
anti-cancer agents leading to drug resistance in chemotherapy and recurrent metastasis with a poor treatment outcome in clinic.
Working Hypothesis
A persistent induction of the redox protein Trx may lead to drug resistance in the chemotherapy of cancers.
Research Goal
The current research goal is to understand the role of the inducible redox protein thioredoxin in the chemo-resistance between pre-conditioned N cells and control D cells following the treatment of a chemotherapeutic agent etoposide.
3
Chapter 2: Literature Review
2.1 Neuroblastoma
Neuroblastoma is an extracranial solid tumor that usually occurs in early childhood with high mortality (Maris et al., 2007). It is one of the few human
malignancies that demonstrate spontaneous regression from an undifferentiated state to a completely benign cellular appearance (Bénard et al., 2008). Neuroblastoma cells are derived from the neural crest and usually arise in the adrenal medulla or along the sympathetic chain of the sympathetic nervous system; it usually originates in the adrenal gland but can also develop in nerve tissues of the neck, chest, abdomen, or pelvis (Israel et al., 1994). It is stratified into three risk categories: low, intermediate, and high risk. Low-risk disease is common in infant and usually has good outcomes with observation only or surgery, whereas high-risk disease is difficult to treat
successfully despite intensive multi-agent induction chemotherapy, radiotherapy, surgery and 13-cis retinoic acid (Brodeur, 2003). High-risk patients whom treated with chemotherapy usually have an inadequate response and the outcome is very poor with relapse and metastasis (Marvis, 2010). It is highly likely that metastasis of neuroblastoma cells may be caused by CSCs.
4
2.2 Etoposide and topoisomerase II
Etoposide is a podophyllotoxin which has been developed in 1966 (Slevin, 1991) and is currently used to treat acute myelioid, hodgkin’s disease, lung cancer, breast cancer and ovarian cancer (Hande, 1998). Now is one of chemotherapy drug that used to treat neuroblastoma. DNA topoisomerases are nuclear enzymes with function of DNA replication, transcription, chromosomal segregation and DNA recombination (Froelich-Ammon, 1995). It has two types present in all cells (i) topoisomerase I which makes single strand break (Wang, 1971) and (ii) topoisomerase II with double strands break and pass (Geller et al., 1976).
Etoposide is a topoisomerase II inhibitor used to treat cancers, it forms a ternary complex with DNA and the topoisomerase II enzyme, prevents re-ligation and causes DNA double strands break (Gordaliza et al., 2004). Because of cancer cells are divide more rapidly, they rely on this enzymes more than healthy cells. So that the complex of etoposide-DNA-topoisomerase II forms DNA breaks permanently and caused errors in DNA replication then ultimately triggers apoptosis of the cancer cell (Froelich-Ammon, 1995). In the present study, Hoechst 33258 was used to imaging apoptotic changes in N cells and D cells with different Trx levels following the etoposide treatment.
5
2.3 Cancer Stem Cells (CSC)
2.3.1 Cancer Stem Cell Theory
According to the timeline from Huntly and Gilliand (2005), the CSC theory was first proposed by Rudolph Virchow in 1855. Furth and kahn (1937) show that the injection of leukemia cells into nude mice can develop a solid tumor. By this method, CSCs are found in many kinds of tumors, like brain, breast, colon, ovary, pancreas, prostate, melanoma et al. (Singh et al., 2003; Al-Hajj et al., 2003; O'Brien et al.,2007;Zhang et al., 2007; Li et al., 2007;Maitland and Collins, 2008; Schatton et al., 2008)
Craig, Monica, and Mark (2007) suggest that normal stem cells exist in human body. Ordinarily, they differentiate into progenitors, and then circulated to the tissue and organs, turning into mature cells. But due to the environmental change, after the mutation of the progenitors and mature cells, they will probably turn into cancer stems which will differentiate into a tumor (Figure 1). It has been suggested that the transformation of normal stem cells into CSCs is because of a long-term and repetitive inflammation in some part of the tissues, which makes the environment nearby low in nutrition and oxygen. And cells are forced into mutation to sustain the harsh condition. When the mutation proceeding to a certain degree, CSCs are formed
6
and will probably become a tumor (Heddleston et al., 2010).
Thus, some researchers has done a statistical analysis of a whole tumor, and found the CSCs do not account for most part but only 1% to 30% of all the cells in a tumor, with highly tentative to differentiation and mutation (Phillips, 2006). CSCs have three capabilities (i) proliferation (ii) differentiation and (iii) self-renew which are similar to the normal stem cells, so this kind of cells is called CSCs (Tobias, Natasha, and Markus, 2009).
7
Figure 1. After mutation, normal stem cell may turn into cancer stem cell, which can probably transform to a tumor itself.
(Modified from Li, 2007 and Schatton et al., 2009)
8
2.3.2 Cancer stem cell markers and chemo-resistance
Surgery, chemotherapy and radiotherapy are commonly seen and arranged together in the treatment of cancers. But recurrence and metastasis are usually happen in many cancer cases. As a result, some researchers have proposed a theory that even though cancer cells are killed, CSCs in the tiny part of a tumor cannot be totally
eliminated (Figure 2). Moreover, the center of the tumor will become lack of oxygen and nutrition, which stimulates these cells into drug-resistance and hinders the treatment (Craig, Monica, and Mark, 2007). So it is widely suggested that if we can find the marker of each kind of cancer to target the CSCs and kill it, especially when treatments and target are applied together, the rest of the normal cancer cells will not be able to survive. In the meanwhile, the whole tumor will gradually disappear. Then the cancer will be cured (Tobias, Natasha, and Markus, 2009; Fiona and Norman, 2011).
CSC markers have two significant characteristics (i) every different tumor type has different cell surface marker, but a cell surface marker can appear in different tumor types and (ii) no marker can be used in every tumor type (Table 1). Therefore, it is necessary to find out a marker that can be extensively used (Tobias, Natasha, and Markus, 2009).
9
Figure 2. Cancer stem cell and chemo-resistance
(1) Escape of CSC from Primary cancer after stress induced by chemotherapy (2) Circulating CSC in cardio-vascular system
(3) Invasion of CSC into organs. (metastasis)
10
Table 1. Cancer stem cell markers
Tumor type Cell surface marker(s) Reference Breast CD44+, CD24− /low
Lineage−ESA+
Al-Hajj et al.
CNS Colon
CD133+
CD133+
Singh et al.
O’Brien et al.
ESAhighCD44+ Lineage−
(CD166+)
Dalerba et al
Ewing’s CD133+ Suva et al.
Head and neck CD44+ Lineage− Prince et al.
Melanoma ABCB5+ Schatton et al.
Ovarian CD44+, CD117+ Zhang et al.
Liver CD90+, CD45−(CD44+) Yang et al.
Pancreas CD44+, CD24+, ESA+ Li et al.
CD133+ Hermann et al.
(Modified from Tobias, Natasha, and Markus, 2009)
11
2.4 Thioredoxin (Trx)
2.4.1 Trx system
Thioredoxins (Trx) are a 12 kDa redox-active proteins that contain two cysteines in the conserved center and present in all prokaryotic and eukaryotic organisms. Their redox-active sites are conserved by -Trp-Cys-Gly-Pro-Cys-Lys- (Holmgren, 1985; Eklund, Gleason, and Holmgren, 1991). The oxidized (inactive) form of Trx (Trx-S2) can be reduced by thioredoxin reductase (TrxR) with NADPH exist, and the two cysteines formed by disulfide bond at the active site are turn into a dithiol reduced (active) form of Trx (Trx-SH2) (Holmgren, 1995). The Trx system affect lots of biological functions such as protecting cells against apoptosis,
angiogenesis, DNA replication and cell proliferation, control and regulation of numerous transcription factors activities (Andoh, Chock, and Chiueh, 2002; Laurent, Moore, and Reichard ,1964; Welsh et al., 2002; Luthman et al.,1979; Matthews et al., Abate et al., 1990). It has been observed that Trx levels are relatively high in some cancers which may link to poor treatment outcome, and was 30-times less sensitive to the neurotoxin agent, MPP+ (Andoh, Chock, and Chiueh, 2001). And transfecting of Trx into cancer cells develops chemo-resistance (Yokomizo et al., 1995). Therefore, in the present proposal a new working hypothesis was developed and used to study the
12
redox role of the endogenous Trx in N cells on etoposide-induced apoptotic death and also chemo-resistance in Trx-enriched cancer cells.
2.4.2 Inhibition of Trx system
There are many kinds of Trx inhibitors or TrxR inhibitors used in clinical, such as Azelaic Acid and carmustine, respectively (Schallreuter and Wood, 1987; Schallreuter, Gleason, and Wood, 1990). Because (i) Trx is important in cancer cell survival and TrxR plays a key role in the thioredoxin system (ii) TrxR C-terminal active site can be targeted and inhibited by different electrophilic compounds. Therefore, TrxR has been measure to be a target of anticancer agents (Nguyen et al., 2006). DNCB is an
electrophilic compounds and one of the TrxR inhibitor which is irreversible inhibit TrxR activity (Arner, Bjornstedt, Holmgren, 1995). This function is because of the Cys and Sec in C-terminal active site is alkylated by DNCB (Nordberg, 1998). Thus, DNCB is used to evaluate the effect of Trx in CSC-liked N cell.
13
Chapter 3: Materials and Methods
3.1 Materials
Human Neuroblastoma SH-SY5Y cells (D cells) were kindly provided by Dr.
Carol Thiele (NCI), and the pre-conditioned Human Neuroblastoma SH-SY5Y cells (N
cells) was derived from Human Neuroblastoma SH-SY5Y cells following multiple oxidative stresses. The Dulbecco’s modified Eagle’s medium, Etoposide, Hoechst
33258 (bisbenzimide), DNCB (1-Chloro-2,4-dinitrobenzene) and trypan blue was supplied by Sigma Aldrich (St. Louis, MO). Fetal bovine serum was purchased from BIOLOGICAL INDUSTRIES (Kibbutz Beit Haemek). The human Trx antibody and the actin antibody were obtained from abcam (Cambridge, MA) and Cell Signaling (Beverly, MA), respectively. The goat anti-rabbit IgG secondary antibody was purchased from Millipore (Billerica, MA). Protease inhibitor cocktail and RNase A were from BIONOVAS (Toronto, Ontario).
14
3.2 Cell Cultures
The wild type D cells were cultured in Dulbecco’s modified Eagle’s medium with 10% fetal bovine serum. And the pre-conditioned Human Neuroblastoma
SH-SY5Y cells (N cell) were also cultured in Dulbecco’s modified Eagle’s medium but 5% fetal bovine serum. Both cell lines were incubated in 5% CO2 without phenol red at37°C and changed medium every 2-3 days.
3.3 Nuclear Staining of Apoptotic Cells by Hoechest 33258
Both D and N cells were cultured to 4-5 passage and then seeded into a coated 24-well plate with different density. The Human Neuroblastoma SH-SY5Y cells seeded with 3X104 cells per well and the pre-conditioned Human Neuroblastoma SH-SY5Y cells were seeded with 4X104 cells per well. After 2 to 3 days, Etoposide was added with different concentration (0.85 μM, 8.5 μM, 85 μM and 1.2% DMSO only as the control of 0 μM) and incubated for 24 hours. At the end, both cells were washed by PBS with 3 times and then fixed with 4% paraformaldehyde in PBS at room temperature for 30 min. After PBS washed 3 times, cells were stained by 1 μM Hoechst 33258 fluorescent dye for 5 min at room temperature. The cell nuclear DNA was imaged and photographed with a fluorescent microscope. The apoptotic cells were identified by the present of nuclear condensation and DNA fragmentation.
15
3.4 Trypan Blue Exclusion Assay
Both cells were seeded in 6-cm dish with density of 5X105cells/well(N cell) and 4X105cells/well (D cell). After 2 to 3 days, Etoposide was added with different concentration (0, 0.85, 8.5, 85μM). After incubated with Etoposide for 24 hours, cells
were resuspended and then centrifuged with 900 rpm. After centrifugation, cell pellets were re-suspended with PBS and the stained cells were counted by a heamocytometer.
3.5 Western Blotting
Cells were added cell lysis bufferd 20 mM Hepes-KOH pH 7.5, 10 mM KCl, 1.5 mM MgCl2, 1 mM EDTA, 1 mM dithiothreitol, and 10 μL protease inhibitor and then lysed by sonication. The protein concentration was quatified by BSA kit (Bio-Rad). Lyzed cellular protein extract (20 or 40 μg) was separated by a 20%
SDS-PAGE and then transfer to polyvinylidene difluoride membrane (Millipore).
After PBST (phosphate buffered saline and Tween-20) washed 3 times, polyvinylidene difluoride membrane was blocked by Gelatin- NET (0.25% Gelatin, 0.15 M NaCl, 5 mM EDTA-2Na, 0.05% Tween 20, 50 mM Tris/HCl pH8.0) for 30 min at room
temperature. The target protein was probed by human Trx antibody (1:1000) and actin antibody as control (1:1000) at 4°C overnight. After washing with PBST 3 times, the polyvinylidene difluoride membrane was incubated with 1000X dilution of secondary
16
goat anti-rabbit IgG antibody which was linked with horseradish peroxidase at room temperature for 1 hour. At the end, the target bands that bounded with horseradish peroxidase were reacted with ECL and monitored by Chemiluminescence Imaging System (BioSpectrum 500). The signaling area and intensity was quantified by the Image J.
3.6 MTT Assay for Mitochondria Activity
Both cell lines were cultured to 4-5 passage and then seeded into 24-well plate.
Both Human Neuroblastoma SH-SY5Y cells and the pre-conditioned Human Neuroblastoma SH-SY5Y cells were seeded with 5X104 cells per well. After incubation for 1 to 2 days, etoposide was added into medium with different
concentrations (0.85, 8.5, 85 μM and DMSO only as 0 μM control) and incubated for 24 hours. At the end, both cells were washed by PBS with 3 times, fresh medium
contained 50 μl MTT colorimetric dye (5 mg/ml)was added intoeach well and
incubated for 3-4 hours. After removed the supernatant, cells were washed by PBS with 3 times and resuspeded the formazen by 1mL DMSO. Each well was taken 200 μL and measured O.D 595 by ELISA reader (Nunc, 269620).
17
Chapter 4: Preliminary Results
4.1 Trx expression and N cells
It is known that Trx level is higher in some cancer cell line, such as U-87MG, astroglioma; HeLa, cervical carcinoma; A549, lung carcinoma. The present study investigated whether these Trx-enriched cancer cells are resistant to most of the chemo-therapeutics. Increased Trx is also observed in the human neuroblastoma SH-SY5Y cells following pre-conditioning stress (Andoh, Chock, and Chiueh, 2001).
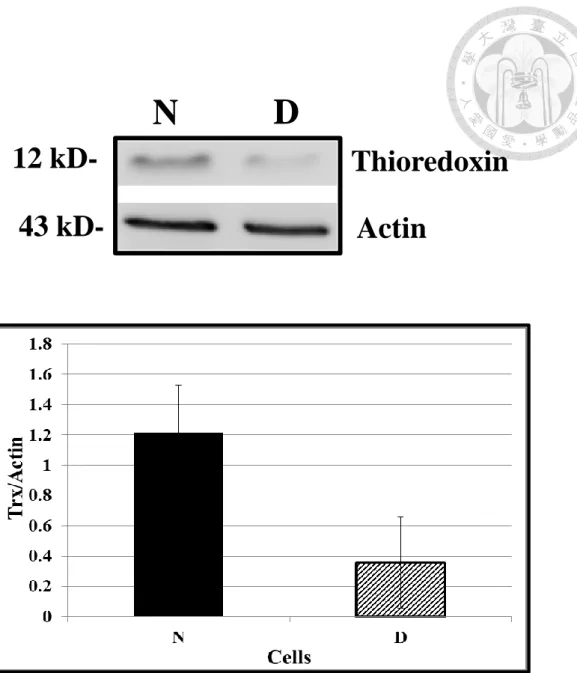
Repeated pre-condition SH-SY5Y cells with increased levels of Trx are less sensitive to oxidative injury and cyto-toxicity. Most of the Trx-enriched and pre-conditioned cells are CD-133 positive (N cells; unpublished observation). The present study used Western blotting (Figure 3), and confirmed that Trx level was higher in the N cells than in the human neuroblastoma SH-SY5Y cells (D cells) by 3 times.
18
Figure 3. The expression of Trx in N cell and D cell by Western blotting
(A) Each land were loaded with 20μg of total protein extraction. The Trx expression was monitored by anti-human Trx polyclonal antibody, and the band was in 12 kD.
Actin as loading control (43kD) was monitored by anti-human Actin monoclonal antibody. Human neuroblastoma SH-SY5Y cells were labeled as D cells; CD-133 positive pre-condition human neuroblastoma SH-SY5Y cells were labeled as N cells.
(B) The expression of Trx was measured by western blotting. (n = 3, mean ± SD)
N D
Actin
12 kD- Thioredoxin
43 kD-
19
4.2 Effects of etoposide
4.2.1 Apoptotic cell changes
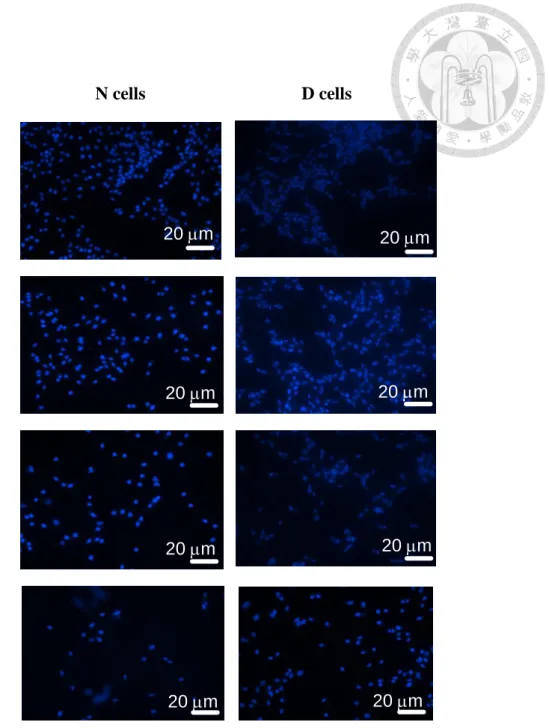
Hoechst 33258 fluorescence dye was used to measure the apoptosis cell changes in N cells and D cells following the treatment with etoposide (0, 0.83, 8.3, 83 μM) for 24 hours. The results of fluoro-microscopy (Figure 4) revealed that D cells were more sensitive than N cells in etoposide-evoked apoptotic changes in nuclei (i.e, nuclear condensation, fragmentation, phenotype changed) concentration dependently.
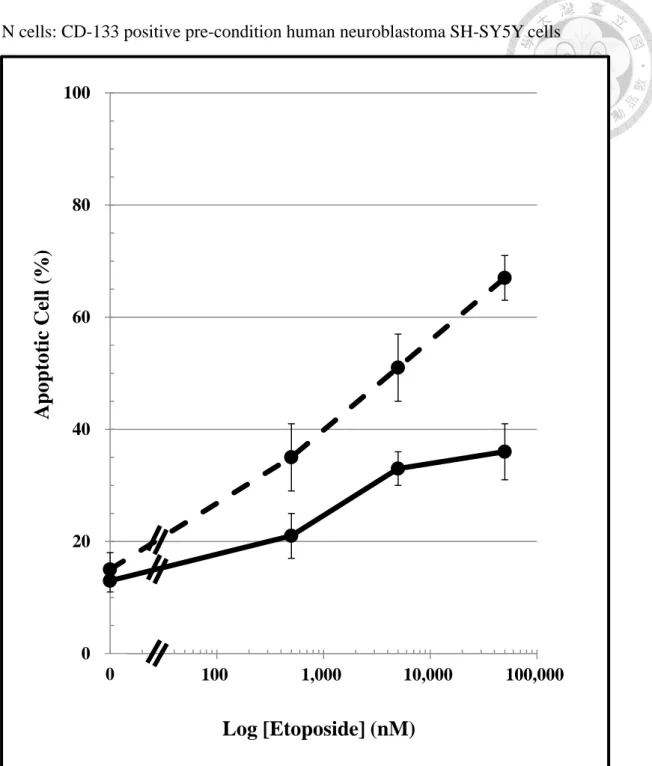
Moreover, etoposide caused a concentration-dependent apoptotic changes in the nuclear of both N cells and D cells. The result of apoptotic changes show that Trx-enriched N cells were less sensitive than D cells following etoposide treatment (Figure 5). The estimated in apoptotic cell changes between N cell and D cell was 1.63 ± 0.15 following the 24-hours treatment with etoposide.
20
Figure 4. Effcts of Etoposide on apoptotic changes in nuclear staining by Hoechst 33258 fluorescent dye in D and N cells.
Typical results of microphotography by the fluorescence microscopy in D cells and N cells (200X) , 24 hours following the treatment with different concentration of etoposide (0.83, 8.3, 83 μM), and control media contained with 1.2% DMSO. Hoechst 33258 nuclear staining for apoptotic changes in nuclei (i.e, nuclear condensation,
fragmentation, size changed). D cells: Human neuroblastoma SH-SY5Y cells
N cells D cells
Control
0.83
8.3
83
20 m 20 m
20 m 20 m
20 m
20 m 20 m
20 m Etoposide
(μM)
21
N cells: CD-133 positive pre-condition human neuroblastoma SH-SY5Y cells
Figure 5. Apoptotic cell death of D cells and N cells evoked by etoposide (0 to 83 μM). For methods, please see the legend of Figure 2.
Each well were taken 4 photos and calculated by Hoechst 33258 nuclear staining for apoptotic changes in nuclei. (n = 3, mean ± SD).
N cells, CD-133 positive pre-condition human neuroblastoma SH-SY5Y cells 0
20 40 60 80 100
10 100 1,000 10,000 100,000
A po pto ti c C el l (% )
Log [Etoposide] (nM)
0
22
D cells, Human neuroblastoma SH-SY5Y cell
4.2.2 Comparison of cell viability in D and N cells following etoposide treatment
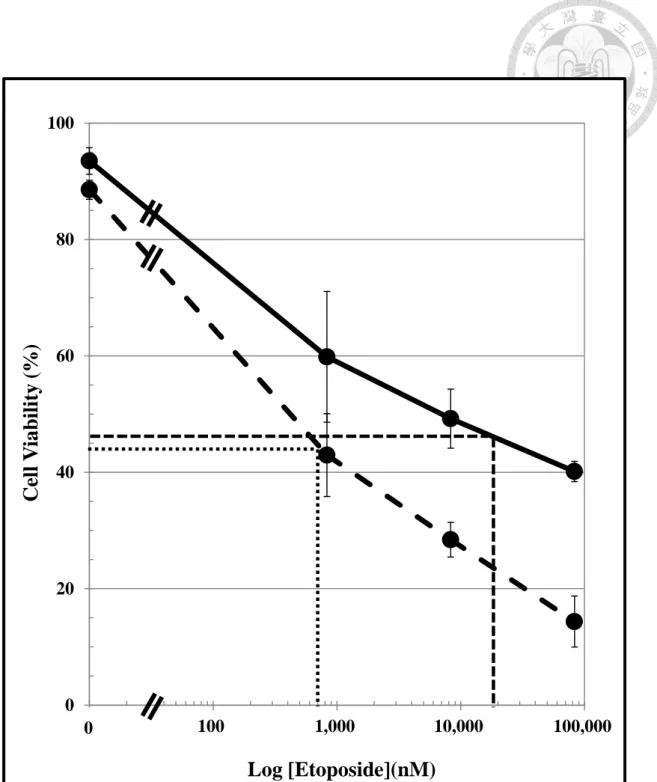
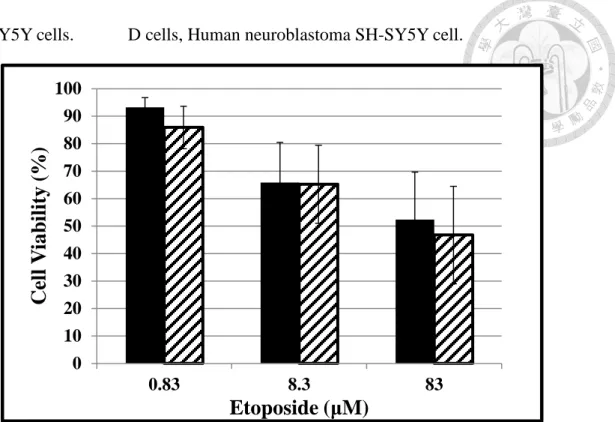
Trypan blue exclusion method was used to measure the concentration-dependent response following a 24-hours treatment of N and D cells with etoposide (0 to 83 μM) (Figure 6). The present concentration-dependent cell viability curve was plotted on a semi-log paper which revealed a linear response curve in both D cells and N cells. The estimated IC50 valuesof etoposide in D cells and N cells were 700 nM and 19,000 nM, respectively. This result shows that N cells were less sensitive to etoposide than D cells by 27-fold. The cell mitochondria viability of both N and D cells has decreased measured by MTT method (Figure 7). But the decreased ratio of both cells was as the same level.
23
Figure 6. Comparison by Trypan blue exclusion method in D cells and N cells following Etoposide treatment (0 to 83 μM).
Cells were treated with etoposie (0 to 83 μM) for 24 hours and the cell viability was measured by the trypan blue exclusion method. (n = 6~8, mean ± SD)
IC50 of D cells ( ) was estimated as 700 nM; IC50 of N cells ( ) was estimated as 1,900 nM. N cells, CD-133 positive pre-condition human neuroblastoma
0 20 40 60 80 100
10 100 1,000 10,000 100,000
C el l V ia bi li ty (% )
Log [Etoposide](nM)
0
24
SH-SY5Y cells. D cells, Human neuroblastoma SH-SY5Y cell.
Figure 7. Effect of etoposide on cell viability, reflected by cell mitochondrial activity was assayed by MTT method in D and N cells.
N cells, CD-133 positive pre-condition human SH-SY5Y cells D cells, Human neuroblastoma SH-SY5Y cells
(n = 12)
MTT, 3-(4,5-dimethylthiazol-2-yl)-2,5diphenyltertrazolium bromide, was measured the activity of cellular enzymes that reduce the tetrazolium dye to its insoluble formazen.
The color of this dye was changed from yellow to purple.
0 10 20 30 40 50 60 70 80 90 100
0.83 8.3 83
C el l V ia bi li ty (% )
Etoposide (μM)
25
4.3 The effect of the inhibitor of redox cycling of Trx on etoposide-induced apoptosis in N cells
4.3.1 Effect of DNCB on etoposide-induced apoptosis in N cells
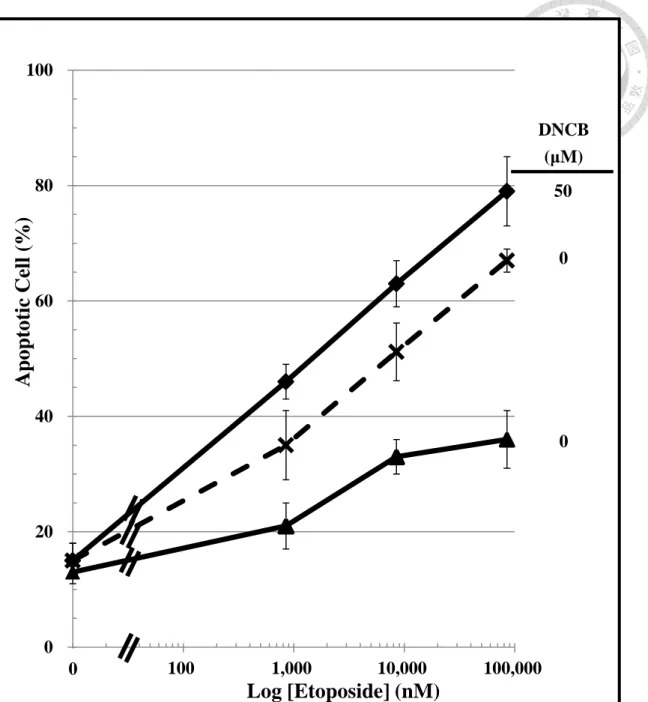
As the results that Trx expression was higher in N cells which were resistant to the etoposide-induced apoptosis and cell death; DNCB was used to block the redox cycling of Trx for understanding the role of endogenous Trx on the chemo-resistance in
oncology. To understand this, the etoposide treated N cells (0 to 83 μM) were co-treat with or without DNCB (50 μM). Etoposide caused a concentration-dependent manner of apoptosis in both N and D cells (Figure 8). Without DNCB co-treatment, etoposide caused a smaller apoptosis change in N cells than D cells by 1.8 times.
Co-treatment of N cells with DNCB increased the sensitivity of N cells to etoposide- induced apoptosis which was similar to untreated D cells.
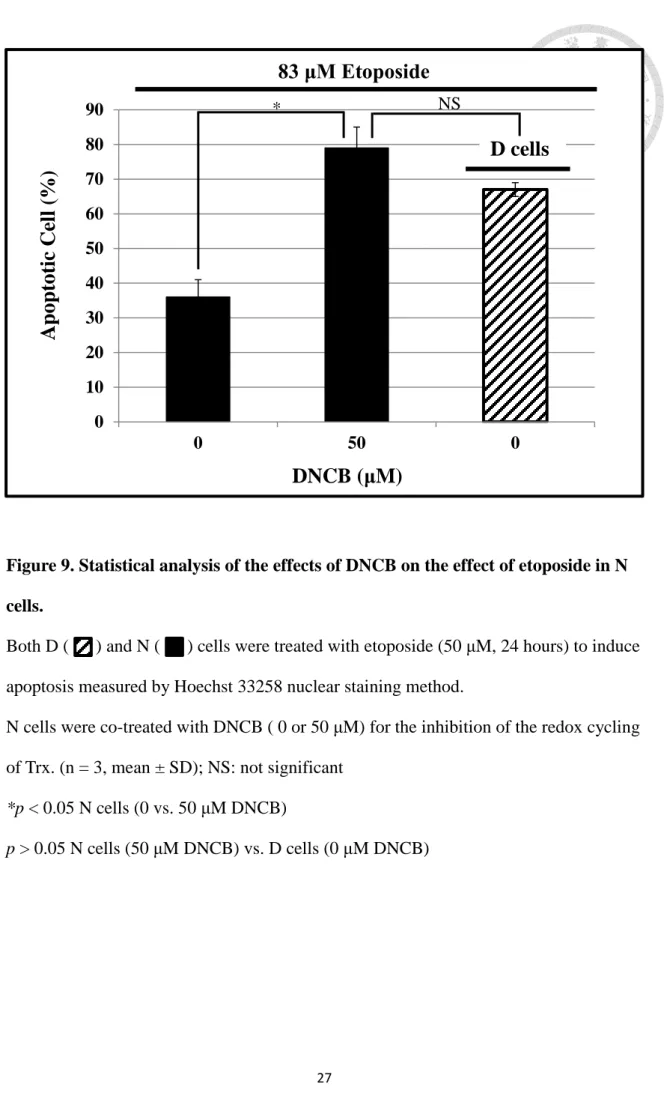
Without DNCB co-treatment (Figure 9), etoposide (83 μM) produced a greater apoptosis in D cells (67%) than N cells (35%). But when the etoposide-treated N cells were co-treated with 50 μM of DNCB, the apoptotic cell population was increased significantly to 79% (p < 0.05), which was not statistically different from D cells with low Trx following treatment of D cells which was treated with etoposide.
26
Figure 8. Effect of endogenous Trx redox cycling on the concentration-dependent apoptosis caused by etoposide in N cells.
N cells ( ), CD-133 positive pre-conditioned human SH-SY5Y cells were co-treated with 0 or 50 μM DNCB and etoposide. Human neuroblastoma SH-SY5Y cells (D cells, ) were used as control. Each well was calculated by Hoechst 33258 nuclear staining for apoptotic changes in nuclei (i.e, nuclear condensation,
fragmentation). DNCB was an inhibitor of Trx reductase which blocks the redox cycling of endogenous Trx in N cells. (n = 3, mean ± SD)
0 20 40 60 80 100
10 100 1,000 10,000 100,000
A po pto ti c C el l (% )
Log [Etoposide] (nM)
50
0
0 DNCB
(μM)
0
27
Figure 9. Statistical analysis of the effects of DNCB on the effect of etoposide in N cells.
Both D ( ) and N ( ) cells were treated with etoposide (50 μM, 24 hours) to induce apoptosis measured by Hoechst 33258 nuclear staining method.
N cells were co-treated with DNCB ( 0 or 50 μM) for the inhibition of the redox cycling of Trx. (n = 3, mean ± SD); NS: not significant
*p < 0.05 N cells (0 vs. 50 μM DNCB)
p > 0.05 N cells (50 μM DNCB) vs. D cells (0 μM DNCB) 0
10 20 30 40 50 60 70 80 90
0 50 0
A po pto ti c C el l (% )
DNCB (μM)
D cells 83 μM Etoposide
* NS
28
4.3.2 Effect of DNCB on Trx levels
The result of western blotting shows that the cellular Trx levels were not changed in the DNCB (50 μM) treated N cells (Figure 10). DNCB is an inhibitor of Trx reductase, so that the redox cycling of Trx was blocked that led to the oxidative form of Trx cannot be reduced back to the biol-active reduced form (Arner, Bjornstedt, and Holmgren, 1995).
29
Figure 10. The expression of Trx in N cell with DNCB (0, 5, 10 and 50 μM) added by Western blotting.
CD-133 positive pre-condition human SH-SY5Y cells (N cells) following the treatment of etoposide (0 to 83μM) for 24 hours. Each land was loaded with 40μg of total protein extraction. The Trx expression was monitored by anti-human Trx polyclonal antibody, and the band was in 12 kD. Actin as loading control (43kD) was monitored by
anti-human Actin monoclonal antibody. (n = 4)
DNCB, 1-Chloro-2,4-dinitrobenzene, which is an inhibitor of TrxR (Thioredoxin reductase).
0
Actin
Thioredoxin 5 10 50
DNCB (μM)
12 kD-
43 kD-
30
4.4 The changes of etoposide-treated D cells
4.4.1 Trx level in etoposide-treated D cells
The Trx expression of human neuroblastoma SH-SY5Y cells (D cells) following the treatment of etoposide (0 to 83 μM) for 24 hours was monitored by Western blotting (Figure 11). It showed that the expression were increased, and were reached highest level at 8.3μM etoposide treated D cells by 3.8 fold. At 83 μM etoposide treated D cells, the expression of Trx was higher than untreated D cells by 2.3 fold.
4.4.2 Effect of etoposide on D cell morphology
Comparison of cell morphology on D and N cells has some differences between two cells (Figure 12). D cells were bigger and had much longer synapse than N cells.
But there has some changed after 83 μM etoposide treatment, D cells were much smaller than before and its synapse were shorter even disappear. Under Hoechst 33258 staining in nuclear, D cells nuclear changed from oval to round. D cells looked much more like N cells on morphology and nuclear shape after 83 μM etoposide treatment.
31
Figure 11. The expression of Trx in D cell with etoposide treatment (0 to 83 μM) by Western blotting.
Human neuroblastoma SH-SY5Y cells (D cells) treated with etoposide (0 to 83μM) for 24 hours. Each land was loaded with 40μg of total protein extraction and ran of 20%
SDS-PAGE (A). The Trx expression was monitored by anti-human Trx polyclonal antibody, and the band was in 12 kD. Actin as loading control (43kD) was monitored by anti-human Actin monoclonal antibody. (B) The expression of Trx was measured by western blotting. (n = 3, mean ± SD) *p < 0.05
0 0.5 1 1.5 2 2.5 3 3.5 4
0 0.83 8.3 83
T rx /A cti n
Etoposide (μM) 0
Actin
Thioredoxin 83
8.3 Etoposide (M)
12 kD-
43 kD-
0.83 (A)
(B)
*
*
32
Etoposide
(μM) White Light UV
0 (D cell)
83 (D cell)
0 (N cell)
Figure 12. The phenol type under white light and Hoehst 33258 nuclear staining of cell nuclear following etoposide treatment (0 or 83 μM) for 24 hours in N and D cells
20 μm
20 μm 20 μm
20 μm
20 μm 20 μm
33
Chapter 5: Discussion
The present results support a new research idea that increases the expression of Trx can prevent cancer cells from apoptotic cell death caused by etoposide (Figure 13).
Etoposide form ternary complex with the topoisomerase II leading to DNA double strands break which cause cell apoptosis and related death as demonstrated in the present study that supports its indication (Gordaliza et al., 2004). Andoh , Chock , and Chiueh, (2002) suggest that Trx prevents apoptosis by (i) increasing mitochondrial bcl2, (ii) decreasing pro-apoptotic cytochrome C and (iii) inhibition of pro-apoptoic caspase-3 and -9 enzymatic activities. In fact, Trx prevents apoptosis and cell death caused by etoposide in N cells (Figure 6) through a redox cycling mechanism of Trx which was blocked by DNCB (Figure 7). The exact molecular event of anti-apoptotic mechanism of Trx reminds to elucidate further in the near future.
5.1 Role of Trx on etoposide-treated N cells.
Etoposide is one of the chemotherapy drugs for the treatment of multiple cancers via DNA double strands break (Geller et al., 1976) leading to apoptosis (Figure 5).
Trx is expressed in cancer cells which might be responsible for poor treatment outcome (Andoh , Chock , and Chiueh, 2002). Consistently, it has been reported that
transfected Trx into cancer cells can increase cell survival (Yokomizo et al., 1995).
34
The pre-conditioned human neuroblastoma SH-SY5Y cells contain high levels of endogenous Trx (Andoh, Chock, and Chiueh , 2002). The present results confirm that Trx expression was higher in the preconditioned N cells than the wild type D cells by 3 times (Figure 3). Furthermore, Trx-enriched N cells were less sensitive than D cells after etoposide-induced apoptotic change (Figure 5) and cell viability (Figure 6).
The results were in agreement with the prior report of transfecting Trx into cancer cells leading to less apoptosis cell changes after chemotherapy (Geller et al., 1976). On the other hands, N cells were less sensitive than D cells by 27-fold by in cell death caused by etoposide (Figure 7). The etoposide-induced LD50 in N cells and D cells was approximate 27-fold (N cells > D cells) which is consistent with a previous report on apoptosis and cytotoxicity caused by a neurotoxin MPP+ in human neuroblastoma cells with or without preconditioning (Andoh , Chock, and Chiueh , 2002).
DNCB is a Trx reductase inhibitor which can block the redox cycling of
endogenous Trx and thus related biological activities (Arner, Bornstedt, and Holmgren , 1995). Compared to low-Trx D cells, N cells had fewer cells under apoptosis if cells were treated with 83 μM etoposide. On the contrary, etoposide-treated N cells had more cells under apoptosis if N cells were co-treated with 50 μM DNCB (Figure 8) to block the redox cycling of Trx. This result infers that Trx play a critical role in the development of chemo-resistance following etoposide treatment. The
35
concentration-dependent manner of etoposide-induced apoptosis was not different in naïve D cells and N cells treated with 50 μM DNCB but much less in the naïve N cells
(Figure 9). The Western blotting of Trx expression showed that Trx level was not changed in DNCB-treated N cells (Figure 10), DNCB only affect the redox cycling of Trx by blocking Trx reductase. The oxidized form of Trx cannot be reduced to the active reduced form (Elias and Holmgren, 2000). These results are consistent with previous reports that reductive form of Trx could play a critical functional role in cell anti-oxidant, anti-apoptosis and anti-DNA damage (Andoh, Chock, and Chiueh, 2002).
The result of MTT method (Figure 7) was not as the same as the trypan blue exclusion method, that there was no significant difference between N and D cells.
According to previous reports, MTT method measures the activity of mitochondria as an index of the cell viability (Gerlier and Thomasset, 1986). The negative result of MTT shows that etoposide might have no effect on cell mitochondria activity.
Etoposide-induced cell death was not due to alteration of mitochondrial activity reflecting by a negative MTT assay. But etoposide may lead to DNA damage and apoptosis resulting in cell death of cancer cells.
36
5.2 D cell may promote it cell survival by increased Trx expression
There are some kinds of cancers are resistant to chemotherapy. Neuroblastoma is one of them with poor survival outcome in the clinical after treatment and with high relapse rate (Marvis, 2010). The Trx level in the chemo-resistance cancer cells is higher than others that has been observed in neuroblastoma (Andoh, Chock, and Chiueh, 2002), but the Trx level after chemotherapy has not been report before. Here we have observed that Trx level was increased by 3.7 in etoposide-treated D cells (Figure 11) while morphology of D cells was altered to mimicking N cells in etoposide-treated D cells (Figure 12). The results shows that (i) cells may prevent from etoposide-induced DNA damage by increased its level and may have effect on the cell morphology that changed from D cell to N cell-like or (ii) etoposide may spare the Trx-enriched cells.
On the other hands, actin has founded to be a pro-apoptosis signal in the extrinsic apoptosis cell death pathway (Desouza M, Gunning PW, and Stehn JR., 2012).
Therefore, the increase of Trx in etoposide-treated D cells could be an error measured because of the decrease of actin. This error measured of Trx level in etoposide-treated D cells can be tested by other negative control protein, GAPDH, or measured RNA level by Real-time PCR. The other chemo-drug, vincristine (data not showed), also showed that N cells were less sensitive to the tubulin-target apoptosis than D cells, but
37
Trx level has not changed after treatment with vincristine (0 to 2.5 μM). Cancer cells may elevate Trx levels to protect itself from DNA-damage stress and apoptosis evoked by etoposide but not vincristine-induced tubulin stress.
38
Figure 13. Trx Pathway Blocks etoposid-induced DNA Breaks
(1) Etoposide forms a ternary complex with DNA and Topoisomerase II lead to DNA double strand breaks.
(2) Trx may inhibit the DNA break and apoptosis caused by etoposide.
(3) DNCB is a Trx reductase inhibitor, it blocks the redox cycling of Trx so that the reduced form of Trx cannot executive it function to inhibit the DNA break and apoptosis caused by etoposide.
(2) (1)
Etoposide
Topoisomerase II
(i) DNA double strand breaks
(ii) Apoptosis
(3)
DNCB
Trx-SH
2Trx-S
2Trx reductase
Redox Cycling
Trx peroxidase
Trx
39
Chapter 6: References
Abate C, Patel L, Rauscher FJ, Curran T (1990) Redox regulation of fos and jun DNA-binding activity in vitro. Science; 249:1157-1161.
Al-Hajj M, Wicha MS, Benito-Hernandez A, Morrison SJ, Clarke MF (2003) Prospective identification of umorigenic breast cancer cells. Proceedings of the National Academy of Sciences of the United States of America 100: 3983-3988.
Andoh T, Chock PB, Chiueh CC (2002) The Roles of Thioredoxin in Protection against Oxidative Stress-induced Apoptosis in SH-SY5Y Cells. J Biol Chem 277,
9655-9660.
Arner ESJ, Bornstedt M, Holmgren A (1995) 1-Chloro-2,4-dinitrobenzene is an
irreversible inhibitor of human thioredoxin reductase. Loss of thioredoxin disulfide reductase activity is accompanied by a large increase in NADPH oxidase activity. J Biol Chem 270:3479-3482.
Arner ESJ, and Holmgren A (2000) Physiological functions of thioredoxin and thioredoxin reductase. Eur. J. Biochem 267: 6102-6109.
Bénard J, Raguénez G, Kauffmann A, et al. (2008). MYCN-non-amplified metastatic neuroblastoma with good prognosis and spontaneous regression: a molecular portrait of stage 4S. Mol Oncol 2: 261–71.
Brodeur GM (2003) Neuroblastoma: biological insights into a clinical enigma.
Nat Rev Cancer 3: 203–216.
Desouza M, Gunning PW, Stehn JR (2012). The actin cytoskeleton as a sensor and mediator of apoptosis. Bioarchitecture 2:75-87.
Eklund H, Gleason FK, Holmgren A (1991) Structural and functional relations among thioredoxins of different species. Proteins 11:13-28.
40
Froelich-Ammon SJ, Osheroff N. (1995) Topoisomerase poisons: harnessing the dark side of enzyme mechanism. J Biol Chem 270: 21429-21432.
Furth J, Kahn MC (1937) The transmission of leukaemia of mice with a single cell. Am J Cancer 31:276–282.
Gellert M, Mitzuuchi K, O’Dea MH, Nash HA (1976) An enzyme that introduces superhelical turns into DNA. Proc Natl Acad Sci USA 73: 3872-2876.
Gerlier D and Thomasset N (1986) Use of MTT colorimetric assay to measure cell activation. Journal of Immunological Methods 94: 57-63.
Gordaliza M, García PA, del Corral JM, Castro MA, Gómez-Zurita MA (2004)
Podophyllotoxin: distribution, sources, applications and new cytotoxic derivatives.
Toxicon 44: 441–59.
Hande KR (1998) Etoposide: Four decades of development of a topoisomerase II inhibitor. Eur. J. Cancer 34: 1514–1521.
Heddleston JM, Li Z, Lathia JD, Bao S, Hjelmeland AB and Rich JN (2010) Hypoxia inducible factors in cancer stem cells .British Journal of Cancer .102: 789–795.
Holmgren A (1985) Thioredoxin. Annu Rev Biochem 54:237-271.
Holmgren A (1995) Thioredoxin structure and mechanism: conformational changes on oxidation of the active-site sulfhydryls to a disulfide. Structure; 3:239-243.
Huntly BJ, Gilliland DG (2005) Leukaemia stemcells and the evolution of cancer-stem-cell research. Nat Rev Cancer 5:311–321.
Israel M A, Hay R J, Park, JG, and Gazdar, A (1994) Atlas of Human Tumor Cell Lines. San Diego: Academic Press, pp. 42–78.
Jordan CT, Guzman ML, and Noble M (2007) Cancer stem cell. N Engl J Med 355:
1253-1261.
Laurent TC, Moore EC, Reichard P (1964) Enzymatic synthesis of
41
deoxyribonucleotides. IV. Isolation and characterizationof thioredoxin, the Hydrogen donor from escherichia Coli. J Biol Chem 239:3436-3444.
Li C, Heidt DG, Dalerba P, Burant CF, Zhang L, Adsay V, Wicha M, Clarke MF,
Simeone DM (2007) Identification of pancreatic cancer stem cells. Cancer Res 67:
1030–7.
Luthman M, Eriksson S, Holmgren A, Thelander L (1979) Glutathione-dependent hydrogen donor system for calf thymusribonucleoside-diphosphate reductase.
Proc Natl Acad Sci USA; 76:2158-2162.
Maitland NJ, Collins AT (June 2008) Prostate cancer stem cells: a new target for therapy.
J Clin Oncol. 26: 2862–70
Maris JM, Hogarty MD, Bagatell R, Cohn SL (2007) Neuroblastoma. Lancet 369:
2106–2120.
Maris JM (2010) Recent advances in neuroblastoma. N Engl J Med 362: 2106-2120.
Matthews JR, Wakasugi N, Virelizier JL, Yodoi J, Hay RT (1992) Thioredoxin regulates the DNA binding activity of NFB by reduction of a disulphide bond involving cysteine 62. Nucleic Acids Res 20:3821-3830.
Nguyen P, Awwad RT, Smart DD, Spitz DR, Gius D (2006). Thioredoxin reductase as a novel molecular target for cancer therapy. Cancer Lett 236:164-174.
Nordberg J, Zhong L, Holmgren A, Arner ES (1998). Mammalian thioredoxin reductase is irreversibly inhibited by dinitrohalobenzenes by alkylation of both the redox active selenocysteine and its neighboring cysteine residue. J Biol Chem
273:10835-10342
O'Brien CA, Pollett A, Gallinger S, Dick JE (2007). A human colon cancer cell capable of initiating tumour growth in immunodeficient mice. Nature 445: 106–10.
Ruth H, Angelika E, Tomoro H, Jane EM, Naohiko I, Patricia F,Anna MC, Audrey EE,
42
and Garrett MB (2002). Resistance to Chemotherapy Mediated by TrkB in Neuroblastomas. Cancer Res 62:6462-6466
Schallreuter KU and Wood JM (1987). Azelaic acid as a competitive inhibitor of thioredoxin reductase in human melanoma cells. Cancer Lett 36: 297–305.
Schallreuter KU, Gleason FK, Wood JM (1990). The mechanism of action of the nitrosourea anti-tumor drugs on thioredoxin reductase, glutathione reductase and ribonucleotide reductase. Biochim Biophys Acta 1054:14-20.
Schatton T, Murphy GF, Frank, NY, Yamaura K, Waaga-Gasser AM, Gasser M, Zhan Q, Jordan S, Duncan LM, Weishaupt C, Fuhlbrigge RC, Kupper TS, Sayegh MH, Frank MH (2008). Identification of cells initiating human melanomas. Nature 451:
345–9.
Schatton T, Frank NY, Frank MH (2009) Identification and targeting of cancer stem cells. Bioessays 31: 1038–1049.
Singh SK, Clarke ID, Terasaki M, Bonn VE, Hawkins C, Squire J, Dirks PB (2003) Identification of a cancer stem cell in human brain tumors. Cancer Res 63: 5821–8.
Slevin M (1991) The clinical pharmacology of etoposide. Cancer 67: 319-329.
Wang JC (1971) Interaction between DNA and E. coli: protein omega. J Mol Biol 55:
523-533.
Welsh SJ, Bellamy WT, Briehl MM, Powis G (2002) The redox protein thioredoxin-1 (Trx-1) increases hypoxia-inducible factor 1alpha protein expression: Trx-1 overexpression results in increased vascular endothelial growth factor production and enhanced tumor angiogenesis. Cancer Res 62:5089-5095.
Yokomizo A, Ono M, Nanri H, Makino Y, Ohga T, Wada M, Okamoto T, Yodoi J, Kuwano M, Kohno K (1995) Cellular levels of thioredoxin associated with drug sensitivity to cisplatin, mitomycin C, doxorubicin, and etoposide. Cancer Res
43
55:4293-6.
Zhang S, Balch C, Chan MW, Lai HC, Matei D, Schilder JM, Yan PS, Huang TH, Nephew KP (2008). Identification and characterization of ovarian cancer-initiating cells from primary human tumors. Cancer Res 68: 4311–20.
問與答
論文口試摘要
本論文口試 2013 年 6 月 24 日上午 10 點於生化科技所 509 室舉行,承蒙 下列五位老師:莊榮輝教授、闕狀卿教授 (本論文共同指導教授)、許秀蘊教 授、楊健志副教授以及黃楓婷助理教授審核。口試摘要匯集書面補充回答後,
依照提問順序加以記錄及整理。
許秀蘊老師:
1. Wild type chemoresistance cell line 是否在 hypoxia 狀態下從 D cell 篩選出 來?Trx 及 CD133 表現?
回答: D cell 為 wild type 也就是一般 commercial 的 cell line,其 Trx 表現量 較低或是沒有表現; N cell 則是由 D cell 經過許多 stress 篩選出來的 cell line,
其會表現較高量的 Trx。CD133 則是細胞表面的抗原,現今則是作為腦神經 CSC 的特殊表面抗原指標;經本實驗室鄔承佑學長的實驗發現,D cell 不會 表現 CD133 而 N cell 則會表現。
2. Etoposide 濃度參考為何?
回答: Etoposide 濃度為參考一篇同樣以 neuroblastoma 作為研究材料之 paper (Ruth et al.,2002)。
3. IC50的單位為何用 nM?
回答: 起初是以 μM 做 semi-log 的圖,但是因為後來發現要比較精準計算
IC50的話,以 nM 較好做圖計算,因此才以 nM 作圖呈現。
楊健志老師:
1. 利用 Trx/Actin ratio 做比較是否適當?
回答: 由於沒看過 paper 有做過,在加藥後進行 Western blotting 比較蛋白質 量的差異,因此這部分算是新的嘗試。由於在高濃度作用下 (Figure 11),
細胞大部分都死亡,所以一次需要收集多盤的細胞,定量上也有困難。所 以 Figure 11 中以 Trx/Actin ratio 作為定量的結果,結果可能是因為 (i) Trx
表現量確實有改變或是 (ii) 因為 Actin 為 apoptosis pathway 上游之 signal,
所以 Actin 的量下降了導致以 Trx/Actin ratio 上升。之後可嘗試改變 internal control 或是利用 real-time PCR 定 RNA 量是否改變來做確定。
2. Hoechst 33258 判定細胞死活的標準?
回答: 以 Figure 4 中表示,以細胞核碎裂、細胞核萎縮、細胞核大小改變座 位細胞凋亡現象的依據。
3. Thioredoxin reductase inhibitor 對於 normal cell 也有作用,如何解決?
回答: 在與闕老師討論時,我也有想到這問題。因此在想是否可與現今發展 之 CSC marker 做結合,即像是 DNCB 與 CSC marker 之 Antibody 結合,使 藥物作用能有專一性。
4. 如何確保永久繼代 N cell?
回答: 有使用低濃度 FBS 作篩選,作為給 N cell 的 stress。也有嘗試過將 N cell 養回 D cell 的 medium 中,也不會產生變異,具有穩定性。
黃楓婷老師:
1. Etoposide concentration 8.3~83 μM 差距很大,是否恰當?
回答: 一開始我是以 paper 中的濃度 (8.3 μM)去做初步測試,並以上下兩倍
差的作一曲線,結果顯示不是很理想,看不太出藥物作用的差異,因此最後 在老師建議下,把間距拉為 10 被之後才看得出藥物對細胞的影響,也才做 得出 semi-log 的圖 (Figure 5)。
2. 現在使用的濃度是否符合臨床藥物的濃度?
回答: 之前和老師有換算過,是符合的。
3. DNCB 對於 Trx 是否有專一性?
回答: DNCB 是作用於 Trx reductase 的抑制劑,對於 Trx reductase 有專一性。
莊榮輝老師:
1. DNCB 對於人體是否有毒性?
回答: DNCB 目前未使用在臨床上,所以不知對人體毒性為何,但是臨床上有 其他的 Trx reductase 抑制劑在使用。在細胞加藥的過程中觀察,單一只加入 DNCB 對於細胞影響不是很大,細胞凋亡和 control 比沒有差很多,所以以此 作為判斷的話,DNCB 對於人體毒性可能還好。
2. 在加藥實驗的部分,是否可能是 medium 裡的 Trx 作用?你是否有測過 medium 裡的 Trx?
回答: 目前沒有做過,但是的確有可能,因為 Trx 已經被證實會被分泌到細胞 外,作為 cell comunication 的 signal,未來可以嘗試使用 medium 作 Western blotting 來做為參考。
3. Trx 與 CSC 之間的關係是必要的嗎?Trx 在 N cell 一直表現之原因?
回答: 目前還沒有做到這 DNA 的部分,但的確在一般細胞沒有受到 stress 是 不表現 Trx,因此 N cell 會穩定表現 Trx 的確是很特殊的狀況,由於 N cell 與 CSC 類似,所以在經過 stress 後可能改變了 Trx 的基因表現,這也可以是未來 的研究方向。
闕狀卿老師:
1. Drug-resistant patient 投 DNCB 藥物?你認為可否做輔助治療?
回答: 我認為是可以的,以 Figure 8 的結果顯示,在抑制 Trx 後可有效地殺死 N cell,也就是可以使難以治療並且表現較高 Trx 之癌症細胞。
2. 和 2002 年 Andoh 的研究發現比較,有何新的發現?
回答: 此次主要發現抑制 Trx 後,可使 N cell 有效的被殺死外 (Figure 8),另 外還有發現 D cell 在 etoposide 作用下產生的變異,像是 Trx 表現量的改變 (Figure 11),以及 cell morphology 改變的現象 (Figure 12)