Research Express@NCKU - Articles Digest
Research Express@NCKU Volume 14 Issue 3 - June 4, 2010 [ http://research.ncku.edu.tw/re/articles/e/20100604/4.html ]
Epigenetic Silencing of CEBPD Activity by YY1/PcG/
DNMTs Complex
Chiung-Yuan Ko
1, Hey-Chi Hsu
5, Meng-Ru Shen
2, Wen-Chang Chang
2,4, and Ju-Ming Wang
3,4*1Institute of Basic Medical Sciences and 2Department of Pharmacology, College of Medicine, Institute of Biosignal Transduction and 3Institute of Bioinformation, College of Bioscience and Biotechnology, and 4Center for Gene Regulation and Signal Transduction Research, National Cheng Kung University and the 5Department of Pathology, College of Medicine, National Taiwan University wwwjm4721@yahoo.com.tw
J. Biol. Chem. Nov 2008, 283(45):30919-30932.
I
t is known that chronic inflammation participates in the process of many human complex diseases. However, the molecular mechanisms during the process remain less studied. In addition, many “missing links” in the progession of chronic inflammation to cancer formation are unidentified. Therefore, we recently focus on an inflammation- responsive factor, CCAAT/enhancer binding Protein δ (CEBPD), to characterize its function in inflammation and the relationship between inflammation and tumorigenesis.CEBPD is one of the C/EBP family members. In addition to the function in inflammation
and differentiation, overexpression of CEBPD is able to induce cancer cell apoptosis and growth arrest.
Furthermore, the activated CEBPD has been observed in many inflammation-related diseases including Alzheimer’s disease, atherosclerosis, type 2 diabetes, rheumatoid arthritis and asthma. However, the CEBPD biology, including how it can be regulated, its downstream targets and the detailed molecular mechanisms, in these diseases remains unclear.
Our previous studies reported the importance and molecular mechanisms of CEBPD function in inflammation and lipogenesis (1, 2, 3), but, as mentioned above, CEBPD inhibits the growth and induces apoptosis of cancer cells.
These observations suggest that overexpression of CEBPD takes a disadvantage in tumorigenesis.
Fig.1 Opposite expression pattern of SUZ12 and CEBPD in human cervical cancer and HCC.
CEBPD, therefore, has been suggested to be a potential tumor suppressor recently. Interestingly, several studies demonstrated that CEBPD gene expression is down-regulated and “loss of function” alterations in CEBPD gene expression were observed in human cancers, such as in breast cancer and leukemia. Taken together, these discoveries make us start to think about
some issues, including the effect of existence of CEBPD in cancer cells, whether CEBPD is a bona fide tumor suppressor and how CEBPD is downregulated in cancer cells. In this current study, we confirmed CEBPD indeed can inhibit the growth and promote apoptosis of cancer cells, and also we further demonstrated that gene silencing of CEBPD gene is also observed in patients with cervical cancer or hepatocellular carcinoma (Fig. 1). These interesting results enforce us to dissect the molecular mechanism of gene silencing of CEBPD in cancer cells.
The loss of tumor suppressor plays an important
1 of 3
Research Express@NCKU - Articles Digest
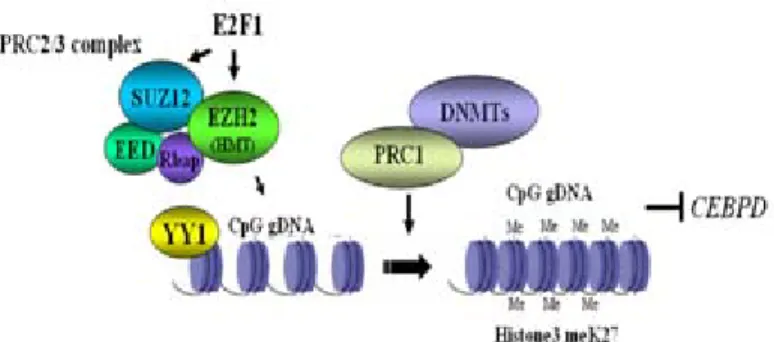
Fig. 2. Oncogene SUZ12/EZH2 participates in the gene silencing of CCAAT/enhancer binding Protein δ (CEBPD) gene. The epigenetic factors, SUZ12 and EZH2, were recruited onto CEBPD promoter through YY1. E2F1 can induce
expression of SUZ12 and EZH, the later one contains histone methyltransferase (HMT) activity. It can promote histone methyaltion of CEBPD promoter. In addition, the SUZ12/
EZH2 complex can further recruit the interaction of PRC1/
DNA methyltransferases complex, resulting in the methylation of CpG islands on CEBPD promoter. Therefore, the gene silencing of CEBPD can be done by these two methylation paths, which takes an advantage for progression of
tumorigenesis.
role to benefit the oncogene’s function in efficiently inducing cancer progression. Recent reports showed that Polycomb group (PcG) proteins, including SUZ12 and EZH2, are epigenetic chromatin modifiers involved in the maintenance of embryonic and adult stem cells and in cancer development. However, the details of suppression of tumor suppressor responds to high expression of PcG proteins in cancer cells is less studied. Following our previous study published in 2005, which described how CEBPD can be upregulated in cells (4), we next focus on dissecting the molecular mechanism of CEBPD gene silencing in cancer cells. Herein, Yin-Yang- 1 (YY1) physically interacts with SUZ12 to serve as a mediator to recruit the SUZ12 interacting protein EZH2, a histone 3
methyaltransferase. Such recruitment will result in histone methylation to participate in the process of CEBPD gene silencing. In addition, this PcG complex can further recruit DNA methyltransferase through the PRC1-mediated manner to cause the hypermethylation of
CEBPD promoter region. These two methylation paths of epigenetic modification can attenuate the sensitivity of CEBPD promoter responds to external stimulation. Therefore, the down- regulated and “loss of function” alterations of CEBPD gene expression in cancer cells can take a advantage for tumorigenic progression (Fig. 2) This paper was accepted and published on 2008 JBC (5).
On the other hand, biologists are interested in a fundamental question of the priority between histone methylation and DNA methylation in maintenance of gene insactivation. In addition to our interesting discovery in how CEBPD expression can be inactivated in cancer cells, we provide an in vivo model of how histone methylation prefers to DNA methyaltion in gene inactivation for biologists to investigate further.
References:
1. Wang JM, Ko CY, Chen LC, Wang WL, Chang WC. Functional role of NF-IL6b (CEBPD) •and its sumoylation and acetylation modifications in promoter activation of cyclooxygenase 2 gene. Nuc. Acids Res. 2006, 34(1):217-231.
2. Wang WL, Lee YC, Yang WM, Chang WC, Wang JM. Sumoylation of LAP1 is involved in the HDAC4- mediated repression of COX-2 transcription. Nuc. Acids Res. 2008, 36 (19): 6066-6079.
3. Lai PH, Wang WL, Ko CY, Lee YC, Yang WM, Shen TW, Chang WC and Wang JM. HDAC1/HDAC3 modulates PPARG2 transcription through the sumoylated CEBPD in hepatic lipogenesis. BBA-Mol. Cell.
Res. 2008, 1783: 1803-1814.
4. Wang JM, Tseng JT, Chang WC. Induction of human NF-IL6b (CEBPD) by epidermal growth factor is
2 of 3
Research Express@NCKU - Articles Digest
mediated through p38 signaling pathway and CREB activation in A431 cells. Mol. Biol. Cell 2005, 16 (7):3365-3376.
5. Ko CY, Hsu HC, Shen MR, Chang WC, and Wang JM. Epigenetic silencing of CEBPD activity by YY1/
PcG/DNMTs complex. J. Biol. Chem. 2008, 283(45):30919-30932.
3 of 3