Basic and Translational Science
Association Between
Survivin Gene Promoter
ⴚ31 C/G Polymorphism and Urothelial
Carcinoma Risk in Taiwanese Population
Yuan-Hung Wang, Hung-Yi Chiou, Chang-Te Lin, Hsiao-Yen Hsieh, Chia-Chang Wu,
Cheng-Da Hsu, and Cheng-Huang Shen
OBJECTIVES To investigate the association between survivin gene promoter ⫺31 C/G polymorphism and urothelial carcinoma (UC) risk in a Taiwanese population.
METHODS A total of 190 patients with pathologically confirmed UC and 210 unrelated controls without cancer were recruited at Chiayi Christian Hospital from August 2002 to May 2007. The ⫺31 C/G polymorphism in the survivin gene promoter was determined using polymerase chain reaction-restriction fragment length polymorphism analysis.
RESULTS Compared with study subjects carrying the G/G genotype, significantly increased UC risks were found for individuals carrying the C/G genotype (odds ratio 2.8; 95% confidence interval [CI] 1.7-4.6) and those with the C/C genotype (odds ratio 4.0; 95% CI 2.3-7.2). Those carrying the C/C or C/G genotype had a significantly increased UC risk of 3.2 (95% CI 1.9-5.2) compared with those with the G/G genotype. Among heavy smokers (ⱖ30 pack-years), we found a significantly increased UC risk of 3.8 (95% CI 1.3-11.3) for individuals with the C/C or C/G genotype compared with those with the G/G genotype. Furthermore, patients with UC carrying the C/C genotype had a significantly greater prevalence of muscle-invasive (Stage T2-T4), high-grade (G3), or invasive, high-grade tumor compared with those carrying the G/G genotype. CONCLUSIONS These findings suggest that the ⫺31 C/G polymorphism of the survivin gene promoter is associated with both the clinical tumor stage and the pathologic tumor grade and might be involved in the development of UC. UROLOGY73: 670 – 674, 2009. © 2009 Published by Elsevier Inc.
A
poptosis, also known as programmed cell death, is an important mechanism to control cell growth and division. Defects in apoptosis are involved in carcinogenesis through prolonging cell sur-vival, promoting accumulation of transforming mutations, and enhancing resistance to therapy.1Survivin is a novel member of the inhibitor of apoptosis protein family and possesses antiapoptosis-effective pathways through direct and/or indirect influence on an initiator (caspase-9) and on effectors (caspase-3 and caspase-7).2Survivin is abundantly expressed in embryonic tissues and in various human ma-lignancies, but it is almost undetectable in normal or well-differentiated adult tissues.3Survivin is thought to play animportant role in carcinogenesis and is associated with a poor clinical outcome in various malignancies.4
Urothelial carcinoma (UC) is the second most common cancer and the second leading cause of death among ma-lignancies of the genitourinary tract system.5 UC usually
arises from the urothelium with transitional cell differenti-ation, including that of the renal pelvis, ureter, and bladder. Cigarettes contain several carcinogens, including polycyclic aromatic hydrocarbons, aromatic amines, and N-nitroso compounds, which are thought to be major risk factors for the development of UC.6,7
Recently, increased survivin expression has been found in various malignancies, including bladder, colorectal, lung, and oral cancer.8-11 A preliminary study reported
that survivin was detected in the urine samples from 46 patients with new or recurrent bladder cancer but was not found in 16 healthy volunteers.12 Another study also
found that greater urine survivin was associated with an increased bladder cancer risk and higher tumor grade.13
In an immunohistochemical analysis, they studied 88 patients with superficial bladder cancer and found sur-vivin expression in tumor cells but not in normal
urothe-This study was supported by grants from the Chiayi Christian Hospital (grant R95-4) and the National Science Council of Taiwan (grants NSC 94-2314-B-038-051 and NSC 95-2314-B-038-047-MY3).
From the Taipei Medical University School of Public Health, Taipei; Departments of Urology and Biomedical Research, Chiayi Christian Hospital, Chiayi; Department of Urology, Taipei Medical University-Shuang Ho Hospital, Taipei; and Department of Life Science, National Chung Cheng University, Chiayi, Taiwan
Reprint requests: Cheng-Huang Shen, M.D., Department of Urology, Chiayi Chris-tian Hospital, 539 Chung Hsiao Road, Chiayi 600 Taiwan. E-mail:b712@cych.org.tw
Submitted: June 21, 2008, accepted (with revisions): September 18, 2008
lium.14However, the clinical application of survivin and its relationship to the stage and grade of UC require additional studies.
The gene coding for survivin is located at chromosome 17q25, and it is composed of 142 amino acids.15A feature of the human survivin gene promoter is the existence of a cell cycle-dependent element and a cell cycle homology region.3Deletion of this promoter region results in a lack of cell cycle-dependent expression in HeLa cells.16 Be-cause of the important role of survivin in carcinogenesis, we proposed that functional polymorphisms of the sur-vivin gene promoter might modulate its gene expression level or enzymatic activity, thereby affecting an individ-ual’s susceptibility to UC. To test this hypothesis, we investigated the association between the⫺31 C/G poly-morphism in the survivin gene promoter and UC risk in a Taiwanese population.
MATERIAL AND METHODS
Study Subjects and Clinical Data
This study recruited a total of 190 patients with UC, who had been diagnosed at the Department of Urology, Chiayi Christian Hospital from August 2002 to May 2007. Pathologic confirma-tion was performed by regular urologic practice, including en-doscopic biopsy and surgical resection of urinary tract tumors. The tumor stage and grade were determined using the 1997 TNM classification and the 2004 World Health Organization classification system, respectively.17The clinical stage was
clas-sified into 2 subgroups: superficial-invasive (Stage T1) and muscle-invasive (Stage T2-T4). The pathologic grade was ini-tially divided into 3 groups (G1, G2, and G3) and then subdi-vided into low grade (G1-G2) and high grade (G3). A total of 210 controls without cancer, who had been frequency-matched with the patients with UC for age (⫾5 years) and sex, were recruited from individuals admitted to the same hospital for a health checkup and who had no previous diagnosis of urologic neoplastic disease or any other malignancy. All subjects received a detailed description of this study and provided written informed consent. All participants were interviewed by a well-trained interviewer using a structured question-naire to collect information, including basic characteristics, cigarette smoking status, and alcohol consumption. The in-stitutional review board of Chiayi Christian Hospital ap-proved this study protocol.
Polymorphism Genotyping
A venous blood sample (6-8 mL) from each participant was drawn into an ethylenediaminetetraacetic acid vial. Genomic DNA was extracted from the peripheral lymphocytes by pro-teinase K digestion and the phenol/chloroform extraction method. Polymerase chain reaction-restriction fragment length polymorphism was used to determine the⫺31 C/G polymor-phism in the survivin gene promoter. Polymerase chain reaction was performed in a final volume of 50 L containing 50 ng genomic DNA, 5 L of 10⫻ polymerase buffer (200 mM Tris-HCl, pH 8.0; 500 mM KCl), 1.5 mM MgCl2, 0.1 mM
dNTPs, 20 pmol/L of forward primer (5=-GTTCTTTGAAAG-CAGTCGAG-3=) and reverse primer (5=-GCCAGTTCTT-GAATGTAGAG-3=), and 1.5 U of Taq polymerase (Invitro-gen, San Diego, CA). The polymerase chain reaction program
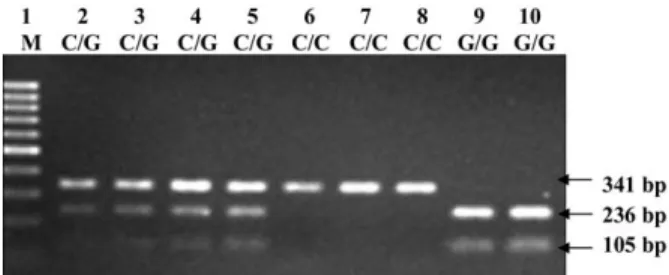
was set on a PTC-150 Minicycler (MJ Research, Watertown, MA), and the thermal cycler was started with an initial turation at 95°C for 5 minutes, followed by 35 cycles of dena-turation at 95°C for 30 s, annealing at 58°C for 90 s, extension at 72°C for 90 s, and completed with a final elongation step at 72°C for 5 minutes. The expected 341-bp product was then digested by the restriction enzyme EcoO109I (New England Biolabs, Ipswich, MA) at 37°C overnight. The G allele of the 341-bp product was cleaved by the enzyme and resulted in 236-and 105-bp fragments; the C allele was not cleaved (Fig. 1).
Statistical Analysis
A measure of lifetime smoking was estimated in terms of pack-years of cigarette smoking, calculated using the following for-mula: pack-years ⫽ (cigarettes daily/20) ⫻ (smoked years).18
For alcohol consumption, “ever drinkers” were recognized as those who had consumed alcoholⱖ3 d/wk for ⱖ6 months; all others were regarded as “never drinkers.” Student’s t test was used to determine whether a significant difference existed in the age distribution between those with UC and controls. A good-ness-of-fit2test was used to test the Hardy-Weinberg
equilib-rium by comparing the observed genotype frequencies with the expected frequencies among the controls.19 The correlation
between the ⫺31 C/G polymorphism in the survivin gene promoter and the clinical stage or pathologic grade of UC was also examined using the2test. The statistical package
Statis-tical Analysis Systems, version 9.1 (SAS Institute, Cary, NC), was used for all analyses with 2-tailed probabilities. The differ-ences between the compared groups were considered significant if P⬍ .05.
RESULTS
The distribution of the selected characteristics for those with UC and the controls is listed inTable 1. No signif-icant differences were found between the patients with UC and the controls in the distribution of age, sex, or alcohol consumption. The prevalence of heavy smokers (ⱖ30 pack-years) was significantly greater in the patients with UC (29.0%) than in the controls (19.5%; P ⫽ .039). Of the UC cases, 34.7% were muscle-invasive and 35.8% were high-grade (G3) tumors.
Figure 1. Polymerase chain reaction-restriction fragment length polymorphism analysis to detect⫺31 C/G polymor-phism of survivin promoter. Polymerase chain reaction prod-ucts (341-bp) digested with restriction enzyme EcoO109I and analyzed by 2% agarose gel. Lane 1, 100-bp DNA ladder (MBI Fermentas); lanes 2-5, C/G heterozygotic; lanes 6-8, homozygotic for C allele; lanes 9 and 10, homozygotic for G allele.
The observed genotype frequencies of the ⫺31 C/G polymorphism in the controls were in Hardy-Weinberg equilibrium (2 ⫽ 0.087, P ⫽ .231). The genotype
frequency distribution and risk estimate of the survivin gene promoter ⫺31 C/G polymorphism are listed in
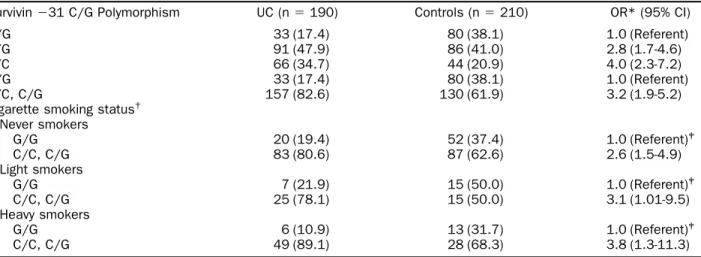
Table 2. Compared with the study subjects carrying the G/G genotype, significantly increased UC risks were found for those carrying the C/G genotype (odds ratio 2.8, 95% confidence interval [CI] 1.7-4.6) and those carrying the C/C genotype (odds ratio 4.0, 95% CI 2.3-7.2). In addition, subjects with the C/C or C/G
genotype had a significantly increased UC risk (odds ratio 3.2, 95% CI 1.9-5.2) compared with those with the G/G genotype. We also investigated the interac-tion between the ⫺31 C/G polymorphism of the sur-vivin gene promoter and cigarette smoking. Among light smokers, those carrying the C/C or C/G genotype had a significantly greater risk of UC of 3.1 (95% CI 1.01-9.5) compared with those with the G/G genotype. For heavy smokers, subjects carrying the C/C or C/G genotype had a significantly increased risk of UC of 3.8 (95% CI 1.3-11.3).
Table 1. Distribution of selected characteristics
Variable UC (n⫽ 190) Controls (n⫽ 210) P Value
Age (y) .145*
⬍55 31 (16.3) 38 (18.1) 55-69 100 (52.6) 125 (59.5) ⱖ70 59 (31.1) 47 (22.4)
Mean⫾ SD age (y) 63.8⫾ 8.0 62.4⫾ 8.9 .098†
Sex .117*
Female 66 (34.7) 89 (42.4) Male 124 (65.3) 121 (57.6)
Cigarette smoking (pack-years) .039* 0 103 (54.2) 139 (66.2) 1-29 32 (16.8) 30 (14.3) ⱖ30 55 (29.0) 41 (19.5) Alcohol consumption .939* Never 166 (87.4) 184 (87.6) Ever 24 (12.6) 26 (12.4) Clinical stage Superficial-invasive (T1) 124 (65.3) Muscle-invasive (T2-T4) 66 (34.7) Pathologic grade 1 45 (23.7) 2 77 (40.5) 3 68 (35.8) UC⫽ urothelial carcinoma.
Data presented as numbers, with percentages in parentheses, unless noted otherwise. *2
test.
†
Student’s t test.
Table 2. Distribution and risk estimate of survivin gene promoter⫺31 C/G polymorphism stratified by cigarette smoking status
Survivin⫺31 C/G Polymorphism UC (n⫽ 190) Controls (n⫽ 210) OR* (95% CI) G/G 33 (17.4) 80 (38.1) 1.0 (Referent) C/G 91 (47.9) 86 (41.0) 2.8 (1.7-4.6) C/C 66 (34.7) 44 (20.9) 4.0 (2.3-7.2) G/G 33 (17.4) 80 (38.1) 1.0 (Referent) C/C, C/G 157 (82.6) 130 (61.9) 3.2 (1.9-5.2) Cigarette smoking status†
Never smokers G/G 20 (19.4) 52 (37.4) 1.0 (Referent)‡ C/C, C/G 83 (80.6) 87 (62.6) 2.6 (1.5-4.9) Light smokers G/G 7 (21.9) 15 (50.0) 1.0 (Referent)‡ C/C, C/G 25 (78.1) 15 (50.0) 3.1 (1.01-9.5) Heavy smokers G/G 6 (10.9) 13 (31.7) 1.0 (Referent)‡ C/C, C/G 49 (89.1) 28 (68.3) 3.8 (1.3-11.3)
UC⫽ urothelial carcinoma; OR ⫽ odds ratio; CI ⫽ confidence interval. Data presented as numbers, with percentages in parentheses. * Adjusted by age, sex, and cigarette smoking status.
†
Light smokers: 1-29 pack-years; heavy smokers:ⱖ 30 pack-years.
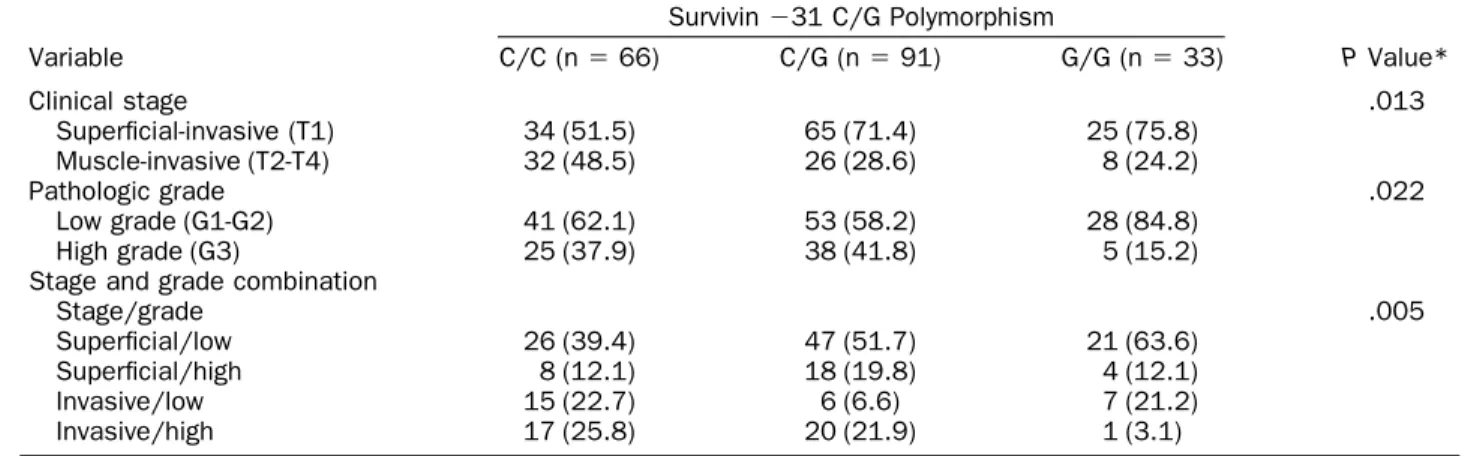
The relationships between the ⫺31 C/G polymor-phism of the survivin gene promoter and the clinical stage and pathologic grade are given in Table 3. The prevalence of muscle-invasive tumor was significantly greater in patients with UC carrying the C/C genotype (48.5%) than in those carrying the G/G genotype (24.2%; P⫽ .013). The pathologic grade was also signif-icantly different. We found that the prevalence of high-grade (G3) tumor was significantly greater in patients with UC with the C/C genotype (37.9%) than in those with the G/G genotype (15.2%; P ⫽ .022). After com-bining the clinical stage and pathologic grade, we ob-served that patients with UC carrying the C/C genotype had a significantly greater prevalence of muscle-invasive, high-grade tumor (25.8%) compared with those carrying the G/G genotype (3.1%).
COMMENT
Recently, many studies have shown that polymorphisms of the survivin gene promoter may modulate the expres-sion of survivin in various malignancies.20-23 In the
present study, we investigated the association between the survivin gene promoter⫺31 C/G polymorphism and UC risk in a Taiwanese population.
A major finding of this study was the significant asso-ciation between the survivin gene promoter ⫺31 C/G polymorphism and UC risk. We observed that the fre-quency of the C/C and C/G genotypes was greater in patients with UC (34.7% and 47.9%, respectively) than in controls (20.9% and 41.0%, respectively). This finding is inconsistent with the findings from a study by Jang et al.21 regarding the ⫺31 C/G polymorphism in the
sur-vivin gene promoter. They observed that the frequency of the C/C and C/G genotypes was 31.6% and 44.5% in patients with lung cancer and 25.3% and 50.3% in con-trols, respectively. They also found that subjects withⱖ1 ⫺31 G allele had a significantly decreased lung cancer risk. Cheng et al.22showed that the frequency of the C/C
and C/G genotypes was 39.6% and 39.6% in patients with gastric cancer and 11.9% and 41.8% in controls,
respectively. A cervical cancer study found that the fre-quency of the C/C and C/G genotypes was 8.0% and 36.0% in patients with cervical cancer and 14.0% and 39.0% in controls, respectively.20 More studies are
needed to elucidate the functional effects of the ⫺31 C/G polymorphism on various malignancies.
The⫺31 C/G polymorphism, which is located at the cell cycle-dependent element/cell cycle homology region repressor binding site, is likely associated with the tran-scription of survivin by modifying the binding domain of the cell cycle-dependent element/cell cycle homology region repressor.22The effect of this⫺31 C/G
polymor-phism in the survivin gene promoter was also investi-gated using a luciferase assay, with the finding that the ⫺31 G allele significantly decreased promoter activity compared with the ⫺31 C allele in HeLa and CHO cells.21Xu et al.23proposed that the⫺31 C/G genotype might increase both the mRNA and the protein levels of survivin.
A significantly increased UC risk was found for heavy smokers carrying the C/C or C/G genotype compared with those carrying the G/G genotype in the present study. More than 4700 compounds have been identified in cigarette smoke, and nicotine is 1 of the major additive components. Although nicotine is not referred to as a carcinogen, it is usually regarded as a “tumor enhancer” by deregulating apoptosis, angiogenesis, and cell-medi-ated immunity.24Dasgupta et al.25reported that nicotine
can cause the dissociation of retinoblastoma tumor sup-pressor protein from survivin promoter in A549 cells and negatively affects the apoptotic potential of chemother-apeutic drugs. Thus, we speculated that subjects who had been exposed to nicotine and carried the C/C or C/G genotype of the survivin gene would be susceptible to UC by way of the nicotine-enhanced antiapoptotic activity. We also found that the survivin gene promoter ⫺31 C/G polymorphism was associated with both clinical stage and pathologic grade. We observed that the prev-alence of invasive, high-grade tumors was significantly greater among patients with UC carrying the C/C
geno-Table 3. Survivin gene promoter⫺31 C/G polymorphism distribution stratified by clinical stage and pathologic grade Variable Survivin⫺31 C/G Polymorphism P Value* C/C (n⫽ 66) C/G (n⫽ 91) G/G (n⫽ 33) Clinical stage .013 Superficial-invasive (T1) 34 (51.5) 65 (71.4) 25 (75.8) Muscle-invasive (T2-T4) 32 (48.5) 26 (28.6) 8 (24.2) Pathologic grade .022 Low grade (G1-G2) 41 (62.1) 53 (58.2) 28 (84.8) High grade (G3) 25 (37.9) 38 (41.8) 5 (15.2) Stage and grade combination
Stage/grade .005
Superficial/low 26 (39.4) 47 (51.7) 21 (63.6) Superficial/high 8 (12.1) 18 (19.8) 4 (12.1) Invasive/low 15 (22.7) 6 (6.6) 7 (21.2) Invasive/high 17 (25.8) 20 (21.9) 1 (3.1)
Data presented as numbers, with percentages in parentheses. *2
type than in those with the G/G genotype. Previous studies have reported that the expression of survivin was significantly increased in patients with high-grade tu-mors.26-29 These results suggest that survivin gene pro-moter⫺31 C/G polymorphism might not only result in survivin overexpression, but also disrupt the regulation of apoptosis. Therefore, it is reasonable to speculate that the ⫺31 C/G polymorphism in the survivin gene promoter might be involved in tumor initiation, promotion, and progression.
CONCLUSIONS
To our knowledge, this is the first report to investigate the association between survivin gene promoter⫺31 C/G polymorphism and UC risk. We found that survivin gene promoter⫺31 C/G polymorphism was significantly asso-ciated with the development of UC, especially among heavy smokers. In addition, muscle-invasive and high-grade tumors were more prevalent for the ⫺31 C/C genotype than for the⫺31 G/G genotype of the survivin gene promoter. Future studies with larger sample sizes in different ethnic groups are needed to clarify the relation-ship between survivin gene promoter⫺31 C/G polymor-phism and UC.
References
1. Melet A, Song K, Bucur O, et al. Apoptotic pathways in tumor progression and therapy. Adv Exp Med Biol. 2008;615:47-79. 2. Reed JC. The survivin saga goes in vivo. J Clin Invest. 2001;108:
965-969.
3. Sah NK, Khan Z, Khan GJ, et al. Structural, functional and therapeutic biology of survivin. Cancer Lett. 2006;244:164-171. 4. Duffy MJ, O’Donovan N, Brennan DJ, et al. Survivin: a promising
tumor biomarker. Cancer Lett. 2007;249:49-60.
5. Shen CH, Wang YH, Wang WC, et al. Inducible nitric oxide synthase promoter polymorphism, cigarette smoking, and urothelial carcinoma risk. Urology. 2007;69:1001-1006.
6. Samanic C, Kogevinas M, Dosemeci M, et al. Smoking and bladder cancer in Spain: effects of tobacco type, timing, environmental tobacco smoke, and gender. Cancer Epidemiol Biomarkers Prev. 2006;15:1348-1354.
7. Strope SA, Montie JE. The causal role of cigarette smoking in bladder cancer initiation and progression, and the role of urologists in smoking cessation. J Urol. 2008;180:31-37.
8. Karam JA, Lotan Y, Ashfaq R, et al. Survivin expression in patients with non- muscle-invasive urothelial cell carcinoma of the bladder.
Urology. 2007;70:482-486.
9. Ponnelle T, Chapusot C, Martin L, et al. Cellular localisation of survivin: impact on the prognosis in colorectal cancer. J Cancer Res
Clin Oncol. 2005;131:504-510.
10. Ulukus EC, Kargi HA, Sis B, et al. Survivin expression in non-small-cell lung carcinomas: correlation with apoptosis and other apoptosis-related proteins, clinicopathologic prognostic factors and prognosis. Appl Immunohistochem Mol Morphol. 2007;15:31-37. 11. Lin CY, Hung HC, Kuo RC, et al. Survivin expression predicts
poorer prognosis in patients with areca quid chewing-related oral squamous cell carcinoma in Taiwan. Oral Oncol. 2005;41:645-654. 12. Smith SD, Wheeler MA, Plescia J, et al. Urine detection of survivin and diagnosis of bladder cancer. JAMA. 2001;285:324-328.
13. Shariat SF, Casella R, Khoddami SM, et al. Urine detection of survivin is a sensitive marker for the noninvasive diagnosis of bladder cancer. J Urol. 2004;171:626-630.
14. Ku JH, Kwak C, Lee HS, et al. Expression of survivin, a novel inhibitor of apoptosis, in superficial transitional cell carcinoma of the bladder. J Urol. 2004;171:631-635.
15. Chiou SK, Jones MK, Tarnawski AS. Survivin—an anti-apoptosis protein: its biological roles and implications for cancer and beyond.
Med Sci Monit. 2003;9:PI25-PI29.
16. Li F, Ambrosini G, Chu EY, et al. Control of apoptosis and mitotic spindle checkpoint by survivin. Nature. 1998;396:580-584. 17. Pasin E, Josephson DY, Mitra AP, et al. Superficial bladder cancer:
an update on etiology, molecular development, classification, and natural history. Rev Urol. 2008;10:31-43.
18. Chiu BC, Lynch CF, Cerhan JR, et al. Cigarette smoking and risk of bladder, pancreas, kidney, and colorectal cancers in Iowa. Ann
Epidemiol. 2001;11:28-37.
19. Wittke-Thompson JK, Pluzhnikov A, Cox NJ. Rational inferences about departures from Hardy-Weinberg equilibrium. Am J Hum
Genet. 2005;76:967-986.
20. Borbély AA, Murvai M, Szarka K, et al. Survivin promoter poly-morphism and cervical carcinogenesis. J Clin Pathol. 2007;60:303-306.
21. Jang JS, Kim KM, Kang KH, et al. Polymorphisms in the survivin gene and the risk of lung cancer. Lung Cancer. 2008;60:31-39. 22. Cheng ZJ, Hu LH, Huang SJ. Correlation of⫺31 G/C
polymor-phisms of survivin promoter to tumorigenesis of gastric carcinoma.
Ai Zheng. 2008;27:258-263.
23. Xu Y, Fang F, Ludewig G, et al. A mutation found in the promoter region of the human survivin gene is correlated to overexpression of survivin in cancer cells. DNA Cell Biol. 2004;23:527-537. 24. Zeidler R, Albermann K, Lang S. Nicotine and apoptosis. Apoptosis.
2007;12:1927-1943.
25. Dasgupta P, Kinkade R, Joshi B, et al. Nicotine inhibits apoptosis induced by chemotherapeutic drugs by up-regulating XIAP and survivin. Proc Natl Acad Sci USA. 2006;103:6332-6337.
26. Pina-Cabral L, Santos L, Mesquita B, et al. Detection of survivin mRNA in urine of patients with superficial urothelial cell carcino-mas Clin Transl Oncol. 2007;9:731-736.
27. Schultz IJ, Kiemeney LA, Witjes JA, et al. Survivin mRNA ex-pression is elevated in malignant urothelial cell carcinomas and predicts time to recurrence. Anticancer Res. 2003;23:3327-3331. 28. Ohsawa I, Nishimura T, Kondo Y, et al. Detection of urine survivin
in 40 patients with bladder cancer. J Nippon Med Sch. 2004;71:379-383.
29. Wang H, Xi X, Kong X, et al. The expression and significance of survivin mRNA in urinary bladder carcinomas. J Cancer Res Clin