行政院國家科學委員會專題研究計畫成果報告
周邊投予肺炎雙球菌引起腦膜炎之致病機轉 (二)
Pathophysiology of exper imental pneumococcal meningitis induced by
per ipher al inoculation (II)
計畫編號:NSC 89-2314-B-006-015
執行期限:88 年 8 月 1 日至 89 年 7 月 31 日
主持人:劉清泉
執行機構及單位名稱:
國立成功大學醫學院小兒學科
E-mail: liucc@mail.ncku.edu.tw
行政院國家科學委員會專題研究計畫成果報告
周邊投予肺炎雙球菌引起腦膜炎之致病機轉 (二)
Pathophysiology of exper imental pneumococcal meningitis induced by
per ipher al inoculation (II)
計畫編號:NSC 89-2314-B-006-015
執行期限:88 年 8 月 1 日至 89 年 7 月 31 日
主持人:劉清泉 執行機構及單位名稱:
國立成功大學醫學院小兒學科
E-mail: liucc@mail.ncku.edu.tw
一、中文摘要
在本研究中,我們成功利用小鼠實驗 動物模式,由周邊(腹腔)注射革蘭氏陽性--肺炎鏈球菌(S. pneumoniae)引發腦膜炎, 並用來研究血行性感染併發細菌性腦膜炎 的致病機轉。 在腹膜腔注射 S. pneumoniae type 6, 小署將在 36 小時內因為細菌增長而死於敗 血性休克。但我們觀察到在注射後 6 小時 細菌可穿過腦-血管屏障(blood-brain barrier, BBB),並可在腦內發現細菌的存在。將腦 組織作 TNF-α的免疫組織染色發現在注射 後 6 到 24 小時皆可有很強的染色反應。由 腹 膜 腔 投 予 anti-TNF-α抗 體 可 阻 斷 S. pneumoniae type 6 血行進入腦內,這顯示 TNF-α在控制腦-血管屏障的開啟扮演重要 的角色。 我們使用腹膜腔投予抗生素 cefazolin 以抑制細菌過度生長,避免小鼠死於敗血 性休克。這個血行性感染引發細菌性腦膜 炎的小鼠動物模式,將有助於我們未來實 驗性細菌性腦膜炎的治療研究。 關鍵詞:細菌性腦膜炎,小鼠動物模式, 肺炎鏈球菌,腦-血管屏障,TNFα,血行 性。 Abstr actAn adult murine model of meningitis was developed to study the pathogenesis of haematogenous infection by S. pneumoniae.
After intraperitoneal injection of S. pneumoniae type 6, the mice would die of
septic shock within 36 h due to bacterium overgrowth. However, the bacteria could cross the BBB and begin to deposit in brain at 6 h post injection. There was strong staining of TNF-α from 6 h to 24 h post infection. Intraperitoneally given with anti-TNF-α antibody could block the entrance of circulatory S. pneumoniae type 6 into brain,
indicating that TNF-α played an important role in controlling the opening of BBB. Cefazolin antibiotic was used to inhibit the bacterial load in vivo, and the mice survived from septic shock, but haematogenous meningitis would develop four days later. This haematogenous meningitis model should facilitate the experimental study on the therapy of bacterial meningitis in vivo.
Keywor ds: bacterial meningitis, murine model,
Streptococcus pneumoniae, blood-brain barrier, TNFα, hematogenous.
Intr oduction
Bacterial meningitis is a bacterial infection that develops inflammatory response in the brain. The central nervous system (CNS) of mammals is considered to be an immunologically privileged site for the lack of lymphatic drainage and the separation from the blood compartment by the BBB (1, 11). The BBB plays an important role in controlling inflammatory cells and macromolecules into the brain by virtue of its selective permeability on its microvascular endothelial cells such as the presence of tight junction, no formation of pinocytotic vesicles, and wrapping by astrocytes (1, 8, 11). The first step of developing bacterial meningitis is the invasion and multiplication of bacteria in the subarachnoidal space of CNS. Once the bacterium multiply in the CNS, the bacterium itself or its degraded products in the brain would stimulate the production and release of proinflammatory mediators such as cytokines and prostaglandins by leukocytes, endothelial cells, astrocytes, microglial cells, and other cells in the CNS, and these subsequently lead to an increase in the permeability of the blood-brain barrier (BBB). This triggers transendothelial migration of neutrophils and leakage of plasma proteins that further damage the brain (2, 11, 12, 22). Clinic evidence suggested that bacterial meningitis resulted from bacteremia or sepsis (3, 10). But how the bacteria enter the CNS is not clearly understood.
Streptococcus pneumoniae is one of the most
common bacteria causing meningitis in elder children and adults. Since antibiotic-resistant strains of S. pneumoniae have increased
rapidly worldwide, the incidence of infection with S. pneumoniae is still associated with a
high mortality rate (14). In order to reduce the infection with S. pneumoniae, especially
the antibiotic-resistant strains, there is an
urgent need to establish the murine infection model to study the therapeutic effect of non-antibiotic new drugs. Although bacteremia-induced meningitis were reported on infant rats or mice that were deficient of the mature structure of BBB (9, 15-16), no haematogenous meningitis model with predictable manner in adult mice was available. In this study, we demonstrated that circulatory S. pneumoniae type 6 could enter
into the brain via the action of TNF-α, and replicated there to induce inflammation if the mice were treated with cefazolin antibiotic to prolong the survival of the septic mice. This haematogenous meningitis model in adult mice is discussed for the study of the therapy in bacterial meningitis.
Mater ials and Methods
Mice. Breeder mice of B6 strain were
purchased from The Jackson Laboratory, Bar Harbor, ME or Charles River Japan, Inc. (Atsugi, Japan). They were maintained on standard laboratory chow and water ad libitum in our animal facility. The animals were raised and cared for following the guidelines set up by the National Science Council of the Republic of China. Eight- to 12-week-old female mice were used in all experiments.
Induction of haematogenous pneumo-coccal meningitis. S. pneumoniae type 6,
isolated clinically from National Cheng Kung University Hospital (Tainan, Taiwan), were grown in Todd Hewitt broth (Difco Laboratories, Detroit, M.I.) for 12 h. The concentration of bacteria was determined with a spectrophotometer (Beckman Instrument, Somerset, N.J.) with an optical density at 600 nm of 1 equal to 108 CFU/ml (23). The 100% lethal dose (LD100) of S.
pneumoniae type 6 by intraperitoneal
injection in B6 mice is 10 CFU. In the antibiotic-treated experiment, groups of six mice were injected intraperitoneally with 50 CFU of S. pneumoniae type 6, and then
mg/kg of body weight) (Fujiawa Taiwan Co., Ltd, Taipei, Taiwan) twice at 12 h and 24 h. The animals were observed every 12 h for a total of 5 days.
Detection of remnant bacteria in the blood and the brain. Groups of four mice
were inoculated with 50 CFU of S. pneumoniae type 6 intraperitoneally. At
various times post injection, the blood was collected with heparin. Then, the mouse was sacrificed by perfusion via cardiac puncture with PBS, the brain was aseptically removed and was homogenized with 3% gelatin (Difco Laboratories, Detroit, M.I.) in PBS. The samples were serial diluted, poured in blood agar plates (Becton Dickinson and company, Cockeysville, Md.) and incubated at 37oC overnight. The number of CFU of S. pneumoniae was quantitated and was
expressed as the mean ± standard deviation per mouse. In the antibody inhibition experiment, 50 µg (in 100 µl) of rabbit anti-TNF-α antiserum (Genzyme diagnostics, Cambridge, Mass.) or normal rabbit serum was administrated intraperitoneally at 5 h post stimulation.
Immunohistochemistry. Groups of four mice were sacrificed by perfusion via cardiac puncture with PBS. Brains were removed and embedded in OCT compound (Miles Inc., Elkhart, Ind.) and were then frozen in liquid nitrogen. Four-µm cryosections were made and were fixed with ice-cold acetone for 3 min. They were then stained with a primary rat anti-TNF-α monoclonal antibody (MAb; MAb MP6-XT3), rat-MIP-1β MAb (MAb A65-2; PharMingen, San Diego, Calif.) or a hamster anti-IL-1β MAb (Genzyme, Cambridge, Mass.). Secondary antibodies were peroxidase-conjugated sheep anti-rat immunoglobulin G (IgG) or goat anti-hamster IgG (Boehringer Mannheim GmbH, Mannheim, Germany). A peroxidase stain with a reddish brown color was developed with an aminoethyl carbazole substrate kit
(ZYMED Laboratories, San Francisco, Calif.) (6).
Results
Anti-TNF-α antibody inhibited the invasion of haematogenous S. pneumoniae
into the brain. The deposition of S. pneumoniae type 6 after its intraperitoneal
injection was traced. S. pneumoniae type 6
was resistant to phagocytosis, and can replicate in mice. The LD100 and LD50 of S.
pneumoniae type 6 in naïve B6 mice were 10
CFU and 5 CFU, respectively. As shown in Table 1, the bacteria grew rapidly in vivo. After intraperitoneal injection with 50 CFU of S. pneumoniae type 6, the number of
bacteria in blood increased from 65 CFU at 3 h to 109 CFU per milliliter at 24 h in each mouse. The mice would all die within 36 h due to septic shock of high bacterial load. Interestingly, bacteria could cross the BBB and begin to deposit in brain at 6 h, the number increased to 105 CFU at 24 h post injection. In the previous results, we found that anti-TNF-α antibody could block the opening of BBB induced by S. pneumoniae
type 14 (N. Tsao, H. P. Hsu, C. M. Wu, C. C. Liu, and H. Y. Lei, submitted for publication). To confirm the role of TNF-α in the infection of S. pneumoniae type 6, we detected the
TNF-α expression by immunohistochemical staining. There was strong staining from 6 h to 24 h after infection (Fig. 1), and its expression time was coincident with the bacteria found in brain. These results suggested that TNF-α might play the role to control the bacteria invasion from periphery into the brain. Further demonstrating the role of TNF-α, the mice were administrated intraperitoneally with anti-TNF-α antibody at 5 h after injection with S. pneumoniae type 6.
As shown in Table 2, anti-TNF-α antibody inhibited the number of bacteria in brain significantly comparing with the normal serum control. No bacteria were found in the brain until 24 h. These data indicated that TNF-α plays an important role in controlling
the entry of bacteria into the brain.
The manifestation of haematogenous S. pneumoniae-induced meningitis. Although
there were 106 CFU of bacteria in the brain at 24 h after intraperitoneal inoculation of 50 CFU of S. pneumoniae type 6, no meningitis
symptom was observed. Probably the mice died before the development of meningitis. The LD50 of S. pneumoniae type 6 in naïve
B6 mice was 5 CFU. After inoculation with 5 CFU of S. pneumoniae type 6, about 40 % of
the mice died within 2 days and additional 10 % of the mice died in the following 1 day, the other surviving mice showed no symptoms of illness (data not shown). In order to control the illness of the mice, we used cefazolin antibiotic, which was known to have useless penetration capability into the brain, to inhibit the overgrowth of bacteria in mice. But the dosage of cefazolin was limited. If the dose of cefazolin was lowered than 3 mg/kg of body weight, the mice would all die due to severe bacteremia, while the dose was heightened than 5 mg/kg of body weight, no mice showed illness any more (data not shown). If cefazolin (3.3 mg/kg of body weight) was given intraperitoneally twice at 12 and 24 h after S. pneumoniae injection,
the mice would all survive and the neutrophilic infiltration developed expectantly beginning at 84 h after injection (Fig. 2 & 3C). IL-1β as well as MIP-1β was also found on the neuron cells (Fig. 3F & I). Counting the bacteria in the circulation and brain revealed that S. pneumoniae gradually
increased to 105 CFU in brain while the number in blood remained at the level of 104 -106 CFU per milliliter (Table 3). S. pneumoniae did multiply in brain and
induced neutrophilic inflammation after intraperitoneal injection at a predictable time. Based on the data, we concluded that the murine haematogenous meningitis induced by S. pneumoniae was demonstrated.
Discussion
Through the years, bacterial meningitis
has remained an infection with a high mortality rate, particularly in very young and elderly patients, despite the availability of effective antibiotic treatments (14, 22). The first step of developing bacterial meningitis is the invasion and multiplication of bacteria in the subarachnoidal space of CNS. Clinically, bacterial meningitis is accompanied with bacteremia and resulted from peripheral infection (3, 10). But how the bacteria cross this tight barrier of BBB into the brain is not clearly understood. Most experimental studies of bacterial meningitis involved the direct inoculation of bacteria or its cell wall components into the cisterna magnum of the animal (13, 18-19, 25-26), but this treatment does not match with the natural course. A haematogenous meningitis model in adult mice was established in this study. TNF-α -mediated action during S. pneumoniae
bacteremia allowed the entrance of the virulent S. pneumoniae type 6 into the brain.
The S. pneumoniae in brain would replicate
and induce neutrophilic inflammation at four days later if cefazolin antibiotic was used to inhibit peripheral septic shock of bacterial overgrowth.
Bacterial meningitis can be induced either by Gram-negative or Gram-positive bacteria (4, 24). The early fatality in children with bacterial meningitis resulted from septic shock (3). The BBB, by virtue of its selective permeability, plays an important role in separating inflammatory cells and macromolecules from periphery into the brain (1, 8, 11). How the bacteria crossing the BBB into the brain became the important issue to intervene the development of meningitis. In this study, the invasion of S. pneumoniae type 6 into the brain was
significantly decreased after treatment with anti-TNF-α antibody. This data suggested that TNF-α played the major role in controlling the bacteria invasion. There was much literature to illustrate the action of TNF-α. Tong et al. found that TNF-α
enhanced the adherence of S. pneumoniae
may facilitate bacteria to further damage epithelium (17). Freyer et al. reported that S. pneumoniae cell wall can stimulate cerebral
endothelial cells to release TNF-α that further regulate inducible nitric oxide synthase and ICAM-1 expression (5). In our previous studies, TNF-α also induced cyclooxygenase 2 that not only increased the vasopermeability of blood-brain barrier but also enhanced the neutrophil survival in
Escherichia coli-induced brain inflammation
(20). Moreover, we also found that TNF-α, but not IL-1β, produced during bacteremia could induce the BBB opening (N. Tsao, H. P. Hsu, C. M. Wu, C. C. Liu, and H. Y. Lei, submitted for publication). Once the BBB was opened, it was permeable to circulatory S. pneumoniae to invade into the CNS.
The bacteria, invaded into the brain, would replicate and then induced chemokine such as MIP-1β in parenchymal brain and adhesion molecule of ICAM-1 on blood vessels to recruit neutrophil infiltration. This process needed at least four days to develop. Since the LD100 of S. pneumoniae type 6 in naïve
B6 mice is 10 CFU, the mice would die of septic shock within 36 h. We had to use antibiotic to inhibit the death from septic shock. The dose and timing of the antibiotic are manipulated to allow the development of meningitis. Although the cefazolin-treated mice would develop meningitis, once no antibiotic was given any more, they would die two weeks later (unpublished data) because of the overgrowth of bacteria in brain. Cefazolin is the first-generation of cephalosporin. When we used the third-generation of cephalosporin (ceftriaxone), under the same condition of two injections at 12 and 24 h, the mice developed meningitis at a later time (e.g., 120 h post injection), and the ceftriaxone-treated mice survived (unpublished data) because of no growth of bacteria in the brain. This is probably due to the more efficient penetration of the third-generation of cephalosporin into the brain to kill the bacteria.
In according to the epidemiologic surveillance of bacterial meningitis, the incidence rates of infection with specific pathogens are most influenced by age. The infection of S. pneumoniae is most common
in older adults (14). Experimental pneumococcal meningitis induced after bacteremia was usually reported on infant rats or mice (9, 15-16). While the infant rats or mice were deficient of mature structure of BBB, so the haematogenous meningitis model did not match with the natural course. Iizawa et al. reported an induction of
haematogenous pneumococcal meningitis in adult mice by intraperitoneal injection of 1 x 104 CFU of S. pneumoniae type 6 (7). In their
model, about half of the mice died in 2 days and additional 20% of mice died in the following 2 days. By day 7, more than 90% of mice die, and the surviving mice showed no symptoms of illness. They had to select the mice by body weight loss. Only mice that lost more than 3 g can develop meningitis. On the contrary, our haematogenous meningitis model is almost 100% and is time-predictable.
Recently, the frequency of meningitis due to the antibiotic-resistant strains of S. pneumoniae has increased rapidly, and the
infection causes the high mortality rate in adults (14). Creating the other compounds, especially the non-antibiotic compounds, became the urgent issue. We have used carboxyfullerene to inhibit the Escherichia coli-induced meningitis (21). Our model of
haematogenous meningitis mimicking the clinical infection can be used for the evaluation of therapy with new drugs or various immunomodulators in bacterial meningitis.
Refer ences
1. Abbott, N. J., and I. A. Romero. 1996. Transporting therapeutics across the blood-brain barrier. Mol. Med. Today 2:106-113. 2. Benveniste, E. N. 1992. Inflammatory
cytokines within the central nervous system: sources, function, and mechanism of action. Am. J. Physiol. 263:C1-C16.
3. Chang, Y. C., C. C. Huang, S. T. Wang, C. C. Liu, and J. J. Tsai. 1998. Risk factors analysis for early fatality in children with acute bacterial meningitis. Pediatr. Neurol. 18:213-217.
4. Durand, M. L., S. B. Calderwood, D. J. Weber, S. I. Miller, F. S. Southwick, V. S. Caviness, and M. N. Swartz. 1993. Acute bacterial meningitis in adults. A. review of 493 episode. N. Engl. J. Med. 328:21-28. 5. Freyer, D., R. Manz, A. Ziegenhorn, M.
Weih, K. Angstwurm, W. D. Docke, A. Meisel, R. R. Schumann, G. Schonfelder, U. Dirnagl, and J. R. Weber. 1999. Cerebral endothelial cells release TNF-alpha after stimulation with cell walls of Streptococcus pneumoniae and regulate inducible nitric
oxide synthase and ICAM-1 expression via autocrine loops. J. Immunol. 63:4308-4314. 6. Ho, T. S., C. Y. Tsai, N. Tsao, N. H. Chow, and H. Y. Lei. 1997. Infiltrated cells in experimental allergic encephalomyelitis by additional intracerebral injection in myelin-basic-protein-sensitized B6 mice. J. Biomed. Sci. 4:300-307.
7. Iizawa, Y., K. Hiroe, M. Nakao, and K. Okonogi. 1998. Therapeutic efficacy of cefozopran in a murine model of haematogenous pneumococcal meningitis. Chemother. 44:265-271.
8. Janzer, R. C., and M. C. Raff. 1987. Astrocytes induce blood-brain barrier properties in endothelial cells. Nature 325:253-257.
9. Kaplan, S. L., E. P. Hawkins, M. W. Kline, G. S. Patrick, and E. O. Mason, Jr. 1989. Invasion of the inner ear by Haemophilus influenzae type b in experimental meningitis. J. Infect. Dis. 159:923-930. 10. Liu, C. C., J. S. Chen, C. H. Lin, Y. J.
Chen, and C. C. Huang. 1993. Bacterial meningitis in infants and children in southern Taiwan: emphasis on
Haemophilus Influenzae type B infection.
J. Formos. Med. Assoc. 92:884-888. 11. Perry, V. H., D. C. Anthony, S. J. Bolton,
and H. C. Brown. 1997. The blood-brain barrier and the inflammatory response. Mol. Med. Today 3:335-341.
12. Pfister, H. W., A. Fontana, M. G. Tauber, A. Tomasz, and W. M. Scheld. 1994. Mechanisms of brain injury in bacterial meningitis. Clin. Infect. Dis. 19:463-479. 13. Quagliarello, V. J., A. Ma, H. Stukenbrok,
and G. E. Palade. 1991. Ultrastructural localization of albumin transport across the cerebral micro- vasculature during experimental meningitis in the rat. J. Exp. Med. 174:657-672.
14. Quagliarello, V. J., and W. M. Scheld. 1997. Treatment of bacterial meningitis. N. Engl. J. Med. 336:708-716.
15. Rodriguez, A. F., S. L. Kaplan, E. P. Hawkins, and E. O. Mason, Jr. 1991. Hematogenous pneumococcal meningitis in the infant rat: description of a model. J. Infect. Dis. 164:1207-1209.
16. Tan, T. Q., C. W. Smith, E. P. Hawkins, E. O. Mason, Jr., and S. L. Kaplan. 1995. Hematogenous bacterial meningitis in an intercellular adhesion molecule-1-deficient infant mouse model. J. Infect. Dis. 171:342-349.
17. Tong, H. H., L. M. Fisher, G. M. Kosunick, and T. E. Demaria. 1999. Effect of tumor necrosis factor alpha and interleukin 1-alpha on the adherence of
Streptococcus pneumoniae to chinchilla
tracheal epithelium. Acta Oto-Laryngologica 119:78-82.
18. Toumanen, E., A. Tomasz, B. Hengstler, and O. Zak. 1985. The relative role of bacterial cell wall and capsule in the induction of inflammation in pneumococcal meningitis. J. Infect. Dis. 151:535-540.
19. Toumanen, E., H. Liu, B. Hengstler, O. Zak, and A. Tomasz. 1985. The induction of meningeal inflammation by components of the pneumococcal cell wall. J. Infect. Dis. 151:859-868.
20. Tsao, N., H. P. Hsu, and H. Y. Lei. 1999. TNFα-induced cyclooxygenase 2 not only increases the vasopermeability of blood-brain barrier but also enhances the
neutrophil survival in Escherichia
coli-iduced brain inflammation. Prostaglandins Lipid Mediat. 57:371-382. 21. Tsao, N., P. P. Kanakamma, T. Y. Luh, C. K. Chou, and H. Y. Lei. 1999. The inhibition of Escherichia coli-induced
meningitis by carboxyfullerene. Antimicro. Agent Chemother. 43:2273-2277.
22. Van Furth, A. M., J. J. Roord, and R. Van Furth. 1996. Roles of proinflammatory and anti-inflammatory cytokines in pathophysiology of bacterial meningitis and effect of adjunctive therapy. Infect. Immun. 64:4883-4890.
23. Wang, S. D., K. J. Huang, Y. S. Lin, and H. Y. Lei. 1994. Sepsis-induced apoptosis of the thymocytes in mice. J. Immunol. 152:5014-5021.
24. Wenger, J. D., A. W. Hightower, R. R. Facklam, S. Gaventa, C. V. Broome, and Bacterial meningitis study group. 1990. Bacterial meningitis in the United State, 1986: reports of a multistate surveillance study. J. Infect. Dis. 162:1316-1323. 25. Wispelwey, B., A. J. Lesse, E. J. Hansen,
and W. M. Scheld. 1988. Haemophilus influenzae lipopolysaccharide-induced blood brain barrier permeability during experimental meningitis in the rat. J. Clin. Invest. 82:1339-1346.
26. Wispelwey, B., E. J. Hansen, and W. M. Scheld. 1989. Haemophilus influenzae
outer membrane vesicle-induced blood-brain barrier permeability during experimental meningitis. Infect. Immun. 57:2559-2562.
Table1. The multiplication of S.pneumoniae type 6 in blood and brain after intraperitoneal injection.
S. pneumoniae type 6
Time(hour) Blood(CFU/ml) Brain(CFU/brain)
3 (6.5±5.0)×10 None
6 (6.8±2.8) ×102 (7.0±2.5) ×102
12 (2.5±1.5) ×105 (4.5±3.1) ×102 24 (2.5±1.0) ×109 (5.5±2.8) ×105
Table2. Anti-TNFα antibody blocked the entrance of S. pneumoniae type 6 into brain.
S. pneumoniae type 6(CFU/brain) Time(hour) W/o anti-TNFα antibody With anti-TNFα
antibody
12 (7.2±5.1)×102 None
24 (1.1±0.8)×106 (8.0±3.5)×102
Table 3. The multiplication of S. pneumoniae
serotype 6 in blood and brain of cefazolin-treated B6 mice.
S. pneumoniae type 6
Time(hour) Blood(CFU/ml) Brain(CFU/brain) 48 (2.70±3.26)×104 (1.38±2.37)×104 72 (1.21±1.36)×106 (2.85±2.47)×103 84 (7.93±14.54)×106 (7.85±11.61)×105 96 (3.37±3.67)×105 (2.38±2.18)×104 108 (8.50±6.09)×105 (5.12±3.01)×105
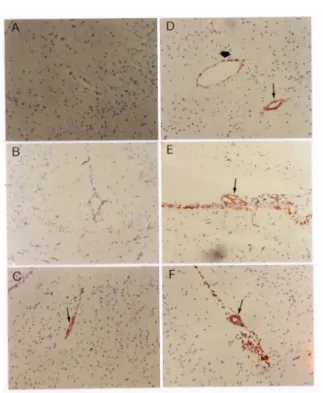
Figur e 1. Induction of TNF-α in brain by injection of S. pneumoniae. Groups of four
mice were inoculated with 50 CFU of S. pneumoniae type 6 per mouse and were
sacrificed at various times post injection (B-F). PBS was used as a control (A). Four-µm cryosections of frozen brain tissues were stained with anti-TNF-α antibody as described in Materials and Methods. A, PBS, 3 h; B, 3 h; C, 6 h; D, 9 h; E, 12 h; F, 24 h (100X). “ Ô “, arteriole; “è “, venule. Arrow indicates positive staining.
0 12 24 36 48 60 72 84 96 108 120 0 1 2 h S c o re S.pneumoniae 6 cefazolin Scor e1 : TNFα expression
Scor e2 : netr ophil infiltr ation, TNFα and IL-1β expression
Figur e 2. Development of meningitis in cefazolin-tr eated miceafter injection with S.pneumoniae type 6.
Figur e 3. Induction of TNF-α, IL-1β and MIP-1β in brain by injection of S. pneumoniae type 6 in cefazolin-tr eated B6 mice. Groups of four mice were inoculated
with 50 CFU of S. pneumoniae type 6 per
mouse, and then intraperitoneally given with cefazolin twice at 12 h and 24 h. The mice were sacrificed at 96 h post injection (C, F, I). PBS-treatment was used as a control (A, D, G), and the mice treated with 50 CFU of S. pneumoniae were also sacrificed at 36 h post
injection (B, E, H). Four-µm cryosections of frozen brain tissues were stained with anti-TNF-α antibody (A-C), anti-IL-1β antibody (D-F), or anti-MIP-1β antibody (G-I)(400 X) as described in Materials and Methods. “Õ”, neuron cell; “ Ô “, arteriole; “▲“, infiltrating neutrophil. Arrow indicates positive staining.