Long-term Survival of A Patient with
Asymptomatic Left Ventricular Pseudoaneurysm after Acute Myocardial Infarction
Chun-Tai Mao, Ming-Feng Li, Yu-Cheng Kao, Wen-Jin Cherng, and Ming-Jui Hung
Department of Cardiology, Chang Gung Memorial Hospital, Chang Gung University College of Medicine, Keelung, Taiwan
Abstract
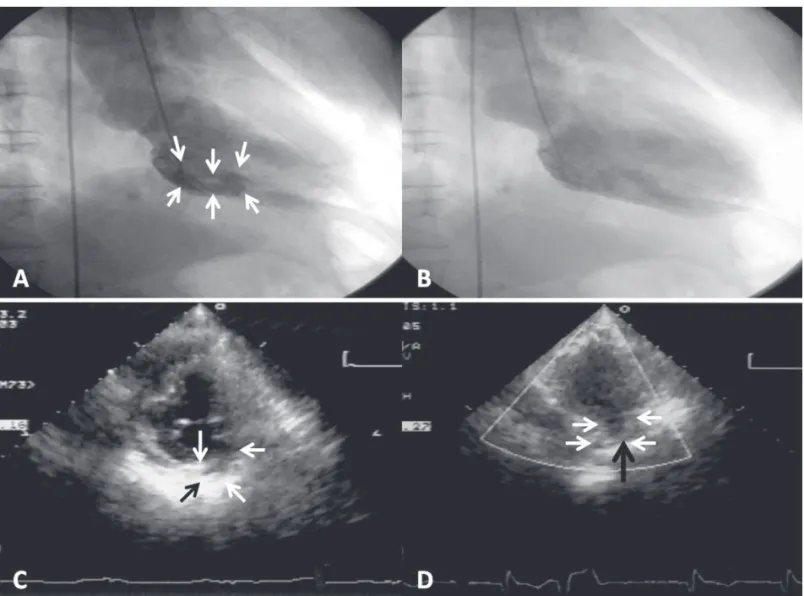
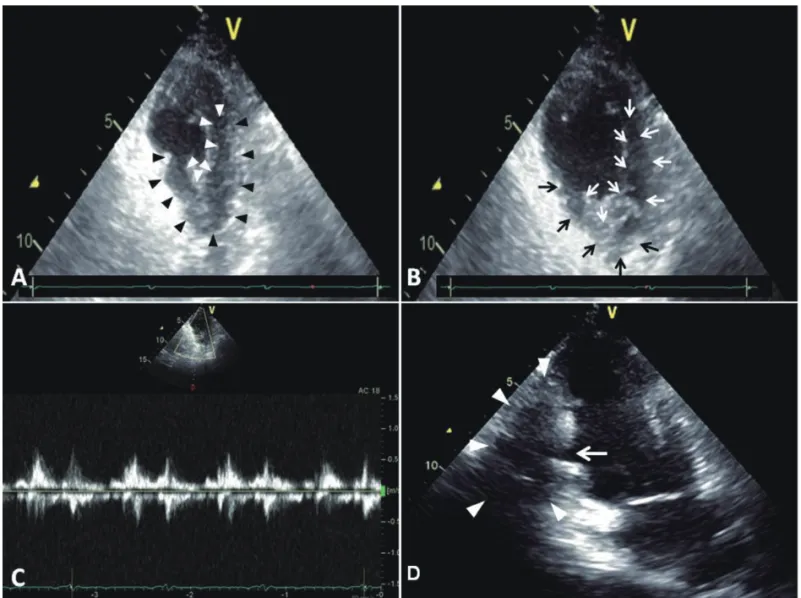
An 82 years old man developed a left ventricular pseudoaneurysm after acute myocardial infarction when he was 72 years old. Coronary angiography showed left main and triple-vessel coronary artery disease. On left ventriculography, a tubular-like pseudoaneurysm was demonstrated that originated from the basal inferoposterior wall of the left ventricle. He underwent coronary artery bypass surgery with no plication of the pseudoaneurysm because the surrounding tissues of pseudoaneurysm were all necrotic. The most recent follow-up transthoracic echocardiography revealed a hypokinetic basal inferior wall, impaired LV contraction with an ejection fraction of 44%, and an inferoposterior wall pseudoaneurysm. The patient was doing well more than 10 years after the myocardial infarction. The prognosis might be determined by the organized thrombi, aggressive pharmacologic treatment, and coronary artery bypass surgery. Although our patient has survived for more than 10 years with a nonsurgically treated post-infarction LV pseudoaneurysm, we could not provide an evidence to support that conservative therapy is enough for every patient with a post-infarction pseudoaneurysm. (J Intern Med Taiwan 2012; 23: 442-448)
Key Words: LV pseudoaneurysms, LV diverticulum, STEMI, CABG, Echo
Introduction
Left ventricular (LV) pseuodaneurysms develop when rupture of the free wall of the left ventricle is contained by pericardial adhesions or scar tissue
1. Surgical resection of pseudoaneurysms is usually recommended because of the risk of spontaneous rupture
2. However, few reports have been published on survival of patients with nonsurgically treated LV pseudoaneurysms
that developed as a result of acute myocardial infarction
3-6. Herein, we present a patient who has survived for more than 10 years with a nonsurgically treated LV pseudoaneurysm that occurred after acute myocardial infarction.
Case report
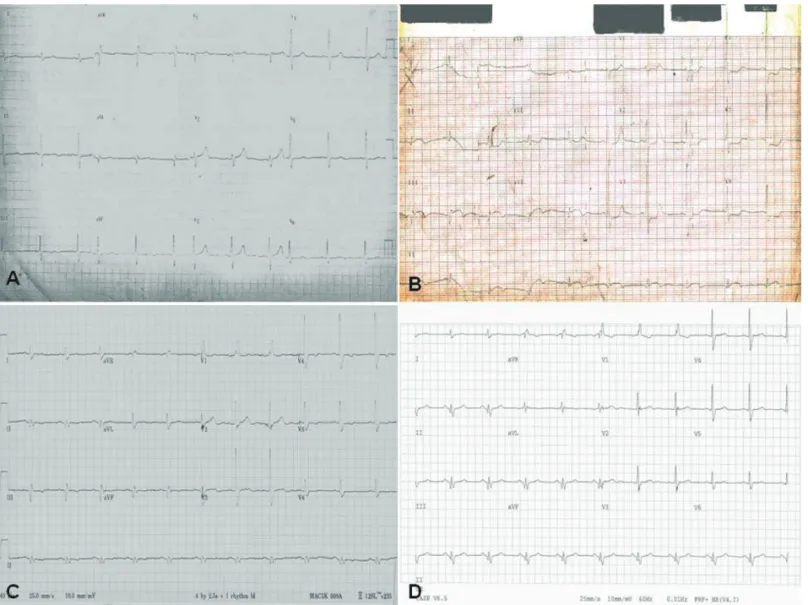
An 82-year-old man with a history of cigarette smoking, hypertension, and dyslipidemia developed an acute inferior myocardial infarction (Fig. 1 A &
Reprint requests and Correspondence:Dr. Ming-Jui Hung
Address:Department of Cardiology, Chang Gung Memorial Hospital, 222 Maijin Road, Keelung 20401, Taiwan