行政院國家科學委員會專題研究計畫 期中進度報告
羊水過少對胎鼠肺臟生長因子、細胞外基質及訊息傳遞的作 用(1/2)
計畫類別: 個別型計畫
計畫編號: NSC94-2314-B-038-025-
執行期間: 94 年 08 月 01 日至 95 年 07 月 31 日 執行單位: 臺北醫學大學醫學系
計畫主持人: 陳中明
共同主持人: 周周周周周周周
報告類型: 精簡報告
處理方式: 本計畫可公開查詢
中 華 民 國 95 年 5 月 8 日
Abstract:
Platelet-derived growth factor (PDGF) is important for alveogenesis of the normally developing lung. The aims of this study were to evaluate the effects of experimental oligohydramnios on lung growth, PDGF and its receptors expressions, and lung morphology in fetal rats. On day 16 of gestation, we anesthetized timed pregnant Sprague-Dawley dams, punctured the uterine wall and fetal membranes of each uterine sac, which resulted in oligohydramnios. Fetuses in the opposite uterine horn served as controls. On days 19 and 21 of gestation, the fetuses were delivered by cesarean section and weighed, and the lungs were dissected free and weighed. Radial saccular count was measured in fetal rat lungs delivered on day 21 of gestation. Oligohydramniotic rats exhibited significantly lower lung/body weight ratios on days 19 and 21 and significantly lower radial saccular counts on day 21 of gestation than did the control rats. Oligohydramnios significantly decreased PDGF-A and -B, and collagen I gene and protein expressions, and elastin content on days 19 and 21 of gestation, and significantly decreased PDGFR-and -gene expressions on day 19 of gestation. These results suggest that decreased PDGF expressions may be important in the pathogenesis of lung hypoplasia induced by oligohydramnios.
Key words: lung growth, collagen, elastin 摘要
血小板生長因子對於正常發育肺臟肺泡的發展很重要。這項研究的目的是評估實驗 性羊水過少對胎鼠的肺生長、肺形態學、血小板生長因子及其接受體的影響。在懷孕週
數第 16 天,我們麻醉懷孕的大白鼠,刺穿子宮囊和胎鼠的膜,導致羊水過少。在另一
角的子宮角裡的胚胎作為控制組。在懷孕週數第19 天及第 21 天,以剖腹產手術取出胎
鼠,秤出生體重,在取出肺、秤肺重。在懷孕週數第21 天出生的胎鼠,測量其肺 Radial
saccular count。與控制組老鼠比,羊水過少的老鼠在懷孕週數第 19 天及第 21 天時有比 較低的肺/體重比率。羊水過少的老鼠在懷孕週數第 19 天及第 21 天時有比較低的血小板 生長因子,collagen I 基因表現和蛋白質表現,elastin 基因表現。這些結果顯示血小板生 長因子表現減少可能在羊水過少引起的肺發育不良的病理變化扮演重要的角色。
關鍵詞:肺生長、膠原蛋白、彈力蛋白
Pulmonary hypoplasia is common in the perinatal period and is a significant cause of death in newborn infants (1). Oligohydramnios is one of the most common associated abnormalities.
Oligohydramnios may retard fetal lung growth and results in pulmonary hypoplasia in experimental animals (2, 3) and in human fetuses with prolonged rupture of the membrane (4) or renal anomalies with decreased fetal urine output (5). However, the exact mechanism by which oligohydramnios alters the fetal respiratory system remains unknown.
Platelet-derived growth factor (PDGF) is important for alveogenesis of the normally developing lung (6). PDGFs are homodimers or heterodimers consisting of two distinct polypeptide chains (A and B), which can be dimerized via sulfhydryl bridges to form three bioactive isoforms (AA, BB, and AB) (7). Physical forces play an important role in regulating fetal lung growth and maturation (8, 9). The main physical force the lung experiences is stretching produced by fetal breathing movements and lung fluid in the airspaces during normal lung development (10). In the fetus, the lungs are filled with a fluid that is secreted from the pulmonary epithelium into the potential airspaces and leaves the lungs via the trachea. The fluid maintains the lungs in an expanded state and provides the tissue with the mechanical stretching that is necessary for normal lung development (11). Mechanical stretching may increase growth factor expression through intracellular signal transduction pathways (12) and has significant impact on the synthesis and secretion of several extracellular matrix (ECM) molecules in fetal lung cell culture (13, 14). However, little is known about the effects of oligohydramnios on the lung PDGF and ECM status in vivo.
MATERIALS AND METHODS
Animals. This study was approved by the Animal Care and Use Committee at Taipei Medical University and was performed with timed pregnant Sprague-Dawley rats. On day 16 of gestation, pregnant dams were anesthetized with pentobarbital (50 mg/kg, i.p.). An abdominal midline incision was made, and the two uterine horns were exposed and kept moist with phosphate-buffered saline. The uterine wall and fetal membranes of each uterine sac in one horn were punctured using a 19-gauge needle, which resulted in immediate visible leakage of amniotic fluid and a decrease in the size of the uterine sac. Fetuses in the opposite uterine horn served as controls. The uterus was returned to the abdomen and the abdominal incision was repaired. On days 19 and 21 of gestation, the fetuses were delivered by cesarean section and allowed not to breathe. The fetuses were weighed and killed by pentobarbital (100 mg/kg, i.p.); lungs were removed, dissected free, weighed, and values were expressed as the ratio (%) of lung/body weights.
Reverse transcription PCR. The left lung was ground into a powder in liquid nitrogen, and gene expressions were measured with RT-PCR. The PCRs were carried out with the primers shown in Table 1. At least five samples on each gestational day were analyzed for each gene in each group.
Western blot analysis. Proteins (30 g) were separated on SDS-PAGE under a non-reducing condition and electro-transferred to a polyvinylidene difluoride membrane (ImmobilonP, Millipore, Bedford, MA, USA).
Lung collagen and elastin content. The Sircol collagen and Fastin elastin assay (Biocolor, Belfast, UK)wasperformed following themanufacturer’sinstructionsand modified from Philips et al. (15).
Statistical analysis. Results are presented as the means SEM. Comparisons between control and oligohydramnios groups at equivalent gestational age were made using unpaired Student’st-test. Differences were considered significant at p < 0.05.
RESULTS
There were three and six pregnant dams used on days 19 and 21 of gestation, respectively.
Control fetuses were all alive at the time of the cesarean section. Three of 26 (11.5%) and 30 of 53 (56.6%) oligohydramniotic fetuses were dead on days 19 and 21 of gestation, respectively.
Body weight, lung weight, and the lung/body weight ratio (%).Oligohydramniotic rats exhibited significantly lower lung/body weight ratios on day 19 of gestation and lower lung weights and lung/body weight ratios on day 21 of gestation when compared with control rats (Table 2).
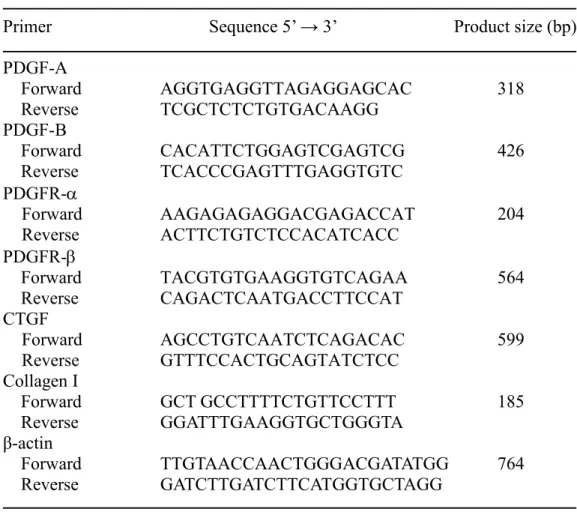
PDGF and PDGFR gene expressions. Oligohydramnios significantly decreased PDGF-A -B, R-, and -R-gene expressions on day 19 of gestation (Fig. 1A). On day 21 of gestation, oligohydramnios also decreased PDGF and PDGFR gene expressions and the values reached statistical significance for PDGF-A and -B only (Fig. 1B).
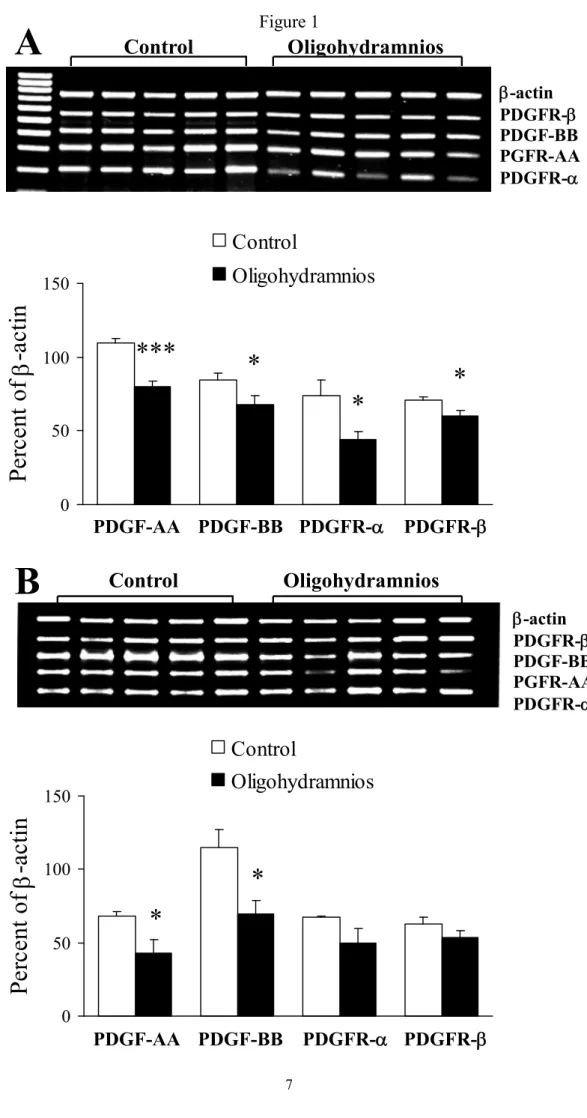
Western blot analysis of PDGF. PDGF-AA and -BB decreased in fetal lung tissue exposed to oligohydramnios on day 16 and harvested on days 19 (Fig. 2A) and 21 (Fig. 2B) of gestation.
CTGF and collagen type I gene expressions. In this study we measured only collagen type I gene expression because it constitutes greater than 65% of the total lung collagen.
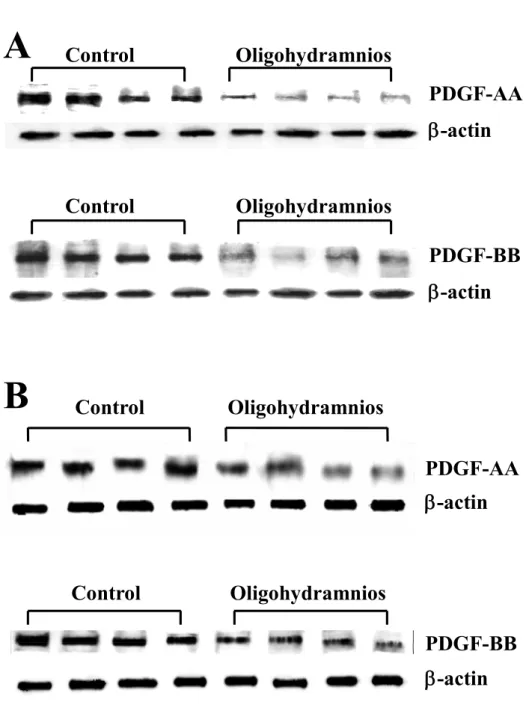
Oligohydramnios significantly decreased collagen I gene expressions on days 19 (Fig. 3A) and 21 (Fig. 3B) of gestation and had no significant effect on CTGF gene expressions.
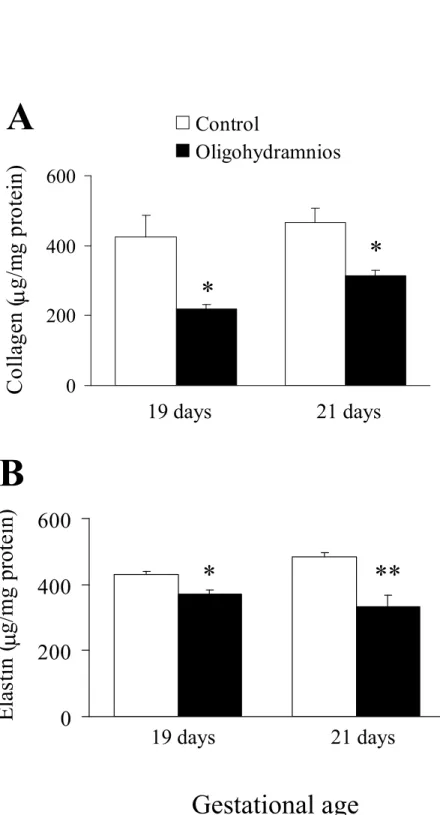
Lung collagen and elastin content. Oligohydramnios significantly decreased lung collagen and elastin content when compared to the control rats on days 19 and 21 of gestation (Fig. 4).
DISCUSSION
Oligohydramnios may retard fetal lung growth and result in pulmonary hypoplasia (2-5).
Pulmonary hypoplasia was defined as a low lung weight for a given body weight. The reported effects of oligohydramnios during late gestation on lung morphometry have been inconsistent in rodents (16, 17). We found that oligohydramnios created on day 16 of gestation decreased lung/body weight ratio on days 19 and 21 of gestation and decreased the radial saccular count on day 21 of gestation. In our preliminary experiment, we found that pulmonary hypoplasia induced by oligohydramnios was not consistently found when we used a finer needle.
PDGF is a powerful stimulator of fibroblast chemotaxis, proliferation, and collagen production (18, 19). Han et al. reported that both PDGF and its receptors are present in fetal rat lung (20, 21). Buch et al. investigated the ontogeny of PDGF gene expression in fetal rat lung epithelial cells and found that PDGF-A gene expression declined with advancing gestation, whereas PDGF-B gene expression increased (22). The effects of oligohydramnios on PDGF gene expression in fetal lung tissues are not well characterized. By using antisense PDGF-A oligonucleotides and antisense PDGF-B oligodeoxynucleotides in embryonic rat lung explant culture, Souza et al. found that PDGF-AA and -BB play critical roles in early lung branching morphogenesis and lung growth, respectively (23, 24).
CTGF, a cysteine-rich, heparin-binding protein, has been implicated in fibroblast proliferation, cellular adhesion, angiogenesis, and ECM synthesis (26). In this study, we found decreased total collagen contents and comparable CTGF expressions on days 19 and 21 of gestation. PDGF is a powerful stimulator of fibroblast chemotaxis and collagen production (18, 19).PDGF overexpression produces fibrosing alveolitis in rats (27), and PDGF inhibition by receptor tyrosine kinase inhibitors attenuates radiation-induced pulmonary fibrosis in mice (28). Those and our findings suggest that CTGF is not a major factor regulating collagen production, and that PDGF plays a pivotal role in the pathogenesis of decreased collagen content in hypoplastic lung induced by oligohydramnios.
Two major stimuli to fetal lung growth result from stretching due either to 1) intermittent and repetitive fetal breathing movements, or 2) a constant distending pressure when fetal breathing movements are absent (10). The constant distending pressure is produced by the secretion of lung fluid and the resistance to outflow in the upper airway (29).
Oligohydramnios does not influence fetal breathing movements but it does decrease the volume of fluid within the potential airways and airspaces (3, 30). The fluid maintains the lungs in an expanded state and provides mechanical stretching for the tissue that is necessary
for normal lung development (11). Mechanical stretching increases growth factors expressions through intracellular signal transduction pathways (12).
In conclusion, the results of this study showed that maternal oligohydramnios during late gestation decreased PDGF and its receptor expressions, decreased total collagen and elastin contents, and arrested lung development in fetal rat lungs. These results suggest that PDGF expressions may be important in the pathogenesis of oligohydramnios-induced lung hypoplasia. The underlying mechanism is unclear, and its elucidation may provide useful therapeutic strategies for pulmonary hypoplasia induced by oligohydramnios.
REFERENCES
1. Husain AN, Hessel RG 1993 Neonatal pulmonary hypoplasia: an autopsy study of 25 cases. Pediatr Pathol 13:475-484
2. Adzick NS, Harrison MR, Glick PL, Villa RL, Finkbeiner W 1984 Experimental pulmonary hypoplasia and oligohydramnios: relative contributions of lung fluid and fetal breathing movements. J Pediatr Surg 19:658-665
3. Dickson KA, Harding R 1989 Decline in lung liquid volume and secretion rate during oligohydramnios in fetal sheep. J Appl Physiol 67:2401-2407
4. ThibeaultDW,Beatty EC,HallRT,Bowen SK,O’NellDH1985 Neonatal pulmonary hypoplasia with premature rupture of fetal membranes and oligohydramnios. J Pediatr 107:273-277
5. Hislop A, Hey E, Reid L 1979 The lungs in congenital bilateral renal agenesis and dysplasia. Arch Dis Child 54:32-38
6. Lindahl P, Bostrom H, Karlsson L, Hellstrom M, Kalen M, Betsholtz C 1999 Role of platelet-derived growth factors in angiogenesis and alveogenesis. Curr Top Pathol 93:27-33
7. Ross R 1989 Platelet-derived growth factor. Lancet 1:1179-1182
8. Harding R, Hooper SB 1996 Regulation of lung expansion and lung growth before birth. J Appl Physiol 81:209-224
9. Joe P, Wallen LD, Chapin CJ, Lee CH, Allen L, Han VK, Dobbs LG, Hawgood S, Kitterman JA 1997 Effects of mechanical factors on growth and maturation of the lung in fetal sheep. Am J Physiol 272:L95-L105
10. Kitterman JA 1996 The effects of mechanical forces on fetal lung growth. Clin Perinatol 23:727-740
11. Alcorn D, Adamson TM, Lambert TF, Maloney JE, Ritchie BC, Robinson PM 1977 Morphological effects of chronic tracheal ligation and drainage in the fetal lamb lung. J Anat 123:649-660
12. Liu M, Tanswell AK, Post M 1999 Mechanical force-induced signal transduction in lung cells. Am J Physiol 277:L667-L683
13. Xu J, Liu M, Post M 1999 Differential regulation of extracellular matrix molecules by mechanical strain of fetal lung cells. Am J Physiol 276:L728-L735
14. Breen EC 2000 Mechanical strain increases type I collagen expression in pulmonary fibroblasts in vitro. J Appl Physiol 88:203-209
15. Phillips RJ, Burdick MD, Hong K, Lutz MA, Murray LA, Xue YY, Belperio JA, Keane MP, Strieter RM 2004 Circulating fibrocytes traffic to the lungs in response to CXCL12 and mediate fibrosis. J Clin Invest 114:438-446
16. Blachford KG, Thurlbeck WM 1987 Lung growth and maturation in experimental oligohydramnios in the rat. Pediatr Pulmonol 3:328-333
17. Collins MH, Moessinger AC, Kleinerman J, James LS, Blanc WA 1986 Morphometry of hypoplastic fetal guinea pig lungs following amniotic fluid leak. Pediatr Res 20:955-960 18. Osornio-Vargas AR, Goodell AL, Hernandez-Rodriguez NA, Brody AR, Coin PG,
Badgett A, Bonner JC 1995 Platelet-derived growth factor (PDGF)-AA, -AB, and -BB
induce differential chemotaxis of early-passage rat lung fibroblasts in vitro. Am J Respir Cell Mol Biol 12:33-40
19. Zhang K, Phan SH 1996 Cytokines and pulmonary fibrosis. Biol Signals 5:232-239 20. Han RNN, Mawdsley C, Souza P, Tanswell AK, Post M 1992 Platelet-derived growth
factors and growth-related genes in rat lung. III. Immunolocalization during fetal development. Pediatr Res 31:323-329
21. Han RNN, Liu J, Tanswell AK, Post M 1993 Ontogeny of platelet-derived growth factor receptor in fetal rat lung. Micros Res Tech 26:381-388
22. Buch S, Jassal D, Cannigia I, Edelson J, Han R, Liu J, Tanswell K, Post M 1994 Ontogeny and regulation of platelet-derived growth factor gene expression in distal fetal rat lung epithelial cells. Am J Respir Cell Mol Biol 11:251-261
23. Souza P, Kuliszewski M, Wang J, Tseu I, Tanswell AK, Post M 1995 PDGF-AA and its receptor influence early lung branching via an epithelial-mesenchymal interaction.
Development 121:2559-2567
24. Souza P, Sedlackova L, Kuliszewski M, Wang J, Liu J, Tseu I, Liu M, Tanswell AK, Post M 1994 Antisense oligodeoxynucleotides targeting PDGF-B mRNA inhibit cell proliferation during embryonic rat lung development. Development 120:2163-2173 25. Moussad EE, Brigstock DR 2000 Connective tissue growth factor: what's in a name? Mol
Genet Metab 71:276-292
26. Schild C, Trueb B 2002 Mechanical stress is required for high level expression of connective tissue growth factor. Exp Cell Res 274:83-91
27. Yoshida M, Sakuma J, Hayashi S, Abe K, Saito I, Harada S, Sakatani M, Yamamoto S, Matsumoto N, Kaneda Y, Kishimoto T 1995 A histologically distinctive interstitial pneumonia induced by overexpression of the interleukin 6, transforming growth factor1, or platelet-derived growth factor B gene. Proc Natl Acad Sci USA 92:9570-9574
28. Abdollahi A, Li M, Ping G, Plathow C, Domhan S, Kiessling F, Lee LB, McMahon G, Grone HJ, Lipson KE, Huber PE 2005 Inhibition of platelet-derived growth factor signaling attenuates pulmonary fibrosis. J Exp Med 201:925-935
29. Harding R, Bocking AD, Sigger JN 1986 Upper airway resistances in fetal sheep: the influence of breathing activity. J Appl Physiol 60:160-165
30. Savich RD, Guerra FA, Lee CC, Padbury JF, Kitterman JA 1992 Effects of acute oligohydramnios on respiratory system of fetal sheep. J Appl Physiol 73:610-617
FIGURE LEGENDS
Figure 1. Platelet-derived growth factor (PDGF) and PDGF receptor (PDGFR) gene expressions in the control and oligohydramniotic rat lungs. (A) Oligohydramnios significantly decreased PDGF-A -B, -R-, and -R-gene expressions compared to those of control rats on day 19 of gestation. (B) Oligohydramnios significantly decreased PDGF-A and -B gene expressions on day 21 of gestation. *p < 0.05,
***p < 0.001 versus the control.
Figure 2. Representative Western blots for PDGF-AA and -BB protein in fetal rat lung exposed to oligohydramnios on day 16 and harvested on days 19 (A) and 21 (B) of gestation. Oligohydramnios decreased PDGF-AA and -BB on days 19 and 21 of gestation.
Figure 3. Connective tissue growth factor (CTGF) and collagen I gene expressions on days 19 (A) and 21 (B) of gestation. Oligohydramnios significantly decreased collagen I gene expressions on days 19 and 21 of gestation and had no significant effect on CTGF gene expressions. **p < 0.01 versus the control.
Figure 4. (A) Total collagen and (B) elastin content in fetal rat lungs. Oligohydramnios significantly decreased lung tissue collagen and elastin content on days 19 and 21 of gestation. *p < 0.05, **p < 0.01 versus the control.
Table 1. Oligonucleotide sequences of the primers used
Primer Sequence5’→ 3’ Product size (bp)
PDGF-A
Forward AGGTGAGGTTAGAGGAGCAC 318
Reverse TCGCTCTCTGTGACAAGG
PDGF-B
Forward CACATTCTGGAGTCGAGTCG 426
Reverse TCACCCGAGTTTGAGGTGTC
PDGFR-
Forward AAGAGAGAGGACGAGACCAT 204
Reverse ACTTCTGTCTCCACATCACC
PDGFR-
Forward TACGTGTGAAGGTGTCAGAA 564
Reverse CAGACTCAATGACCTTCCAT
CTGF
Forward AGCCTGTCAATCTCAGACAC 599
Reverse GTTTCCACTGCAGTATCTCC
Collagen I
Forward GCT GCCTTTTCTGTTCCTTT 185
Reverse GGATTTGAAGGTGCTGGGTA
β-actin
Forward TTGTAACCAACTGGGACGATATGG 764
Reverse GATCTTGATCTTCATGGTGCTAGG
Table 2. Body weight, lung weight, and lung/body weight ratio in the control and oligohydramniotic rats
Treatment n Gestational Body weight Lung weight Lung/body weight
age (d) (g) (g) (%)
Control 16 19 2.02 ± 0.13 0.08 ± 0.01 3.71 ± 0.10
Oligohydramnios 23 19 2.08 ± 0.11 0.07 ± 0.00 3.18 ± 0.10***
Control 27 21 4.10 ± 0.12 0.15 ± 0.01 3.65 ± 0.11
Oligohydramnios 23 21 3.88 ± 0.20 0.12 ± 0.01** 3.04 ± 0.08***
**p < 0.01, ***p < 0.001 versus the control group at each gestational age.
Figure 1
0 50 100 150
Pe rc en to
f b
-a ct in
Control
Oligohydramnios
0 50 100 150
Pe rc en to
f b
-a ct in
Control
Oligohydramnios
-actin PDGFR-
PDGF-BB PGFR-AA PDGFR-
PDGF-AA PDGF-BB PDGFR- PDGFR-
-actin PDGFR-
PDGF-BB PGFR-AA PDGFR-
PDGF-AA PDGF-BB PDGFR- PDGFR-
Control Oligohydramnios
A
B
*** *
* *
*
*
Control Oligohydramnios
Figure 2
PDGF-AA
-actin Control
PDGF-BB
-actin Control Oligohydramnios
Oligohydramnios
Control
PDGF-AA
-actin
Control Oligohydramnios
PDGF-BB Oligohydramnios
-actin
A
B
Figure 3
0 50 100 150
Pe rc en to
f b
-a ct in
Control
Oligohydramnios
0 25 50 75
Pe rc en to
f b
-a ct in
Control
Oligohydramnios
CTGF Collagen I
**
CTGF Collagen I
**
-actin CTGF Collagen I
-actin CTGF Collagen I
Control Oligohydramnios
A
B Control Oligohydramnios
Figure 4
0 200 400 600
C ol la ge n
( m g/ m g pr ot ei n)
Control
Oligohydramnios