Original Article
Risk factors and mortality of adults with lung cancer admitted to the intensive care unit
Chih-Cheng Lai1#, Chung-Han Ho2,3#, Chin-Ming Chen4,5, Shyh-Ren Chiang5,6, Chien-Ming Chao1, Wei- Lun Liu7, Jhi-Joung Wang2, Ching-Chieh Yang8,9,10, Kuo-Chen Cheng6,11
1Department of Intensive Care Medicine, Chi Mei Medical Center, Liouying; 2Department of Medical Research, Chi Mei Medical Center, Tainan;
3Department of Hospital and Health Care Administration, Chia-Nan University of Pharmacy and Science, Tainan; 4Department of Intensive Care Medicine, Chi Mei Medical Center, Tainan; 5Chia Nan University of Pharmacy and Science, Tainan; 6Internal Medicine, Chi Mei Medical Center, Tainan; 7Department of Emergency and Critical Care Medicine, Fu Jen Catholic University Hospital, New Taipei; 8Department of Radiation Oncology, Chi-Mei Medical Center, Tainan; 9Institute of Biomedical Sciences, National Sun Yat-Sen University, Kaohsiung; 10Department of Biotechnology, Chia-Nan University of Pharmacy and Science, Tainan; 11Department of Safety, Health, and Environmental Engineering, Chung Hwa University of Medical Technology, Tainan
Contributions: (I) Conception and design: CC Lai, CH Ho, KC Cheng; (II) Administrative support: CC Yang, JJ Wang; (III) Provision of study materials or patients: JJ Wang, KC Cheng; (IV) Collection and assembly of data: CH Ho; (V) Data analysis and interpretation: CC Lai, CM Chen, SR Chiang, CM Chao, WL Liu, CC Yang, KC Cheng; (VI) Manuscript writing: All authors; (VII) Final approval of manuscript: All authors.
#These authors contributed equally to this work.
Correspondence to: Kuo-Chen Cheng. Department of Internal Medicine, Chi Mei Medical Center, 901 Zhonghua Road, Yongkang District, Tainan City 71044. Email: kcg.cheng@gmail.com.
Background: This study aims to investigate lung cancer patients’ risk factors for: intensive care unit (ICU) admission, infectious complications and organ dysfunction in the ICU, and prognosis after ICU admission.
Methods: The records of all patients with lung-cancer catastrophic-illness cards admitted to the ICU between 2003 and 2012 were reviewed. The primary endpoint was 1-year mortality.
Results: We finally analyzed the records of index-date-, age-, and sex-matched ICU-admitted (ICU+) lung cancer patients (n=17,687) and ICU-non-admitted (ICU–) lung cancer patients (n=35,374). The overall 1-year mortality rate was significantly (P<0.0001) higher for ICU+ patients (49.91%) than for ICU– patients (44.86%). Most ICU+ patients (56.16%) had infectious complications and organ dysfunction (52.33%), and overall, 6,893 (38.97%) had sepsis. Independent mortality risk factors were age (≥75 years) [adjusted hazard ratio (AHR), 1.22; 95% confidence interval (CI), 1.16–1.29], male sex: (AHR, 1.18; 95% CI, 1.13–1.23), recent radiotherapy (AHR, 1.09; 95% CI, 1.04–1.15), infectious complications (AHR: 1.23; 95% CI: 1.17–
1.29), organ dysfunction (AHR, 1.57; 95% CI, 1.50–1.65), and hospital level (regional hospital: AHR, 1.11;
95% CI, 1.06–1.16; local hospital: AHR, 1.28; 95% CI, 1.18–1.37).
Conclusions: ICU admission for lung cancer patients is associated with higher mortality. Several risk factors of mortality for ICU+ patients should help physicians provide patients personalized and better- informed lung cancer therapy decisions.
Keywords: Lung cancer; intensive care unit; mortality; risk factor; sepsis
Submitted Feb 28, 2018. Accepted for publication Jun 22, 2018.
doi: 10.21037/jtd.2018.06.165
View this article at: http://dx.doi.org/10.21037/jtd.2018.06.165
Introduction
Despite improvements in early diagnosis and appropriate treatments such as chemotherapy, targeted therapy, and even immunotherapy for lung cancer, improvements in long-term survival of patients with lung cancer have been limited, and lung cancer remains a devastating disease (1). It is now the leading cause of cancer-related death worldwide (2,3): it leads to 1.6 million deaths annually (4,5). More than 158,000 patients died from lung cancer in the United States in 2016, which accounted for 27% of all cancer deaths (4,5).
Much work needs to be done to improve the outcome of lung cancer patients. In addition to early detection and advanced treatment, intensive care unit (ICU) strategies and treatments are important because ICU admission is not uncommon for lung cancer patients with malignancy- related complications or with underlying comorbidities (6).
Factors associated with poor ICU outcomes for these patients include mechanical ventilation (MV) (7-12), poor pre-event performance status (PS) (10,13,14), high admission Acute Physiologic and Chronic Health Evaluation (APACHE) III scores with vasopressor use (11), and refractory disease (8,10,11,15). However, most of the previous studies were single-center, and their data cannot be generalized across ICUs. Moreover, few studies have examined the risk factors for lung cancer patients admitted to the ICU. Therefore, we investigated the risk factors for admission to the ICU, the infectious complications, organ dysfunction during ICU hospitalizations, and the prognosis after ICU admission among patients with lung cancer.
Methods Data source
This retrospective, population-based cohort study used Taiwan’s National Health Insurance Research Database (NHIRD). Taiwan’s NHI program is a single-payer compulsory system currently enrolls more than 23 million of the country’s legal residents (>99.7% of Taiwan’s population (16). The NHIRD provides detailed healthcare services information about the clinical visits of each beneficiary. It uses International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) diagnostic and procedure codes. We used the NHIRD’s lung cancer database, which was constructed from the records of all patients with lung cancer and a catastrophic illness card to select cases for our study. In the NHIRD, biopsy and histological verifications are required before a definite
diagnosis of carcinoma can be made and a catastrophic illness card can be obtained. Thus, because we used these criteria, the lung cancer cases selected for our study were considered valid and definite. The lung cancer database is a longitudinal subset of the NHIRD; it contains data related to inpatient and outpatient medical care, diagnoses, surgical procedures, and prescribed medications from 2003 to 2012.
The study was approved by the Institutional Review Board (IRB no: 10411-E01) of Chi Mei Medical Center. Because the data used in this study are de-identified, and have been released to the public for research, the IRB waived informed consent.
Case selection and definition
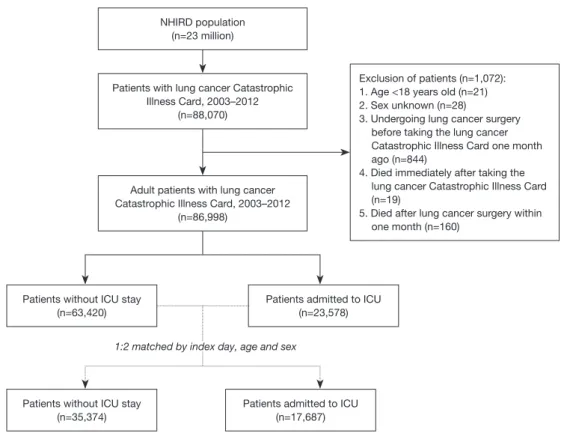
The lung cancer database included 88,070 patients with a lung cancer catastrophic illness card. After excluding patients <18 years old, of unknown sex, who underwent lung cancer surgery just one month before receiving the lung cancer catastrophic illness card, who died immediately after undergoing lung cancer catastrophic illness care, or who died within one month after lung cancer surgery, 86,998 cases were selected for our study: 23,578 had been admitted to the ICU between January 1, 2003, and December 31, 2012. ICU admission was defined according to medical expenditure applications, and the date of ICU admission was defined as the index date. To investigate the risk factor of ICU admission, each case admitted to the ICU was index-date-, age-, and sex-matched with two cases not admitted to the ICU. Finally, the records of 17,687 cases admitted to the ICU and 35,374 cases not admitted to the ICU were selected for analysis (Figure 1).
Baseline variables
To be as comprehensive as possible when adjusting for factors that might affect outcome, we considered the following as potential confounders: sex, age, hospital accreditation, and underlying comorbidities. Comorbidities were defined according to the ICD-9-CM including diabetes mellitus (DM) [ICD-9-CM codes: 250], hyperlipidemia [272], hypertension [401–405], stroke [430–438], chronic obstructive pulmonary disease (COPD) [490–496], chronic liver disease [571], and chronic kidney disease (CKD) [585] within 1 year before their index admission. We also collected information about several other possible confounders—anticancer treatments ≤1 year of ICU admission: surgery, chemotherapy, radiotherapy, and
targeted therapy; infectious diseases; and organ dysfunction by using ICD coding.
Endpoint
The primary endpoint of the study was one-year mortality.
The definition of mortality was based on the death record from the inpatient claim dataset. Patients were followed from January 1, 2003, until the earliest onset of one of two occurrences, whichever came first: mortality or the end of the study, December 31, 2013.
Statistical analysis
All categorical variables are presented as frequencies with percentages, and the Pearson’s χ2 test or Fisher’s exact test was used to compare the differences between lung cancer patients admitted (ICU+) and not admitted (ICU–) to the ICU. Continuous variables are presented as means ± standard deviation (SD) or median with interquartile range (IQR), and a Student’s t-test or Wilcoxon rank-sum test was used to compare age and time to mortality or follow-
up time. In addition, Kaplan-Meier survival curves were used for the 1-year survival rate during the study, and a log-rank test was used to compare risks between ICU+ and ICU– patients. The ratio of 1-year mortality between ICU+ and ICU– patients was estimated using Cox proportional regression models adjusted for age, sex, comorbidities, lung cancer surgery, recent anticancer treatment, infectious diseases, organ dysfunction, and hospital level. A multiple Cox regression analysis was used to find the potential predictors of 1-year mortality among ICU+ patients. SAS 9.4 (SAS Institute Inc., Cary, NC, USA) was used for all analyses. Significance was set at P<0.05. Kaplan-Meier survival curves were plotted using STATA 12 (Stata Corp., College Station, TX, USA).
Results
Clinical characteristics
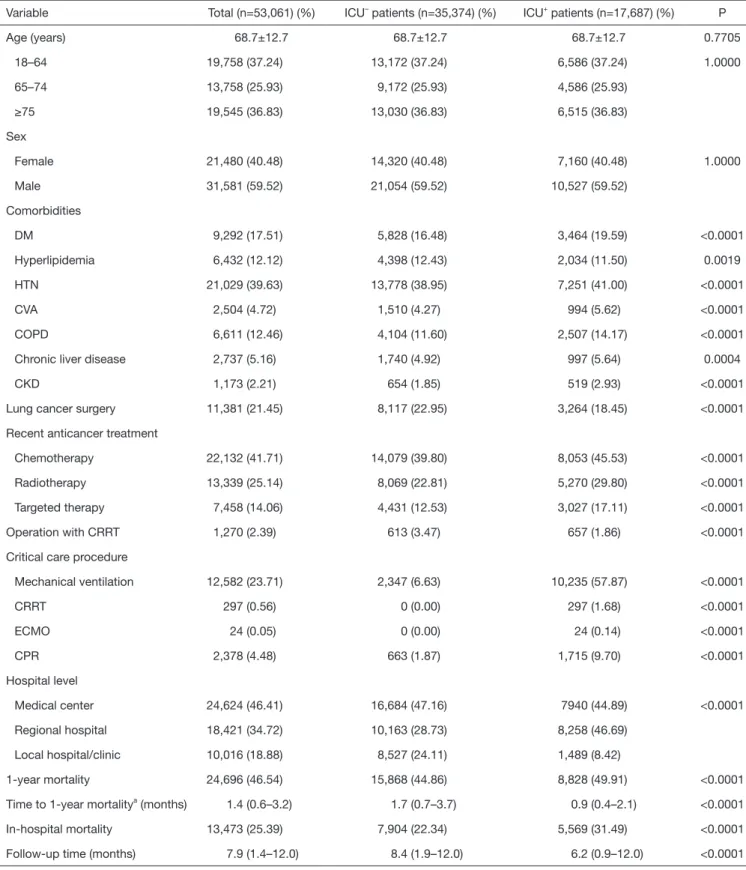
ICU+ patients had more comorbidities—DM, HTN, strokes, COPD, chronic liver disease, and CKD—than did ICU– patients (Table 1). In contrast, ICU– patients had more hyperlipidemia than did ICU+ patients. ICU+ patients were
NHIRD population (n=23 million)
Patients with lung cancer Catastrophic Illness Card, 2003–2012
(n=88,070)
Exclusion of patients (n=1,072):
1. Age <18 years old (n=21) 2. Sex unknown (n=28)
3. Undergoing lung cancer surgery before taking the lung cancer Catastrophic Illness Card one month ago (n=844)
4. Died immediately after taking the lung cancer Catastrophic Illness Card (n=19)
5. Died after lung cancer surgery within one month (n=160)
Adult patients with lung cancer Catastrophic Illness Card, 2003–2012
(n=86,998)
Patients without ICU stay (n=35,374)
Patients admitted to ICU (n=17,687) Patients without ICU stay
(n=63,420)
Patients admitted to ICU (n=23,578) 1:2 matched by index day, age and sex
Figure 1 Algorithm for patient enrollment.
Table 1 Characteristics of lung cancer patients
Variable Total (n=53,061) (%) ICU– patients (n=35,374) (%) ICU+ patients (n=17,687) (%) P
Age (years) 68.7±12.7 68.7±12.7 68.7±12.7 0.7705
18–64 19,758 (37.24) 13,172 (37.24) 6,586 (37.24) 1.0000
65–74 13,758 (25.93) 9,172 (25.93) 4,586 (25.93)
≥75 19,545 (36.83) 13,030 (36.83) 6,515 (36.83)
Sex
Female 21,480 (40.48) 14,320 (40.48) 7,160 (40.48) 1.0000
Male 31,581 (59.52) 21,054 (59.52) 10,527 (59.52)
Comorbidities
DM 9,292 (17.51) 5,828 (16.48) 3,464 (19.59) <0.0001
Hyperlipidemia 6,432 (12.12) 4,398 (12.43) 2,034 (11.50) 0.0019
HTN 21,029 (39.63) 13,778 (38.95) 7,251 (41.00) <0.0001
CVA 2,504 (4.72) 1,510 (4.27) 994 (5.62) <0.0001
COPD 6,611 (12.46) 4,104 (11.60) 2,507 (14.17) <0.0001
Chronic liver disease 2,737 (5.16) 1,740 (4.92) 997 (5.64) 0.0004
CKD 1,173 (2.21) 654 (1.85) 519 (2.93) <0.0001
Lung cancer surgery 11,381 (21.45) 8,117 (22.95) 3,264 (18.45) <0.0001
Recent anticancer treatment
Chemotherapy 22,132 (41.71) 14,079 (39.80) 8,053 (45.53) <0.0001
Radiotherapy 13,339 (25.14) 8,069 (22.81) 5,270 (29.80) <0.0001
Targeted therapy 7,458 (14.06) 4,431 (12.53) 3,027 (17.11) <0.0001
Operation with CRRT 1,270 (2.39) 613 (3.47) 657 (1.86) <0.0001
Critical care procedure
Mechanical ventilation 12,582 (23.71) 2,347 (6.63) 10,235 (57.87) <0.0001
CRRT 297 (0.56) 0 (0.00) 297 (1.68) <0.0001
ECMO 24 (0.05) 0 (0.00) 24 (0.14) <0.0001
CPR 2,378 (4.48) 663 (1.87) 1,715 (9.70) <0.0001
Hospital level
Medical center 24,624 (46.41) 16,684 (47.16) 7940 (44.89) <0.0001
Regional hospital 18,421 (34.72) 10,163 (28.73) 8,258 (46.69)
Local hospital/clinic 10,016 (18.88) 8,527 (24.11) 1,489 (8.42)
1-year mortality 24,696 (46.54) 15,868 (44.86) 8,828 (49.91) <0.0001
Time to 1-year mortalitya (months) 1.4 (0.6–3.2) 1.7 (0.7–3.7) 0.9 (0.4–2.1) <0.0001
In-hospital mortality 13,473 (25.39) 7,904 (22.34) 5,569 (31.49) <0.0001
Follow-up time (months) 7.9 (1.4–12.0) 8.4 (1.9–12.0) 6.2 (0.9–12.0) <0.0001
Data are shown as n (%), mean ± SD or median (Q1–Q3). aLength of ICU stay (ICU+ patients) or length of hospital stay (ICU– patients) to 1-year mortality. DM, diabetes mellitus; HTN, hypertension; CVA, cerebralvascular accident; COPD, chronic obstructive pulmonary disease; CKD, chronic kidney disease; CRRT, continuous renal replacement therapy; ECMO, extracorporeal membrane oxygenation; CPR, cardiopulmonary resuscitation.
more likely to be treated with chemotherapy, radiotherapy, and targeted therapy than were ICU– patients. In contrast, ICU– patients were more likely to have undergone prior lung cancer surgery than were ICU+ patients. Regarding critical care procedures, ICU+ patients are more likely to receive MV, continuous renal replacement therapy (CRRT), extracorporeal membrane oxygenation (ECMO), and cardiopulmonary resuscitation (CPR) than ICU- patients.
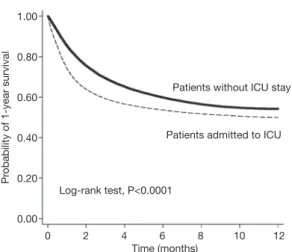
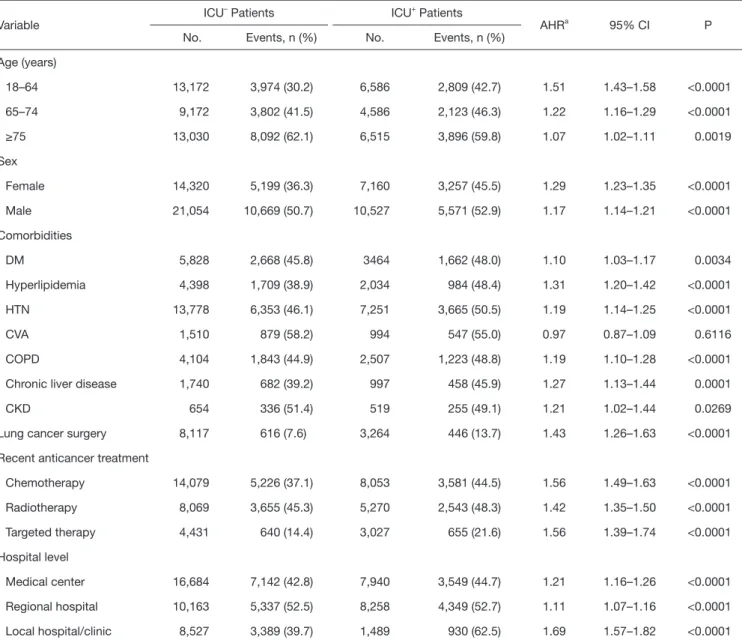
ICU+ patients were more frequently treated in a medical center or regional hospital than were ICU– patients. The overall 1-year mortality rate was significantly higher for ICU+ patients than for ICU– patients (49.91% vs. 44.86%, respectively; P<0.0001), and the time to one-year mortality was significantly shorter for ICU+ patients than for ICU– patients (P<0.0001). Kaplan-Meier survival analysis showed that ICU+ patients had a lower 1-year survival rate than did ICU– patients (Log rank test, P<0.001) (Figure 2). In the subgroup analysis, for ICU+ patients remained significant in each subgroup, irrespective of age, sex, comorbidities, recent anticancer treatment, and hospital level (Table 2).
Complications of ICU+ lung cancer patients
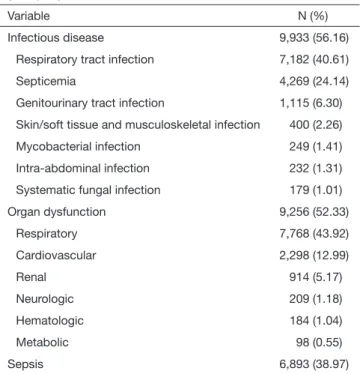
Of the 17,687 ICU+ lung cancer patients, the median length of ICU stay was 4 days (IQR: 2–11 days). More than half of these patients had an infectious disease (n=9,933, 56.16%):
respiratory tract infections were most common (n=7,182, 40.61%), and septicemia (n=4,269, 24.14%). Almost as many (n=9,256, 52.33%) had an organ dysfunction:
respiratory organ dysfunction was most common (n=7,768, 43.92%), and cardiovascular system (n=2,298, 12.99%)
(Table 3). Overall, 6,893 (38.97%) patients had sepsis during their ICU stay.
Risk factors for 1-year mortality among ICU+ patients A multivariate analysis indicated that independent risk factors for mortality for ICU+ lung cancer patients were age (≥75 years) [adjusted hazard ratio (AHR), 1.22; 95%
CI, 1.16–1.29], male sex (AHR, 1.18; 95% CI, 1.13–1.23), recent radiotherapy (AHR, 1.09; 95% CI, 1.04–1.15), infectious diseases (AHR, 1.23; 95% CI, 1.17–1.29), organ dysfunction (AHR, 1.57; 95% CI, 1.50–1.65), and lower- level hospitals (Table 4). In contrast, ICU+ patients treated with recent lung cancer surgery (AHR, 0.19; 95% CI, 0.17–0.21), chemotherapy (AHR, 0.84; 95% CI, 0.80–0.88), or targeted therapy (AHR, 0.32; 95% CI, 0.30–0.35) were associated with lower mortality (Table 4).
Discussion
This is the first national population-based study to specifically investigate the outcomes of ICU+ lung cancer patients. We have several significant findings. First, in almost all subgroup analyses, the overall 1-year mortality rate was significantly higher, and the time to 1-year mortality was significant shorter, for ICU+ patients. Survival analysis showed that patients admitted to ICU had lower probability of 1-year survival rate than patients without ICU admission. The differences regarding survival rate become evident just soon after ICU admission. The poor impact of ICU admission on the 1-year mortality remained significant in almost all of subgroup analysis.
Second, we found that about 40% of ICU+ lung cancer patients had sepsis, and more than 50% had an organ dysfunction: respiratory tract infections and respiratory organ dysfunction were most common. This finding is consistent the previous study (9) of 143 lung cancer which showed that the main reasons for ICU admission were sepsis (44%) and acute respiratory failure (31%). This is reasonable (9,17,18). Overall, this kind of information will help intensivists improve treatment for ICU+ lung cancer patients.
Third, we identified several factors that indicate a poor prognosis: age, male sex, recent radiotherapy, infectious diseases, organ dysfunction, and low hospital level, some of have already been reported (9,19). Chang et al. (19) enrolling 143 patients with lung cancer and pneumonia-
Probability of 1-year survival
1.00
0.80
0.60 0.40
0.20
0.00
0 2 4 6 8 10 12
Time (months)
Patients without ICU stay
Patients admitted to ICU
Log-rank test, P<0.0001
Figure 2 Kaplan-Meier plot: 1-year mortality.
Table 2 Specific subgroup analysis for 1-year mortality in ICU– and ICU+ patients (n=53,061)
Variable ICU– Patients ICU+ Patients
AHRa 95% CI P
No. Events, n (%) No. Events, n (%)
Age (years)
18–64 13,172 3,974 (30.2) 6,586 2,809 (42.7) 1.51 1.43–1.58 <0.0001
65–74 9,172 3,802 (41.5) 4,586 2,123 (46.3) 1.22 1.16–1.29 <0.0001
≥75 13,030 8,092 (62.1) 6,515 3,896 (59.8) 1.07 1.02–1.11 0.0019
Sex
Female 14,320 5,199 (36.3) 7,160 3,257 (45.5) 1.29 1.23–1.35 <0.0001
Male 21,054 10,669 (50.7) 10,527 5,571 (52.9) 1.17 1.14–1.21 <0.0001
Comorbidities
DM 5,828 2,668 (45.8) 3464 1,662 (48.0) 1.10 1.03–1.17 0.0034
Hyperlipidemia 4,398 1,709 (38.9) 2,034 984 (48.4) 1.31 1.20–1.42 <0.0001
HTN 13,778 6,353 (46.1) 7,251 3,665 (50.5) 1.19 1.14–1.25 <0.0001
CVA 1,510 879 (58.2) 994 547 (55.0) 0.97 0.87–1.09 0.6116
COPD 4,104 1,843 (44.9) 2,507 1,223 (48.8) 1.19 1.10–1.28 <0.0001
Chronic liver disease 1,740 682 (39.2) 997 458 (45.9) 1.27 1.13–1.44 0.0001
CKD 654 336 (51.4) 519 255 (49.1) 1.21 1.02–1.44 0.0269
Lung cancer surgery 8,117 616 (7.6) 3,264 446 (13.7) 1.43 1.26–1.63 <0.0001
Recent anticancer treatment
Chemotherapy 14,079 5,226 (37.1) 8,053 3,581 (44.5) 1.56 1.49–1.63 <0.0001
Radiotherapy 8,069 3,655 (45.3) 5,270 2,543 (48.3) 1.42 1.35–1.50 <0.0001
Targeted therapy 4,431 640 (14.4) 3,027 655 (21.6) 1.56 1.39–1.74 <0.0001
Hospital level
Medical center 16,684 7,142 (42.8) 7,940 3,549 (44.7) 1.21 1.16–1.26 <0.0001
Regional hospital 10,163 5,337 (52.5) 8,258 4,349 (52.7) 1.11 1.07–1.16 <0.0001
Local hospital/clinic 8,527 3,389 (39.7) 1,489 930 (62.5) 1.69 1.57–1.82 <0.0001
AHR, adjusted hazard ratio, adjusted for age, gender sex, comorbidities, lung cancer surgery, recent anticancer treatment, and hospital level. DM, diabetes mellitus; HTN, hypertension; CVA, cerebralvascular accident; COPD, chronic obstructive pulmonary disease; CKD, chronic kidney disease.
induced respiratory failure in a medical intensive care unit showed that history of radiotherapy (OR =2.80; 95% CI, 1.15–6.78) was associated with increased mortality. Soares et al. (9) found that number of organ failures (OR =1.96;
95% CI, 1.38–2.79) was associated with increased mortality.
Previous study (20) has showed that hospital volume can affect the outcome of cancer patients undergoing anti- cancer treatment. In this study, we found that hospital level was independently associated with mortality. Even sicker patients referred to medical centers had better survival
rates than did patients in regional and local hospitals. The finding is consistent with previous evidence (20) that larger hospital volume or specialty focus is associated with the better outcome of cancer patients.
Finally, we found that recent surgery, chemotherapy, and targeted therapy all lowered the mortality risk, which is consistent with other claims (6). The similar finding was noted in previous study (6) that anticancer therapy in the ICU might provide better short-term ICU survival for treatment-naïve, critically ill lung cancer patients. It
is possible that the patients who were chosen to receive anti-cancer therapy may have better performance status and could tolerate the treatment-related complication than the patients who physicians cannot recommend anti- cancer therapy such as chemotherapy or surgery due to their poorer performance status. Taking all these findings together suggest that, although anticancer treatment causes complications, it will positively affect survival for some patients. However, additional studies are required to determine which specific populations will benefit from which anticancer treatments.
This study has several strengths. We studied a large sample from Taiwan’s general population, did a long-term follow-up, and included the full range of hospital levels from local clinics to medical centers. These strengths should make our findings generalizable to most Taiwanese.
However, a retrospective insurance claims database analysis also has several inherent limitations. First, the NHIRD information is provided using ICD-9 coding, not actual medical records. However, this limitation might not affect our findings, because lung cancer in this study was Table 3 Infectious diseases and organ dysfunction in ICU+ patients
(n=17,687)a
Variable N (%)
Infectious disease 9,933 (56.16)
Respiratory tract infection 7,182 (40.61)
Septicemia 4,269 (24.14)
Genitourinary tract infection 1,115 (6.30) Skin/soft tissue and musculoskeletal infection 400 (2.26)
Mycobacterial infection 249 (1.41)
Intra-abdominal infection 232 (1.31)
Systematic fungal infection 179 (1.01)
Organ dysfunction 9,256 (52.33)
Respiratory 7,768 (43.92)
Cardiovascular 2,298 (12.99)
Renal 914 (5.17)
Neurologic 209 (1.18)
Hematologic 184 (1.04)
Metabolic 98 (0.55)
Sepsis 6,893 (38.97)
a, the median (IQR) ICU length of stay was 4 (2 to 11) days.
Table 4 The risk of 1-year mortality in ICU+ patients (n=17,687)
Variable No. Events, n (%) Univariate HR (95% CI) AHRa (95% CI) P
Age (years)
18–64 6,586 2,809 (42.7) 1.00 (Reference) 1.00 (Reference) –
65–74 4,586 2,123 (46.3) 1.13 (1.07–1.19) 1.03 (0.97–1.10) 0.2767
≥75 6,515 3,896 (59.8) 1.63 (1.56–1.71) 1.22 (1.16–1.29) <0.0001
Sex
Female 7,160 3,257 (45.5) 1.00 (Reference) 1.00 (Reference) –
Male 10,527 5,571 (52.9) 1.24 (1.19–1.30) 1.18 (1.13–1.23) <0.0001
Lung cancer surgery 3,264 446 (13.7) 0.17 (0.16–0.19) 0.19 (0.17–0.21) <0.0001
Recent anticancer treatment
Chemotherapy 8,053 3,581 (44.5) 0.80 (0.76–0.83) 0.84 (0.80–0.88) <0.0001
Radiotherapy 5,270 2,543 (48.3) 0.99 (0.95–1.04) 1.09 (1.04–1.15) 0.0004
Targeted therapy 3,027 655 (21.6) 0.32 (0.30–0.35) 0.32 (0.30–0.35) <0.0001
Infectious disease 9,933 5,636 (56.7) 1.63 (1.56–1.70) 1.23 (1.17–1.29) <0.0001
Organ dysfunction 9,256 5,479 (59.2) 1.91 (1.83–1.99) 1.57 (1.50–1.65) <0.0001
Hospital level
Medical center 7,940 3,549 (44.7) 1.00 (Reference) 1.00 (Reference) –
Regional hospital 8,258 4,349 (52.7) 1.27 (1.21–1.32) 1.11 (1.06–1.16) <0.0001
Local hospital/clinic 1,489 930 (62.5) 1.74 (1.62–1.88) 1.28 (1.18–1.37) <0.0001
aAHR, adjusted hazard ratio (adjusted for age, gender sex, comorbidities, lung cancer, surgery, recent anticancer treatment, MV, CRRT, ECMO, CPR, and hospital level). MV, mechanical ventilation; CRRT, continuous renal replacement therapy; ECMO, extracorporeal membrane oxygenation; CPR, cardiopulmonary resuscitation.
based on a catastrophic illness card that can be obtained only after the diagnosis is confirmed by a biopsy and histological testing. Second, we chose only first-episode ICU+ patients admitted more than once during the study period, but patients with lung cancer might be readmitted in short time-spans for anticancer treatment-related complications. Third, the criteria for ICU admission vary by hospital, especially for hospitals at different levels. This might increase heterogeneity into the study cohort. Finally, the NHIRD does not contain data about specific histologic types or stages of cancer, specific cause of death, Eastern Cooperative Oncology Group (ECOG) performance status, and we did not assess the effect of different anticancer treatments and the number of cancer-related mortality.
Conclusions
ICU admission for lung cancer patients is associated with higher mortality. Several risk factors of mortality after ICU admission identified in this study can help physicians provide personalized and better-informed decisions for managing lung cancer.
Acknowledgements None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The study was approved by the Institutional Review Board (IRB No: 10411-E01) of Chi Mei Medical Center. Because the data used in this study are de-identified, and have been released to the public for research, the IRB waived informed consent.
References
1. Sugimura H, Yang P. Long-term survivorship in lung cancer: a review. Chest 2006;129:1088-97.
2. Heuvers ME, Hegmans JP, Stricker BH, et al. Improving lung cancer survival; time to move on. BMC Pulm Med 2012;12:77.
3. Vachani A, Sequist LV, Spira A. AJRCCM: 100-Year Anniversary. The Shifting Landscape for Lung Cancer:
Past, Present, and Future. Am J Respir Crit Care Med
2017;195:1150-60.
4. Miller KD, Siegel RL, Lin CC, et al. Cancer treatment and survivorship statistics, 2016. CA Cancer J Clin 2016;66:271-89.
5. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016.
CA Cancer J Clin 2016;66:7-30.
6. Chen YF, Lin JW, Ho CC, et al. Outcomes of cancer therapy administered to treatment-naive lung cancer patients in the intensive care unit. J Cancer 2017;8:1995-2003.
7. Lin YC, Tsai YH, Huang CC, et al. Outcome of lung cancer patients with acute respiratory failure requiring mechanical ventilation. Respir Med 2004;98:43-51.
8. Reichner CA, Thompson JA, O'Brien S, et al. Outcome and code status of lung cancer patients admitted to the medical ICU. Chest 2006;130:719-23.
9. Soares M, Darmon M, Salluh JIF, et al. Prognosis of lung cancer patients with life-threatening complications. Chest 2007;131:840-6.
10. Roques S, Parrot A, Lavole A, et al. Six-month prognosis of patients with lung cancer admitted to the intensive care unit. Intensive Care Med 2009;35:2044-50.
11. Adam AK, Soubani AO. Outcome and prognostic factors of lung cancer patients admitted to the medical intensive care unit. Eur Respir J 2008;31:47-53.
12. Müller AM, Gazzana MB, Silva DR. Outcomes for patients with lung cancer admitted to intensive care units.
Rev Bras Ter Intensiva 2013;25:12-6.
13. Boussat S, El'rini T, Dubiez A, et al. Predictive factors of death in primary lung cancer patients on admission to the intensive care unit. Intensive Care Med 2000;26:1811-6.
14. Toffart AC, Minet C, Raynard B, et al. Use of intensive care in patients with nonresectable lung cancer. Chest 2011;139:101-8.
15. Kim YJ, Kim MJ, Cho YJ, et al. Who should be admitted to the intensive care unit? The outcome of intensive care unit admission in stage IIIB-IV lung cancer patients. Med Oncol 2014;31:847.
16. Wen CP, Tsai SP, Chung WS. A 10-year experience with universal health insurance in Taiwan: measuring changes in health and health disparity. Ann Intern Med 2008;148:258-67.
17. Rosolem MM, Rabello LS, Lisboa T, et al. Critically ill patients with cancer and sepsis: clinical course and prognostic factors. J Crit Care 2012;27:301-7.
18. Torres VB, Azevedo LC, Silva UV, et al. Sepsis-Associated Outcomes in Critically Ill Patients with Malignancies. Ann Am Thorac Soc 2015;12:1185-92.
19. Chang Y, Huh JW, Hong SB, et al. Outcomes and
prognostic factors of patients with lung cancer and pneumonia-induced respiratory failure in a medical intensive care unit: a single-center study. J Crit Care 2014;29:414-9.
20. Hillner BE, Smith TJ, Desch CE. Hospital and physician volume or specialization and outcomes in cancer
treatment: importance in quality of cancer care. J Clin Oncol 2000;18:2327-40.
Cite this article as: Lai CC, Ho CH, Chen CM, Chiang SR, Chao CM, Liu WL, Wang JJ, Yang CC, Cheng KC. Risk factors and mortality of adults with lung cancer admitted to the intensive care unit. J Thorac Dis 2018;10(7):4118-4126. doi:
10.21037/jtd.2018.06.165