嘉南藥理科技大學專題研究計畫成果報告
化妝品用之高分散型奈米碳黑之研發與評估
計畫類別:□個別型計畫 ■整合型計畫 計畫編號:CNCS9507
執行期間:95 年 1 月 1 日至 95 年 12 月 31 日
計畫主持人:李佳芬 教授
執行單位:化粧品應用與管理系
中華民國 96 年 3 月 8 日
Abstract
The purpose of this study was to modify the surface characteristics of CB so as to prevent the aggregation of CB to provide the dispersibilities in either H2O or organic solvent. In this study, five kinds of hydrophilic TEMPO-terminated polymer, hydrophobic TEMPO-terminated polymer and amphiphilic TEMPO-terminated block copolymer were synthesized. The five kinds of TEMPO-terminated polymers were: (1) poly(4-acetoxystyrene) (PAS-T) (2) poly(4-hydroxystyrene) (PHS-T) (3) polystyrene (PS-T) (4) poly(4-acetoxystyrene)-block-polystyrene (PAS-b-PS-T) (5) poly(4-hydroxystyrene)-block-polystyrene (PHS-b-PS-T). These TEMPO-terminated polymers with desired molecular weights and specific structures were synthesized by using the method of living radical polymerization in the presence of 2,2,6,6-tetramethylpiperidinyl-1-oxy (TEMPO). These TEMPO-terminated polymers and TEMPO-terminated block copolymers were grafted onto the surface of CB through a reaction of polymer radicals trapped by CB, so as to obtain the TEMPO-terminated polymer / CB and TEMPO-terminated block copolymer / CB composite nanoparticles. Various variables such as reaction time, reaction temperature, amount of TEMPO-terminated polymer, molecular weight of TEMPO-terminated polymer and amount of CB all of which influenced the grafting efficiency were investigated. Besides, the stability of the composite nanoparticles, which dispersed in H2O or organic solvent, was investigated by laser light scattering.
The amphiphilic composite nanoparticles, PHS-T/CB and PHS-b-PS-T/CB, which dispersed well in both H2O and organic solvent, were synthesized successfully in this work.
Introduction
Recently, surface-initiated grafted polymerization has been studied but it usually results in a poor control of chain-length and chains structure [1]. The techniques of living radical polymerization has proven very promising for the synthesis of low polydispersity linear polymers, block copolymers and star polymers [2-6]. Living polymerization techniques were successfully applied to surface-initiated graft polymerization to form the inorganic/organic composite material. Eizo Marutani et al. [7]
reported the synthesis of magnetite nanoparticles coated with a well-defined graft polymer by using the method of living radical polymerization. In addition, G. Larnelle et al.[8] synthesized the inorganic/organic composite material consisting of poly(n-butyl acrylate)-b-poly(styrene) (PBA-b-PS) diblock copolymer anchored to silica particles via “grafting from” technique using a living free radical polymerization. They pointed out that the morphology of the composite material was core/double shell structure. The materials obtained have different thermal behavior function of the ratio PBA/PS.
Besides, in order to enhance the application of carbon black (CB) in industry, various kinds of polymers were grafted to the surfaces of CB. P.K.
In this study, TEMPO-terminated polymers, PAS-T, PS-T and TEMPO-terminated block copolymer, PAS-b-PS-T, were synthesized by using the method of living radical polymerization. Then these TEMPO-terminated polymers were grafted onto the surface of CB by a reaction of polymer radicals trapped by CB to form the PAS-T/CB, PS-T/CB and PAS-b-PS-T/CB composite nanoparticles.
Various variables, which influenced the grafting efficiency, were investigated. In addition, NH4OH
was used to proceed the hydrolysis reaction of PAS-T/CB and PAS-b-PS-T/CB to form the PHS-T/CB and PHS-b-PS-T/CB respectively. The stability of the composite nanoparticles, which dispersed in H2O or organic solvents, was investigated.
Experiment Materials
Styrene was distilled under a nitrogen atmosphere and reduced pressure prior to polymerization. Water was redistilled and deionized. The 2,2,6,6-tetramethylpiperidinyl-1-oxy (TEMPO), initiator (benzoyl peroxide (BPO)), 4-acetoxystyrene (AS), styrene (St), and other chemicals were of analytical grade and used without further purification. The carbon black (CB) used was Monarch 900 (CABOT Co., Ltd.). The average diameter of CB was about 15 nm.
Synthesis of PAS-T and PS-T TEMPO-terminated polymers and PAS-b-PSt-T TEMPO-terminated block copolymers
TEMPO (0.018 M), BPO (0.015 M) and AS (5 ml) or (styrene (5 ml)) were charged into a reactor, and then the reaction mixture was pre-heated for 3.5 hours at the temperature of 95℃.
Afterwards, the temperature was raised to 125℃ to synthesize the TEMPO-terminated polymer PAS-T (or PS-T). After the polymerization reaction, PAS-T (or PS-T) was obtained as a precipitate from a large excess of methanol, purified by centrifuge with methanol in order to remove the residual AS (or styrene) monomer. Afterwards, PAS-T (30µ mole) was used as the macro initiator to polymerize styrene (2 ml) at the temperature of 125℃ to form the TEMPO-terminated PAS-b-PSt-T copolymer.
Synthesis of TEMPO-terminated polymers/CB and TEMPO-terminated block copolymer/CB composite particles
TEMPO-terminated polymer (PAS-T or PS-T) (30μmole) or TEMPO-terminated block copolymer (PAS-b-PS-T) (30μmole), CB (0.05 g), and N,N-dimethylformamide (DMF) (20 ml) were charged into a reactor. Then the reaction mixture was heated under stirring at 125℃, and reacted for 12 hours. The TEMPO-terminated polymers or TEMPO-terminated block copolymer were grafted onto the CB surface through the trapping of polymer radicals by carbon black surface to form the TEMPO-terminated polymer/CB composite particles (PAS-T/CB or PS-T/CB) or TEMPO-terminated block copolymer/CB composite particles (PAS-b-PS-T/CB). After the reaction, the reaction mixture was poured into a large excess of methanol to precipitate the TEMPO-terminated polymer/CB composite particle or TEMPO-terminated block copolymer/CB composite particles. In order to remove the ungrafted TEMPO-terminated polymer, the TEMPO-terminated polymer/CB composite particles were dispersed in THF and the dispersive solution was centrifuged. This procedure was repeated until no more TEMPO-terminated polymer could be detected in the supernatant solution.
Besides, NH4OH (10 ml) was used to proceed the hydrolysis reaction of PAS-T/CB and PAS-b-PS-T/CB composite particles for 24 hours to form the PHS-T/CB and PHS-b-PS-T/CB
composite particles respectively at the temperature of 25℃. These composite particles were purified by centrifuge with H2O to remove the residual NH4OH, and then the composite particles were dried under the vacuum condition at the temperature of 50℃.
Results and discussion Synthesis of TEMPO-terminated polymer/CB composite particles
In order to modify the surface characteristics of CB, the PAS-T/CB, PHS-T/CB and PS-T/CB composite particles were synthesized. The effect of molecular weight of PAS on the percentage of PHS-T grafted onto the surface of CB was shown in Table 3. The increase of molecular weight of PAS-T increased the weight percentage of grafting but decreased the mole number of PHS-T chains (Gn) grafted onto CB. The PAS-T with a larger molecular weight was more difficult to graft onto the surface of CB due to the steric hindrance. Hence, although the grafted number of polymer chains decreased, the weight percentage of grafting may still be higher because the grafted polymer chains had a larger molecular weight.
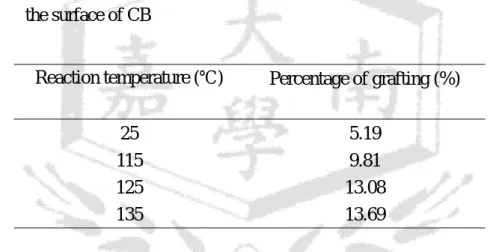
In addition, PS-T polymer was synthesized and then grafted onto the surface of CB. Table 4 showed the percentage of PS-T grafting onto CB at different reaction times. The percentage of grafting increased with increasing the reaction time and gradually leveled off after 18 h. The effect of reaction temperature on the grafting reaction of PS-T with CB was shown in Table 5. It was found that the percentage of PS-T grafting onto the surface of CB increased with the increase of temperature, due to the fact that the mediated TEMPO was easier to escape away from the chain ends of the polymers at higher temperatures, which increased the opportunity of polymer radicals to react with CB, and increased the percentage of PS-T grafted onto the surface of CB. In addition, the increase of the amount of PS-T increased the opportunity of PS-T to react with CB, so as to increase the percentage of PS-T grafted onto the surface of CB as shown in Table 6.
Synthesis of TEMPO-terminated block copolymer/CB composite particles
PAS-b-PSt-T TEMPO-terminated block polymer was synthesized and then grafted onto the surface of carbon black. The molecular weight of PAS-b-PSt-T copolymer influenced the weight percentage of grafting significantly. Table 7 showed the relationship between the molecular weight of PAS-b-PSt-T copolymer and the weight percentage of PAS-b-PSt-T copolymer grafted onto the surface of carbon black. The results showed that the PAS-b-PSt-T with a moderate molecular weight of 12680 had the highest grafting percentage compared to that of smaller or larger molecular weights, 7729 or 17330. Two factors, polymer chain length and steric hindrance, competed to influence the weight percentage of grafting.
Dispersibility of TEMPO-terminated polymer/CB composite particles
The dispersibility of CB was remarkably improved by the grafting of polymers onto the surfaces. The PAS-T/CB composite particles colloidal dispersion in THF was stable as shown in Fig.7.
Also, PHS-T/CB and PS-T/CB composite particles were able to disperse in THF (not shown). Besides, the stability of PHS-T/CB, PAS-T/CB, and PS-T/CB colloidal dispersion in H2O was investigated. It was found that both PS-T/CB and PAS-T/CB composite particles were unable to disperse in H2O, but PHS-T/CB particles were well dispersed in H2O due to the hydrophilic nature of PHS-T. Fig.8 showed the TEM photograph of PHS-T/CB composite particles, which had a uniform particle size and the diameter was about 25 nm. Fig.9 showed the particle sizes of PHS-T/CB composite particles dispersed in H2O, measured by laser light scattering. During the time of measurement, the particle sizes did not change much. Also, the PHS-T/CB colloidal dispersion in H2O was observed as Fig.10.
Both Fig.9 and 10 indicated that the dispersion of the PHS-T/CB particles in water was quite stable.
That is, PHS-T/CB composite particles had amphiphilic properties, which enabled them to disperse well in either organic solvent or H2O.
Dispersibility of TEMPO-terminated block copolymer/CB composite particles
After the grafting of PAS-b-PSt-T TEMPO-terminated copolymers onto the surfaces of CB, the composite particles were forced to disperse in H2O ready for the TEM observation. The TEM photograph of PAS-b-PSt-T/CB composite particles was shown as Fig.11. It showed that the carbon black particles were encapsulated completely by PAS-b-PSt-T copolymer and the size was about 100 nm ~ 250 nm. Through similar dispersibility experiments as above, the PAS-b-PSt-T/CB composite particles were found to be able to disperse well in THF, but unable to disperse well in H2O, due to the hydrophobic property of PAS-b-PSt-T copolymer.
In addition, the PAS-b-PSt-T/CB composite particles were hydrolyzed to form the amphiphilic block copolymer PHS-b-PSt-T/CB composite particles. Fig.12 showed the TEM photograph of the PHS-b-PSt-T/CB composite particles. The particle size was uniformly about 25 nm. Under the detection of laser light scattering, the size of PHS-b-PSt-T/CB composite particles dispersed in H2O was about 120 nm, invariant with time as seen in Fig.13. It indicated that the PHS-b-PSt-T/CB composite particles were able to disperse well in H2O. The sizes of the composite particles measured by laser light scattering were larger than that observed by TEM. Because in the measurement of laser light scattering, the composite particles were in a dispersive state in solution, in which the hydrophilic (or hydrophobic) segments of PHS-b-PSt-T fully extended in H2O (or THF) so that the particle size was significantly enlarged. Similar experiments were done for PHS-b-PSt-T/CB composite particles, which were dispersed in THF, and stability of the dispersive solution evidenced that the PHS-b-PSt-T/CB composite particles were also able to disperse well in THF.
Conclusion
Carbon black is wieldy used in industry while the capability of the carbon black is restricted by the disadvantage of difficult to disperse well in H2O or organic solvent. In this study, the TEMPO-terminated polymer and TEMPO-terminated block copolymer were grafted onto the carbon black to modify the characteristics of the surface of carbon black successfully. Various variables such as molecular weight of TEMPO-terminated polymer, reaction time and reaction temperature, influenced the percentage of grafting significantly. PS-T/CB, PAS-T/CB and PAS-b-PS-T/CB
hydrophobic composite particles dispersed well in THF. Amphiphilic PHS-T/CB and PHS-b-PS-T/CB composite particles dispersed well in both THF and H2O.
Reference
1. Boven G., Oosterling MLCM., Challa G., Schouten AJ., Polymer, 1990; 45: 2231 2. Haruyuki O., Yusuke T., Masahiro T., Masamitsu S., Polymer, 2002; 43: 3155 3. Gabaston LI., Furlong SA., Jackson RA., Arms SP., Polymer, 1999; 40: 4505
4. Astsushi N., Takeshi M., Harumi K., Toshifumi S., Toyoji K., Polymer, 2002; 43: 4835 5. Shim SE., Oh S., Chang YH., Jin MJ., Choe S., Polymer, 2004; 45: 4731
6. Abrol S., Caulfield MJ., Qiao GG., Solomon DH., Polymer, 2001; 42: 5987
7. Marutani E., Yamamoto S., Ninjbadgar T., Tsujii Y., Fukuda T., Takano M., Polymer, 2004; 45:
2231
8. Laruelle G., Parvole J., Francois J., Billon L., Polymer, 2004; 45: 5013
Table 1 Relationship between the molecular weight of PAS and the efficiency of PHS-T grafted onto the surface of CB
Reaction time (hour)
Molecular weight of PAS
Weight percentage of grafting (%)
Mole number of grafting (Gn)
1.5 323 5.50 170.34
2.0 8485 13.70 19.96
3.0 24630 19.52 7.92
(reaction temperature: 125℃, mole of PAS-T: 30μmole, volume of DMF: 20 ml, weight of CB: 0.05 g , grafting time: 12 hrs)
Table 2 Relationship between reaction time of grafting and weight percentage of PS-T grafted onto the surface of CB
Reaction time (hour) Percentage of grafting (%)
3 10.87 6 11.91 12 13.08 18 14.25 24 14.47 (reaction temperature: 125℃, weight of PS-T: 0.19 g, weight of CB: 0.05 g, volume of DMF: 20 ml, molecular weight of PS-T: 5610)
Table 3 Relationship between reaction temperature and weight percentage of PS-T grafted onto the surface of CB
Reaction temperature (℃)
Percentage of grafting (%)
25 5.19 115 9.81 125 13.08 135 13.69 (reaction time: 12 hours, weight of PS-T: 0.19 g, weight of CB: 0.05 g, volume of DMF: 20 ml, molecular weight of PS-T: 5610)
Table 4 Relationship between the weight of PS-T and weight percentage of PS-T grafted onto the surface of CB
Weight of PS-T (g)
Percentage of grafting (%)
0.10 11.90 0.19 13.08 0.30 15.80
(reaction time of grafting: 12 hours, reaction temperature: 125℃, weight of CB: 0.05 g, volume of DMF: 20 ml, molecular weight of PS-T: 5610)
Table 5 The weight percentage of PAS-b-PSt-T copolymer grafted onto the surface of CB
Molecular weight of PAS-b-PSt-T
Weight percentage of PAS-b-PSt-T grafted onto the surface of CB
7729 6.71 %
12680 32.38 %
17330 12.04 %
Fig.1 PAS-T/CB composite particles colloidal dispersion in THF
Fig.2 TEM photograph of PHS-T/CB composite particles
Fig.3 The particle sizes of PHS-T/CB composite particles dispersed in H2O,measured by laser light scattering
Fig.4 PHS-T/CB colloidal dispersion in H2O
Fig.5 The TEM photograph of PAS-b-PSt-T/CB composite particles
Fig.6 The TEM photograph of the PHS-b-PSt-T/CB composite particles
Fig.7 The sizes of PHS-b-PSt-T/CB composite particles which dispersed in H2O, measured by laser light scattering