行政院國家科學委員會專題研究計畫 期中進度報告
山藥多醣生理活性探討(1/2)
計畫類別: 個別型計畫
計畫編號: NSC94-2313-B-038-004-
執行期間: 94 年 08 月 01 日至 95 年 07 月 31 日 執行單位: 臺北醫學大學生藥學研究所
計畫主持人: 侯文琪
報告類型: 精簡報告
處理方式: 本計畫可公開查詢
中 華 民 國 95 年 4 月 13 日
行政院國家科學委員會專題研究計畫成果報告
計畫編號:NSC 94-2313-B-038-004
執行期限:94 年 08 月 01 日至 95 年 07 月 31 日
主持人:侯文琪 執行機構名稱:台北醫學大學生藥所
一、中文摘要
由臺灣產台農一號山藥、台農二號山藥與名間長紅山藥塊莖抽取其粗黏質多醣與經部 分純化後之黏質多醣進行抗氧化活性分析比較。利用分光光度計方法分析 DPPH 自由 基,氫氧自由基與超氧自由基之清除實驗;也利用電子自旋共振儀分析氫氧自由基之 清除實驗。以 50%清除濃度 (IC50) 顯示,台農一號山藥、台農二號山藥與名間長紅山 藥多醣在清除DPPH 自由基而言,純化前後分別為 0.329、0.279; 0.547、0.653 和 0.847、0.631 mg/ml。在清除氫氧自由基而言,純化前後分別為 0.668、1.146; 1.461、
1.096 和 0.946、1.554 mg/ml。在清除超氧自由基而言,純化前後分別為 0.802、0.368;
0.681、0.258; 和 0.086、0.148 mg/ml。利用電子自旋共振儀分析氫氧自由基清除實驗,
純化之台農一號山藥、台農二號山藥與名間長紅山藥多醣之 50%清除濃度為 0.083、
0.47、0.004 mg/ml。以上結果顯示栽培種之間與純化前後之多醣有不同抗氧化活性。
關鍵詞:DPPH 自由基; 電子自旋共振儀; 氫氧自由基; 黏質多醣;超氧自由基;山藥
Abstract
Crude mucilages (CM) and partially purified mucilages (PPM) from three different Taiwanese yam cultivars, including Dioscorea alata L. cv.Tainong 1 (TN1), Dioscorea alata L. cv.Tainong 2 (TN2), and D. alata L. var. purpurea (Roxb.) Ming-Jen (MJ), were used for evaluating the antioxidant effects, including 1,1-diphenyl-2-picrylhydrazyl (DPPH) radical, hydroxyl radical, superoxide radical scavenging activities, and by electron spin resonance (ESR) spectrometry for hydroxyl radical scavenging activities. The IC50 stands for the concentration required for 50 % scavenging activity. The IC50 of CM and PPM against DPPH radical were 0.329, 0.279; 0.547, 0.653; and 0.847, 0.631 mg/ml, respectively, for TN1, TN2 and MJ. The IC50 of CM and PPM against hydroxyl radical by spectrophotometry were 0.668, 1.146; 1.461, 1.096; and 0.946, 1.554 mg/ml, respectively, for TN1, TN2 and MJ. The IC50 of CM and PPM against superoxide radical were 0.802, 0.368; 0.681, 0.258; and 0.086, 0.148 mg/ml, respectively, for TN1, TN2 and MJ. Using ESR to detect hydroxyl radicals, the IC50 of PPM against hydroxyl radical were 0.083, 0.47, and 0.004 mg/ml, respectively, for TN1, TN2 and MJ. The results demonstrated that different cultivars of yams exhibited different antioxidant ability, and the purification process could partially increase the antioxidant activity of the mucilage polysaccharide. Taken together, these results suggest that mucilage polysaccharides of the yam tuber might play an important role on antiradicals and
antioxidants.
Keywords: 1,1-diphenyl-2-picrylhydrazyl (DPPH) radical; electron spin resonance (ESR);
hydroxyl radical; mucilage; superoxide radical; yam 二、緣由與目的
Active (or reactive) oxygen species and free radical-mediated reactions have involved in degenerative or pathological processes such as aging (Harman, 1995), cancer, coronary heart disease and Alzheimer′s disease (Ames, 1983; Smith et al., 1996; Diaz et al., 1997). Meanwhile there are many epidemiological results revealing an association between a diet rich in fresh fruit and vegetable and a decrease in the risk of cardiovascular diseases and certain forms of cancer (Salah et al. 1995) in humans. Several reports were concerned natural compounds in fruit and vegetable for their antioxidant activities, such as phenolic compounds (Rice-Evans et al., 1997), anthocyanin (Espin et al., 2000), echinacoside in Echinaceae root (Hu and Kitts, 2000), methanolic and hot-water extracts of Liriope spicata L. (Hou et al., 2004), water and ethanolic extracts of different sweet potato organs (Huang et al., 2004), the storage proteins of sweet potato root (Hou et al., 2001a), yam tuber (Hou et al., 2001b) and potato tuber (Liu et al., 2003). In cells, there are normally metabolic pathways to degrade free radicals. If the generation rates of free radicals are faster than those of degradation under environmental stresses, cells suffer oxidative stresses. Two distinct pathways, nonenzymatic or enzymatic, were found in plant cells as routes of free radical scavengers. The former included ascorbate (Njus and Kelley, 1993) or chlorogenic acids (Kono et al., 1998) or vitamin E (Halliwell, 1999); the latter included different forms of superoxide dismutase to metabolize superoxide free radical to hydrogen peroxide (Bowler et al., 1992; Lin et al., 1993, Hou et al., 2003).
The hydrogen peroxide produced was further metabolized either by catalase or different forms of peroxidase such as glutathione peroxidase (EC 1.11.1.9).
Yam (Dioscorea species) is a member of the monocotyledonous family Dioscoreaceae and is a staple food in West Africa, Southeast Asia and Caribbean (Akoruda, 1984). Yam was recognized as herbal plants since the tuber dried slices were freqently used as Chinese herbal medicines. The tuber storage proteins of yam, dioscorin, exhibited carbonic anhydrase, trypsin inhibitor activities (Hou et al., 1999a) and both of dehydroascorbate reductase and monodehydroascorbate reductase activities (Hou et al., 1999b). Yam tuber contains mucilages which were reported to be a mannan-protein complex (Misaki et al., 1972; Tsai and Tsai, 1984). Recently, we reported that yam tuber mucilage exhibited angiotensin converting enzyme inhibitory activities (Lee et al., 2003).
In this work we reported that crude mucilages (CM) and partially purified mucilages (PPM) from three different Taiwanese yam cultivars, including Dioscorea alata L. cv.Tainong 1 (TN1), Dioscorea alata L. cv.Tainong 2 (TN2), and D. alata L. var. purpurea (Roxb.) Ming-Jen (MJ), were used for evaluating the antioxidant effects of scavenging DPPH radical, hydroxyl radical, superoxide radical by spectrophotometry, and hydroxyl radical scavenging activity assay by electron spin resonance (ESR) spectrometry.
Materials and Methods Materials
1,1-diphenyl-2-picrylhydrazyl (DPPH), NADH, 5,5-dimethyl-1- pyrroline-N-oxide (DMPO), ferrous sulfate and phenazine methosulfate (PMS) were purchased from Sigma Chemical Co. (St. Louis, MO, USA).
Hydrogen peroxide (33%) was from Wako Pure Chemical Industry (Osaka, Japan). Other chemicals and reagents were from Sigma Chemical Co. (St. Louis, MO, USA).
Extraction and Purificaction of Mucilage from Yam Tuber
Fresh yam tubers of TN1, TN2, and MJ were purchased from a wholesaler. After washing and peeling, the tubers were cut into strips for mucilage extractions and purifications according to the methods of Lee et al.
(2003) with some modifications. Yam tuber was homogenized with four volumes (W/V) of 50 mM Tris-HCl buffer (pH 8.3) containing 1% vitamin C. After centrifugation at 14,000 ×g for 30 min, the supernatants were
mixed with isopropanol to a final concentration of 70%, and stirred quickly at 4 ºC overnight. The precipitates were filtrated and dehydrated with 100% isopropanol, then, rinsed with acetone. After drying at 40 ºC oven, the crude mucilage (CM) was grinded and collected for further purifications by both SDS and heating procedures.
About 1.0 g CM powder was dissloved in 200 ml distilled water and kept in a 50 ºC water bath. Forty ml of 5
% SDS solution (dissloved in 45 % ethanol) was added to the CM solution. The mixture was kept with gentle stirring at 50 ºC for 30 min, then, at room temperature for another 2 hours. After that, the mucilage solution was placed in an ice bath to quickly lower down the temperature in order to precipitate the SDS-protein complex. After centrifugation at 14,000 ×g at 0 ºC for 30 min, the supernatants were precipitated with isopropanol and dried at 40 ºC oven as described earlier. The mucilage was again grinded, dissolved, and then heated at boiling water for 20 min. After centrifugation at 14,000 ×g at 0 ºC for 30 min, the supernatants were mixed with isopropanol to a final concentration of 70%. The partially purified mucilage (PPM) was filtrated, dehydrated, rinsed with acetone, dried, and then collected for further uses.
Scavenging activitiy against 1,1-dipheny-2-picrylhydrazyl (DPPH) radical analyzed by spectrophotometry The scavenging activity of CM and PPM from TN1, TN2, and MJ cultivars against DPPH radical was measured according to the method of Hou et al. (2001a, b). Each 0.3 ml of CM (0.25, 0.5, 1.0 and 1.5 mg/ml) and PPM (0.1, 0.15, 0.3, 0.5 and 1.0 mg/ml) solution was added to 0.1 ml of 1 M Tris-HCl buffer (pH 7.9), and then mixed with 0.6 ml of 100 μM DPPH in methanol for 20 min under light protection at room temperature.
After brief centrifugation at 12,000 ×g for 10 min, the absorbance at 517 nm was measured. Deionized water was used as a blank. The scavenging activity of DPPH radicals (%) was calculated following the equation : (A517blank − A517sample) ÷ A517blank × 100 %. The IC50 stands for the concentration required for 50 % scavenging activity and was calculated from the above equation.
Scavenging activity of CM and PPM against metal ion-dependent hydroxyl radicals
The hydroxyl radical was determined by the deoxyribose method (Halliwell et al., 1987). Every 0.5 ml sample containing different amounts of CM (0.375 , 0.75, and 1.5 mg/ml) and PPM (0.4, 0.8, and 1.6 mg/ml) from TN1, TN2, and MJ cultivars were added to 1.0 ml solution of 20 mM potassium phosphate buffer (pH 7.4), 2.8 mM 2-deoxy-ribose, 104 μM EDTA, 100 μM FeCl3, 100 μM ascorbate and 1mM hydrogen peroxide. The mixtures were incubated for 1 h at 37 ºC. After incubation, equal volume of 0.5% thiobarbituric acid in 10%
trichloroacetic acid was added and the mixtures were boiled at 100 ºC for 15 min. Deionized water was used as a blank experiment. The absorbance at 532 nm was measured. The scavenging activity of hydroxyl radicals (%) was calculated with the equation: (A532blank − A532sample) ÷ A532blank × 100 %. The IC50 stands for the concentration required for 50 % scavenging activity and was calculated from the above equation.
Scavenging Activity against Superoxide Radicals analyzed by Spectrophotometry
The superoxide radical was generated by the PMS-NADH system (Lai et al., 2001). Every 0.2 ml sample containing different amounts of CM (0.125, 0.25, 0.5, and 1.0 mg/ml) and PPM (0.125, 0.5, 1.0 mg/ml) solution from TN1, TN2, and MJ cultivars was added in sequence to 0.2 ml of 630 µM nitroblue tetrazolium, 0.2 ml of 33 µM PMS and 0.2 ml of 156 µM NADH in 100 mM phosphate buffer (pH 7.4). Means of triplicates were measured. Deionized water was used as a blank experiment. The changes of absorbance at 560
nm were recorded during 1 min and expressed as △A560nm/min. The scavenging activity against superoxide radicals was calculated as following: (△A560nm/minblank − △A560nm/minsample) ÷ △A560nm/minblank
× 100 %. The IC50 stands for the concentration of 50% scavenging activity.
Scavenging activities against hydroxyl radical by electron spin resonance spectrometry
The hydroxyl radical was generated by Fenton reaction according to the method of Kohno et al. (1991). The total 500-μl mixture contained different concentrations of PPM solution of TN1 (0.031, 0.062, 0.1, and 0.2 mg/ml), TN2 (0.12, 0.25, 0.5, and 1 mg/ml), and MJ (0.0004, 0.004, and 0.007 mg/ml) cultivars, 5 mM 5,5-dimethyl-1- pyrroline-N-oxide (DMPO), and 0.05 mM ferrous sulfate. After mixing, the solution was transferred to an ESR quartz cell and placed at the cavity of the ESR spectrometer, and then hydrogen peroxide was added to a final concentration of 0.25 mM. Deionized water was used instead of sample solution for blank experiments. After 40 s, the relative intensity of the signal of the DMPO-OH spin adducts was measured. All ESR spectra were recorded at the ambient temperature (298 K) on a Bruker EMX-6/1 EPR spectrometer equipped with WIN-EPR SimFonia software version 1.2. The conditions of ESR spectrometry were as follows:
center field, 345.4 (5.0 mT; microwave power, 8 mW (9.416 GHz); modulation amplitude, 5 G; modulation frequency, 100 kHz; time constant, 0.6 s; scan time,1.5 min.
Results and Discussion
Yam (Dioscorea species) is a member of the monocotyledonous family Dioscoreaceae and is a staple food in West Africa, Southeast Asia and Caribbean (Akoruda, 1984). Yam was recognized as herbal plants since the tuber dried slices were freqently used as Chinese herbal medicines. In this study, three native cultivars of Taiwanese yam were used for mucilage isolation and purification, and then for antioxidant activity assay.
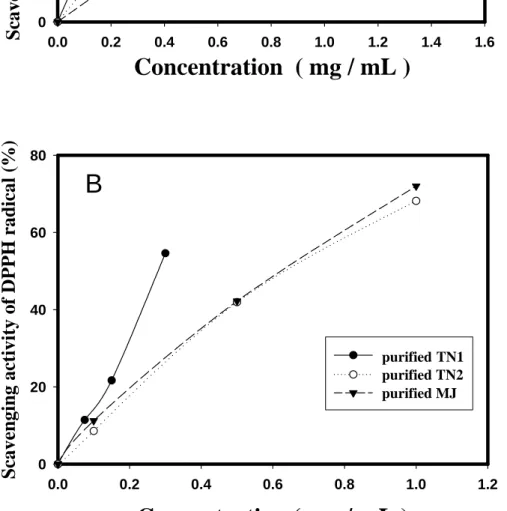
DPPH radicals were widely used in the model system to investigate the scavenging activities of several natural compounds. When DPPH radical was scavenged, the color of the reaction mixture changes from purple to yellow with decreasing of absorbance at wavelength 517 nm. Figure 1A shows the scavenging activity against DPPH radicals of CM from TN1, TN2 ,and MJ yam cultivars. It was found the dose-dependent DPPH radical scavenging activities of CM from three native yam cultivars. The order of DPPH scavenging activity was TN1 >
TN2 > MJ. The IC50 of CM against DPPH radical were 0.329, 0.547, and 0.847 mg/ml, respectively, for TN1, TN2 and MJ cultivars. After being purified with SDS and heated at boiling water, the PPM of three yam cultivars was again assayed for antioxidant activity. Figure 1B shows the scavenging activity against DPPH radicals of PPM from TN1, TN2 ,and MJ cultivars. It was found that the DPPH radical scavenging activities of PPM was better than those of CM from three native yam. The IC50 of PPM against DPPH radical were 0.279, 0.653, and 0.631 mg/ml, respectively, for TN1, TN2 and MJ cultivars. Our previous study reported (Hou et al., 2002) that PPM from Japanese yam also exhibited DPPH scavenging activity, and the IC50 of PPM against DPPH radical was 0.86 mg/ml which was similar to the report of Lai et al. (2001) for Hsian-tsao leaf gum and higher than those of PPM from TN1, TN2 ,and MJ cultivars.
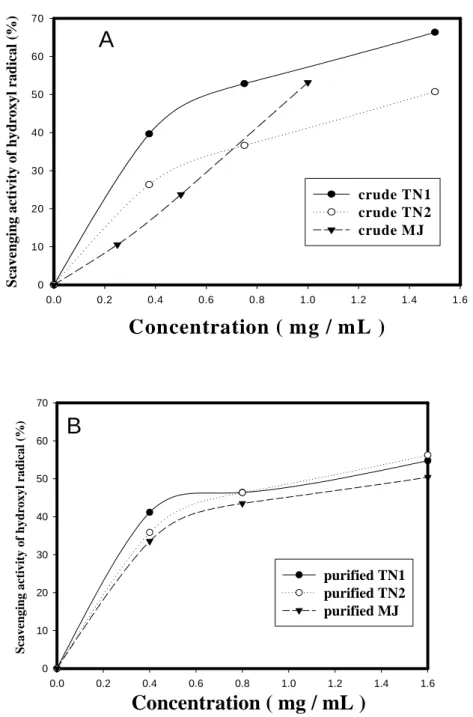
Figure 2 showed the scavenging activity against hydroxyl radical from CM (A) and PPM (B) of TN1, TN2, and MJ yam cultivars. Similar to the results of Figure 1, it was found the dose-dependent hydroxyl radical scavenging activities of CM from three native yam cultivars. The order of hydroxyl radical scavenging activity was TN1 > MJ > TN2 (Figure 2A). The IC50 of CM against hydroxyl radical were 0.668, 1.461, and 0.946 mg/ml, respectively, for TN1, TN2 and MJ cultivars. Figure 2B shows the scavenging activity against hydroxyl radicals of PPM from TN1, TN2 ,and MJ cultivars. The lower hydroxyl radical scavenging activities of PPM was found than those CM from three native yam. The IC50 of PPM against hydroxyl radical were 1.146, 1.096, and 1.554 mg/ml, respectively, for TN1, TN2 and MJ cultivars. Previuosly, we reported that the tuber storage protein, dioscorin, exhibited hydroxyl radical scavenging activity (Hou et al., 2001b). The dioscorin should be removed during SDS and heating treatments and resulted in lesser hydroxyl radical scavenging activity of PPM in three native yam cultivars.
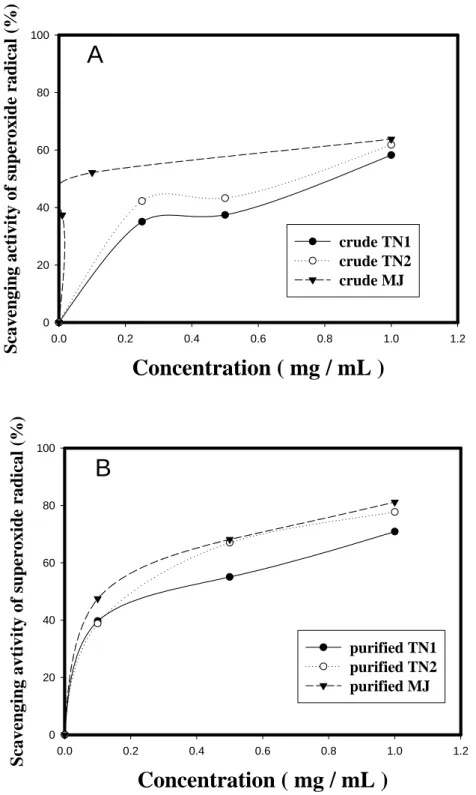
Figure 3 showed the scavenging activity against superoxide radical from CM (A) and PPM (B) of TN1, TN2, and MJ yam cultivars. Similar to the results of Figures 1 and 2, it was found the dose-dependent superoxide radical scavenging activities of CM from three native yam cultivars. The order of superoxide radical scavenging activity was MJ > TN2 > TN1 (Figure 3A). The IC50 of CM against superoxide radical were 0.802, 0.681, and 0.086 mg/ml, respectively, for TN1, TN2 and MJ cultivars.
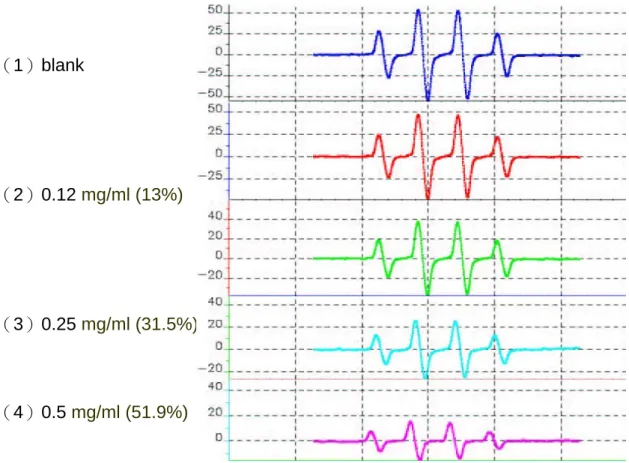
The hydroxyl radical was generated by Fenton reaction and was trapped by DMPO to form DMPO-OH adduct. The intensities of DMPO-OH spin signal in ESR spectrometry were used to evaluate the scavenging activity of PPM of TN1, TN2, and MJ yam cultivars against hydroxyl radical. Figure 4 shows scavenging activities against hydroxyl radicals of (A) TN1 (0.031, 0.062, 0.1, and 0.2 mg/ml), (B) TN2 (0.12, 0.25, 0.5, and 1 mg/ml), and (C) MJ (0.0004, 0.004, and 0.007 mg/ml) cultivars. From the results of Figure 4A, the scavenging activities against hydroxyl radical were 1, 26, 69.6, and 81% at 0.031, 0.062, 0.1, and 0.2 mg/ml, respectively, of PPM from TN1 cultivars. From the results of Figure 4B, the scavenging activities against hydroxyl radical were 13, 31.5, 51.9, and 72.2% at 0.12, 0.25, 0.5, and 1 mg/ml, respectively, of PPM from TN2 cultivars. From the results of Figure 4C, the scavenging activities against hydroxyl radical were 5.5, 50, and 51.8% at 0.0004, 0.004, and 0.007 mg/ml, respectively, of PPM from MJ cultivars. Using ESR to detect hydroxyl radicals, the IC50 of PPM against hydroxyl radical were 0.083, 0.47, and 0.004 mg/ml, respectively, for TN1, TN2 and MJ yam cultivars.
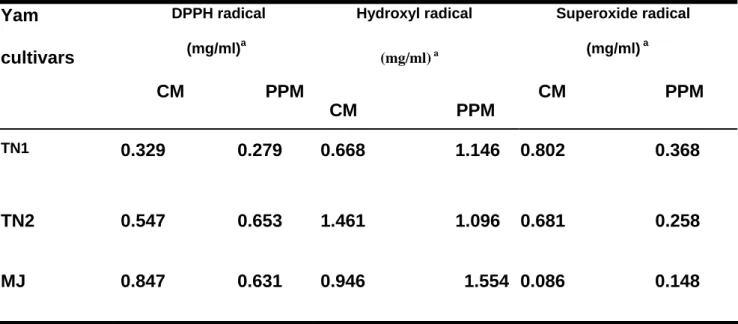
In conclusion, mucilages from three Taiwanese yams exhibited different antioxidant activities against DPPH radicals (Figure 1), hydroxyl radicals (Figure 2, Figure 4), and superoxide radicals (Figure 3) and the purification process could partially increase the antioxidant activity of the mucilage polysaccharide. Table 1 shows the comparison of the antioxidant activity (IC50) of mucilages from TN1, TN2, and MJ yam cultivars before (crude mucilage, CM) and after purification (partially purified mucilage, PPM) against DPPH, hydroxyl and superoxide radicals. Taken together, these results suggest that mucilage polysaccharides of the yam tuber might play an important role on antiradicals and antioxidants.
Acknowledgments. The authors want to thank the financial support (SKH-TMU-94-03) from 新光醫院。
Literature Cited
Ames, B. N. 1983. Dietary carcinogens and anticarcinogens: oxygen radicals and degenerative diseases. Science 221: 1256-1264.
Beauchamp, C. O. and I. Fridovich. 1971. Superoxide dismutase: improved assays and an assay applicable to acrylamide gels. Anal.
Biochem. 44: 276-287.
Bowler, C., M. V. Montagu, and D. Inze. 1992. Superoxide dismutase and stress tolerance. Ann. Rev. Plant Physiol. Plant Mol.
Biol. 43: 83-116.
Diaz, M. N., B. Frei, J. A. Vita, and J. F. Keaney. 1997. Antioxidants and atherosclerotic heart disease. N. Engl. J. Med. 337: 408-416.
Espin, J. C., C. Soler-Rivas, H. J. Wichers, and C. Viguera-Garcia. 2000. Anthocyanin-based natural colorants: a new source of antiradical activity for foodstuff. J. Agric. Food Chem. 48: 1588-1592.
Halliwell, B, J. M. C. Gutteridge, and O. I. Aruoma. 1987. The deoxyribose method: a simple test-tube assay for determination of rate constants for reactions of hydroxyl radicals. Anal. Biochem. 165: 215-219.
Halliwell, B. 1999. Food-derived antioxidants. Evaluating their importance in food and in vivo. Food Sci. Agric. Chem. 1: 67-109.
Harman, D. 1995. Role of antioxidant nutrients in aging: overview. Age 18: 51-62.
Hou, W.C., J. S. Liu, H. J. Chen, T. E. Chen, C. F. Chang, and Y. H. Lin. 1999a. Dioscorin, the major tuber storage protein of yam (Dioscorea batatas Decne), with carbonic anhydrase and trypsin inhibitor activities. J. Agric. Food Chem. 47: 2168-2172.
Hou, W.C., H. J. Chen, and Y. H. Lin. 1999b. Dioscorin, the major tuber storage protein of yam (Dioscorea batatas Decne), with
dehydroascorbate reductase and monodehydroascorbate reductase activities. Plant Sci. 149: 151-156. .
Hou, W. C., Y. C. Chen, H. J. Chen, Y. H. Lin, L. L. Yang, and M. H. Lee. 2001a. Antioxidant activities of trypsin inhibitor, a 33 kDa root storage protein of sweet potato (Ipomoea batatas (L.) Lam cv. Tainong 57). J. Agric. Food Chem. 49: 2978-2981.
Hou, W. C., M. H. Lee, H. J. Chen, W. L. Liang, C. H. Han, Y. W. Liu, and Y. H. Lin. 2001b. Antioxidant activities of dioscorin, the storage protein of yam (Dioscorea batatas Decne) tuber. J. Agric. Food Chem. 49: 4956-4960.
Hou, W. C., F. L. Hsu, and M. H. Lee. 2002. Yam (Dioscorea batatas) tuber mucilage exhibited antioxidant in vitro. Planta Medica 68:
1072-1076.
Hou, W. C., Y. L. Lu, S. Y. Liu, and Y. H. Lin. 2003. Activities of superoxide dismutase and glutathione peroxidase in leaves of different cultivars of Liriope spicata L. on 10% SDS-PAGE gels. Bot. Bull. Acad. Sin., 44: 37-41.
Hou, W. C., W. C. Wu, C. Y. Yang, H. J. Chen, S. Y. Liu, and Y. H. Lin. 2004. Antioxidant activities of methanolic and hot-water extracts from leaves of three cultivars of Mai-Men-Dong (Liriope spicata L.). Bot. Bull. Acad. Sin., 45: 285-290.
Hu, C. and D. D. Kitts. 2000. Studies on the antioxidant activity of Echinaceae root extract. J. Agric. Food Chem. 48: 1466-1472.
Huang, D. J., C. D. Lin, H. J. Chen, abd Y. H. Lin. 2004. Antioxidant and antiproliferative activities of sweet potato (Ipomoea batatas (L.) Lam cv. Tainong 57) constituents. Bot. Bull. Acad. Sin., 45: 179-186.
Kohno, M., M. Yamada, K. Mitsuta, Y. Mizuta, and T. Yoshikawa. 1991. Spin-trapping studies on the reaction of iron complexes with peroxides and the effects of water-soluble antioxidants. Bull. Chem. Soc. Jpn. 64: 1447-1453.
Kono, Y., S. Kashine, T. Yoneyama, Y. Sakamoto, Y. Matsui, and H. Shibata. 1998. Iron chelation by chlorogenic acid as a natural antioxidant. Biosci. Biotechnol. Biochem. 62: 22-27.
Lai, L. S., S. T. Chou, and W. W. Chao. 2001. Studies on the antioxidant activities of Hsian-Tsao (Mesona procumbens Hemsl). J. Agric.
Food Chem. 49: 963-968.
Lee, M. H., Y. S. Lin, Y. H. Lin, F. L. Hsu, and W. C. Hou. 2003. The mucilage of yam (Dioscorea batatas Decne) tuber exhibited angiotensin converting enzyme inhibitory activities. Bot. Bull. Acad. Sin., 44: 267-273.
Lin, C. L., H. J. Chen, and W. C. Hou. 2002. Activity staining of glutathione peroxidase after electrophoresis on native and sodium dodecylsulfate polyacrylamide gels. Electrophoresis 23: 513-516.
Lin, C. T., K. W. Yeh, M. C. Kao, and J. F. Shaw. 1993. Cloning and characterization of a cDNA encoding the copper/zinc-superoxide dismutase from sweet potato tuberous root. Plant Mol. Biol. 3: 911- 913.
Liu, Y. W., C. H. Han, M. H. Lee, F. L. Hsu, and W. C. Hou. 2003. Patatin, the tuber storage protein of potato (Solanum tuberosum L.), exhibits antioxidant activity in vitro. J. Agric. Food Chem. 51: 4389-4393.
Njus, D. and P. M. Kelley. 1993. The secretary-vesicle ascorbate-regenerating system: a chain of concerted H+/e- -transfer reaction.
Arch. Biophys. Acta. 1144: 235-248.
Rice-Evans, C. A., N. J. Miller, and G. Paganga. 1997. Antioxidant properties of phenolic compounds. Trends Plant Sci. 2:
152-159.
Salah, N., N. J. Miller, G. Paganga, L. Tijburg, G. P. Biolwell, and C. Rice-Evans. 1995. Polyphenolic flavonols as scavenger of aqueous phase radicals and as chain breaking antioxidants. Arch. Biochem. Biophys. 322: 339-346.
Smith, M. A., G. Perry, P. L. Richey, L. M. Sayre, V. Anderson, M. F. Beal, and N. Kowal. 1996. Oxidative damage in Alzheimer's . Nature 382: 120-121.
Figure 1
Concentration ( mg / mL )
0.0 0.2 0.4 0.6 0.8 1.0 1.2 1.4 1.6
Scavenging activity of D P PH rad ical (%)
0 20 40 60 80
crude TN1 crude TN2 crude MJ
A
Concentration ( mg / mL )
0.0 0.2 0.4 0.6 0.8 1.0 1.2
Scaven gin g activity of DPPH rad ical (%)
0 20 40 60 80
purifiedTN1 purified TN2 purified MJ
B
Figure 1. The scavenging activity of crude mucilage (CM) (A) and partially purified mucilage (PPM) (B) of TN1, TN2, and MJ yam cultivars against DPPH radical. Means of triplicates were measured.
Deionized water was used as a blank experiment. The scavenging activity of DPPH radical (%) was calculated according to the following equation: (A517blank − A517sample) ÷ A517blank × 100 %.
Figure 2
Concentration ( mg / mL )
0.0 0.2 0.4 0.6 0.8 1.0 1.2 1.4 1.6
Scavenging activity of hydroxyl radical (%)
0 10 20 30 40 50 60 70
crude TN1 crude TN2 crude MJ
A
Concentration ( mg / mL )
0.0 0.2 0.4 0.6 0.8 1.0 1.2 1.4 1.6
Scavenging activity of hydroxyl radical (%)
0 10 20 30 40 50 60 70
purified TN1 purified TN2 purified MJ
B
Figure 2. The scavenging activity of crude mucilage (CM) (A) and partially purified mucilage (PPM) (B) of TN1, TN2, and MJ yam cultivars against hydroxyl radical. Means of triplicates were measured.
Deionized water was used as a blank experiment. The absorbance at 532 nm was measured. The scavenging activity of hydroxyl radicals (%) was calculated with the equation: (A532blank − A532sample) ÷ A532blank × 100 %.
Figure 3
Concentration ( mg / mL )
0.0 0.2 0.4 0.6 0.8 1.0 1.2
Sc avenging activity of superoxide radical (%)
0 20 40 60 80 100
crude TN1 crude TN2 crude MJ
A
Concentration ( mg / mL )
0.0 0.2 0.4 0.6 0.8 1.0 1.2
Scavenging avtivity of superoxide radical (%)
0 20 40 60 80 100
purified TN1 purified TN2 purified MJ
B
Figure 3. The scavenging activity of crude mucilage (CM) (A) and partially purified mucilage (PPM) (B) of TN1, TN2, and MJ yam cultivars against superoxide radical. Means of triplicates were measured.
Deionized water was used as a blank experiment. The changes of absorbance at 560 nm were recorded during 1 min and expressed as △A560nm/min. The scavenging activity of the superoxide radical was calculated as following: ( △ A560nm/minblank − △ A560nm/minsample) ÷ △ A560nm/minblank × 100 %.
Figure 4A
Figure 4. The scavenging activity of partially purified mucilage (PPM) of (A) TN1 (0.031, 0.062, 0.1, and 0.2 mg/ml), (B) TN2 (0.12, 0.25, 0.5, and 1 mg/ml), and (C) MJ (0.0004, 0.004, and 0.007 mg/ml) cultivars against hydroxyl radical. The signal intensities of DMPO-OH adduct were determined by electron spin resonance spectrometry. The scavenging activity against hydroxyl radical was also showed in the figure.
(1)blank
(2)0.031 mg/ml (1%)
(3)0.062 mg/ml (26%)
(4)0.1 mg/ml (69.6%)
(5)0.2 mg/ml (81%)
Figure 4B
(1)blank
(2)0.12 mg/ml (13%)
(3)0.25 mg/ml (31.5%)
(4)0.5 mg/ml (51.9%)
(5)1 mg/ml (72.2%)
Figure 4C
(1) blank
(2)0.0004 mg/ml (5.5%)
(3)0.004 mg/ml(50%)
(4)0.007 mg/ml (51.8%)
Table 1. Comparison of the antioxidant activity of mucilages from TN1, TN2, and MJ
yam cultivars before (crude mucilage, CM) and after purification (partially purified mucilage, PPM) against DPPH, hydroxyl and superoxide radicalsYam cultivars
DPPH radical (mg/ml)a
CM PPM
Hydroxyl radical
(mg/ml) a
CM PPM
Superoxide radical (mg/ml) a
CM PPM
TN1
0.329 0.279 0.668 1.146 0.802 0.368
TN2 0.547 0.653 1.461 1.096 0.681 0.258
MJ 0.847 0.631 0.946 1.554 0.086 0.148
a expressed as the IC50 value