科技部補助專題研究計畫報告
鋅量子點表面對稱性電偶極矩變化與物理吸附,化學反應及熱
效應之關係
報 告 類 別 : 成果報告 計 畫 類 別 : 個別型計畫
計 畫 編 號 : MOST 108-2112-M-006-015- 執 行 期 間 : 108年08月01日至109年07月31日 執 行 單 位 : 國立成功大學物理學系(所)
計 畫 主 持 人 : 羅光耀
計畫參與人員: 碩士班研究生-兼任助理:陳曄 碩士班研究生-兼任助理:顏廷宇 碩士班研究生-兼任助理:林宏信 碩士班研究生-兼任助理:方培丞 碩士班研究生-兼任助理:陳彥維 碩士班研究生-兼任助理:莊耕硯 博士班研究生-兼任助理:張峰銘 博士班研究生-兼任助理:陳偉庭
本研究具有政策應用參考價值:■否 □是,建議提供機關
(勾選「是」者,請列舉建議可提供施政參考之業務主管機關)
本研究具影響公共利益之重大發現:□否 □是
中 華 民 國 109 年 10 月 24 日
中 文 摘 要 : 奈米金屬氧化現象將對於奈米半導體元件的金屬接觸面電性造成影 響,如能對奈米金屬氧化行為確實了解與分析,將有助於未來奈米 元件的發展。由於奈米金屬有較高的表體比例,具高曲率的表面氧 化層會產生較高的內建電場,使得氧化速率大幅提升。這一個實驗 所使用的鋅量子點直徑約18 nm,被均勻地成長在Si(111)上且被 Si(111)表面所侷限。入射光照射的鋅量子點超過109個,因為鋅量 子點具有一致的晶格方向,使得反射二次諧波反應出眾多鋅量子點 的淨電偶極強度。氧化過程時,奈米鋅表面自由電子、氧離子與向 外擴散的鋅離子將形成的不同極化率,觀察反射二次諧波的變化就 可以了解氧化行為的每一個細膩過程。由於氧離子與鋅原子形成Zn- O鍵(弱極化率),與氧化層內的鋅離子向外擴散所呈現的外部鋅離子 (強極化率)的現象發生是漸次發生在眾多鋅量子點上,因此所呈現 的淨極化率強度是兩種競爭下的結果。初期氧化過程的氧離子化、
化學吸附與鋅離子向外擴散與基板溫度與氧氣壓力直接相關,反映 出不同階段的RSHG強度變化。由於氧化開始發生之前需要有氧分子 靠近鋅量子點,然後被離子化後與表面的鋅原子形成鍵結。因此在 發生前照射UVA (365 nm, ~3.4eV)可以斷裂鍵結的Zn-O或阻止氧分 子的離子化,讓氧化現象消失。如移去UV光後,氧化行為又開始進 行。這一個工作使用RSHG來觀察,並可以調控初期氧化條件,可以 觀察到階段性的初期氧化行為。
中 文 關 鍵 詞 : 初期氧化,鋅量子點,反射二次諧波,極化率,奈米金屬,高曲率 英 文 摘 要 : The oxidation behavior of nano-metal causes an impact on
the contact of nano device. How to well realize the actual oxidation of nano metal is helpful to improve the
development of future nano-device. Due to the high surface- to-volume ratio and high curvature of oxide layer on Zn dots, the oxide rate will be speeded. The size of Zn dots is around 18 nm and these dots were uniformly and
constrainedly grown on Si(111) surface. As the Zn dots constrained by Si(111) surface, reflective second harmonic generation (RSHG) exhibits the net polarizability as the 3m symmetry of Si(111) owing to ~109 Zn dots have similar crystal orientation under the irradiation area. During early-stage oxidation, free electrons, oxygen ions and out- diffused Zn ion display the different dipole strength. To observe the evolution of RSHG with time is helpful to realize the detailed phenomena of early-stage oxidation step-by-step. Besides, the variation of RSHG intensity demonstrates the competition between the polarizability of Zn-O binding and Zn ions. The substrate temperature and environmental oxygen pressure directly influenced the early-stage oxidation of Zn dots, and the results were reflected to the RSHG variation. Based on the necessary condition of the early-stage oxidation, oxygen molecules have to close to Zn dots surface and were ionized by the electron tunneling from Zn surface atoms. Under the
irradiation of UVA light (365 nm, ~3.4eV), the early-stage oxidation will be suppressed till the UVA light was
removed. This work presents a possibility to observe the early-stage oxidation behavior of nano-metal with well controlling the enviornments by the RSHG method.
英 文 關 鍵 詞 : early-stage oxidation, Zn dot, reflective second harmonic generation, polarizability, nano metal, high curvature
Abstract
The oxidation behavior of nano-metal causes an impact on the contact of nano device.
How to well realize the actual oxidation of nano metal is helpful to improve the development of future nano-device. Due to the high surface-to-volume ratio and high curvature of oxide layer on Zn dots, the oxide rate will be speeded. The size of Zn dots is around 18 nm and these dots were uniformly and constrainedly grown on Si(111) surface. As the Zn dots constrained by Si(111) surface, reflective second harmonic generation (RSHG) exhibits the net polarizability as the 3m symmetry of Si(111) owing to ~109 Zn dots have similar crystal orientation under the irradiation area. During early- stage oxidation, free electrons, oxygen ions and out-diffused Zn ion display the different dipole strength. To observe the evolution of RSHG with time is helpful to realize the detailed phenomena of early-stage oxidation step-by-step. Besides, the variation of RSHG intensity demonstrates the competition between the polarizability of Zn-O binding and Zn ions. The substrate temperature and environmental oxygen pressure directly influenced the early-stage oxidation of Zn dots, and the results were reflected to the RSHG variation. Based on the necessary condition of the early-stage oxidation, oxygen molecules have to close to Zn dots surface and were ionized by the electron tunneling from Zn surface atoms. Under the irradiation of UVA light (365 nm, ~3.4eV), the early-stage oxidation will be suppressed till the UVA light was removed. This work presents a possibility to observe the early-stage oxidation behavior of nano-metal with well controlling the enviornments by the RSHG method.
Introduction
Accompanying with the development of nano-science and technology, the role of metal nano dots in the field of semiconductor, optics and quantum device are quite important. Due to the size effect, the surface strain will influence the chemical activation energy [1,2]. The electrical properties of nano metal contact will be changed owing to the physical and interfacial properties were modified, such as the Schottky barrier and Ohmic contact [3,4]. The structural evolution of nano-metal dots grown on semiconductor surface via thermal effect generated from actual performance [5,6], and the composition of nano-metal dots due to surface chemical reactions (oxidation and
reduction) on the nano-metal surface will affect the final configuration of the nano- metal/ semiconductor interface. The further relaxation of Zn dot constrainedly grown on Si(111) induced by the thermal effect have proved [7]. The phenomena and mechanism of the early-stage oxidation on the Zn dot surface have to be understood and analyzed. As the first oxide layer forms on the Zn dot surface, the physical adsorption and sequent chemical adsorption would happen. To realize the mechanism of early-stage oxidation would help the chemical reaction of nano-metal in the varied environmental factors. It is a challenge to layer-by-layer observe the early-stage oxidation on nano-metal. Besides, high curvature of nano-metal would enhance the oxidation rate [8], which is a difficulty to study the nano-metal system. To the best of our knowledge, the observation of layered-by-layered oxidation on nano-metal is few reported. How to observe the phenomena of the early-stage oxidation on nano-metal system is the first duty in this subject.
As the early-stage oxidation process happens on the nano-metal surface, oxygen molecules physical absorb on the surface and then be ionized by electrons tunneling through metal surface. Therefore, the first oxidation layer was formed and an electrical field was generated in the layer to drive the ionized metal out-diffuse to the top surface. The out-diffuse metal ion will absorb and ionize oxygen molecules, which creates next oxidation layer. Further oxidation layers will be built via repeated behaviors: oxygen adsorption-oxygen molecule ionization-electron tunneling-metal ion out-diffusion. The oxidation process can be described as Cabrera-Mott theory shown in Fig. 1. If we can slow down the oxidation rate by controlling environment oxygen molecules and substrate temperature, the evolution of layer-by-layer oxidation at the early-stage oxidation would be traced. In order to realize the mechanism of CM theory on metal surface, a sensitive analysis tool is necessary to distinguish and examine each
step in the early-stage oxidation.
Figure 1. (a) CM theory to explain the oxidation by the electron tunneling and diffusion of metal interstitials [9] (b) the step of the early-stage oxidation, (i) oxygen melocules around metal surface, (ii) oxygen molecules physically adsorb on Zn surface, (iii) electron tunnels to ionize oxygen molecules and (iv) metal ion out-diffusion driven by the built-field of single oxidation layer.[10]
The form of oxide layer is fast and dynamic at the early stage, and the reaction will be slow down till the end of the oxidation [9]. The number of oxide layer is strongly dependent on the environment temperature [10]. According each step of the oxidation process, the surface dipole would be changed following the chemical configuration.
The polarizability of the free electrons on the surface atoms of a clean metallic surface mainly contributes to the nonlinear susceptibility [11]. The binding of adsorbate and surface metal atoms will cause the free electron less polarizable and then decrease the nonlinear susceptibility [12]. For crystalline Zn dots, the surface polarizable diploe contributed from surface free electrons follows 3m symmetry of Zn surface, which strength should be stronger than Zn-O binding. As oxygen molecules physically absorb on Zn dot surface, the surface free electrons will be weaken due to van der Walls force between oxygen molecules and Zn atom restrains the freedom of surface electrons. If
oxygen molecules were further ionized and then formed chemical adsorption, Zn-O bond formed with following 3m symmetry of surface Zn crystalline but the polarizability of Zn-O binding is weaker than the one of surface free electrons. The strength evolution of surface dipole on Zn dots will trace the early-stage oxidation process if the oxidation process can be well controlled. Due to larger surface-to volume ratio for Zn dots system, the oxidation rate could be fast in the room temperature and low oxygen pressure.
In this work, we observe the dipole evolution of Zn dots to simulate quasi- atomic revolution on Zn dot surface in the early-stage oxidation using reflective second harmonic generation (RSHG). Though some works report the RSHG variation due to the evolution of adsorbate-fresh metal surface [13-14], however, the detailed of early- stage oxidation on metal dot is few studied, which is based on the relative surface dipole strength at different oxidation stage. As high surface to volume ratio of Zn dots, the evolution of surface dipole via oxidation has higher sensitivity and faster reaction, especially for optical irradiation and analysis. Well controlled oxygen flow and pressure
Based on above advantages, the phenomena of the early-stage oxidation on Zn dots can be observed in the chamber with well-controlled oxygen pressure. The dynamics of electrical dipole evolution on Zn dots in the early-stage oxidation process is clearly inspected and the results directly examine the applicability of CM theory.
Experiments and Methods
Experiments
In order to realize the oxidation of Zn dot at the early stage, collective Zn dot constrained grown on Si(111) were performed via the strategic RF sputtering method [15]. The Zn dot growth of the strategic RF sputtering were performed under the condition of negative substrate bias of −500 V and hydrogen gas (H2). During the
deposition, the ratio of hydrogen (H2) to argon (Ar) was 20% under the working pressure of 1 × 10−2 and RF power of 100 W [16]. Substrate temperature (~250 °C ) was the key issue since the deposition and evaporation rate must keep a balance to stability the size of constrained Zn dots. The substrate temperature of Si(111) was kept at around 250 °C to enhance the reduction of ZnO caused by H2 and the surface diffusion of Zn adatoms [16]. Zn dots was froze to solid phase from liquid phase and the crystal orientation of Zn dots was constrained by oxide-free Si(111) surface. The oxidation process was performed in the chamber after the growth of Zn dots. The detailed oxidation process was controlled by the oxygen pressure and the substrate temperature.
The behavior of rotational-anisotropic RSHG (RA-RSHG) was performed by a programmed rotator combined with the rotator of RF sputtering [15]. A pulsed Nd:YAG laser with the pulse duration of 6 ns and pulse energy of 100 mJ was used as the light source of RSHG experiment. s-polarized RSHG with the irradiation of s-polarized fundamental light (s-s RSHG) was collected and analyzed by the boxcar integrator. As an unavoidable and periodic ground noise would couple into the RSHG signal, we performed a simulation of Fourier filter to purify RSHG signal, especially for the fixed point experiment. Synchrotron XRD were performed at the 17B1 stations of the National Synchrotron Radiation Research Center in Taiwan. The size distribution and micrographs of ZnO/Zn dots were inspected by using FESEM (ZEISS-SUPRA 55-41- 30).
Results and discussion
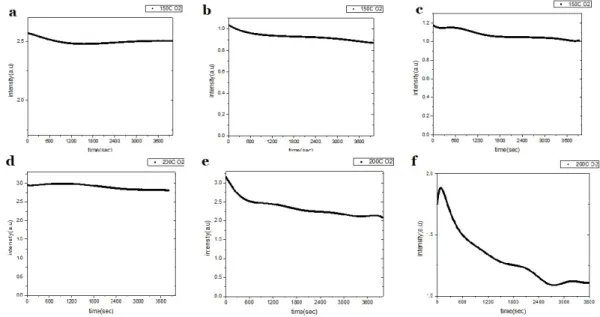
The early-stage oxidation strongly depends on the environment, including substrate temperature and oxygen pressure in the chamber. Fig. 1(a), (b) and (c) exhibits the RSHG intensity variation with time at the substrate temperature of 150oC for the oxygen
pressure of 200, 400 and 800 mtorr, respectively. Fig.1(d), (e) and (f) exhibits the RSHG intensity variation with time at the substrate temperature of 200oC for the oxygen pressure of 200, 400 and 800 mtorr, respectively. The oscillated RSHG intensity exhibits the competition between Zn-O binding and out-diffused Zn ions on the top surface of Zn dots. The oscillated frequency of RSHG intensity depends on the substrate temperature and oxygen pressure, which means the generation of oxidation layer was controlled by the rate of forming Zn-O bonding and sequent out-diffused Zn ions. he RSHG intensity decreases accompanying the oxidation time. The detailed behaviors of early-stage oxidation clearly reflect to the evolution of RSHG intensity.
Fig. 2 the RSHG intensity with time in the early-stage oxidation for the substrate temperature of 150oC and 200oC under different oxygen pressure.
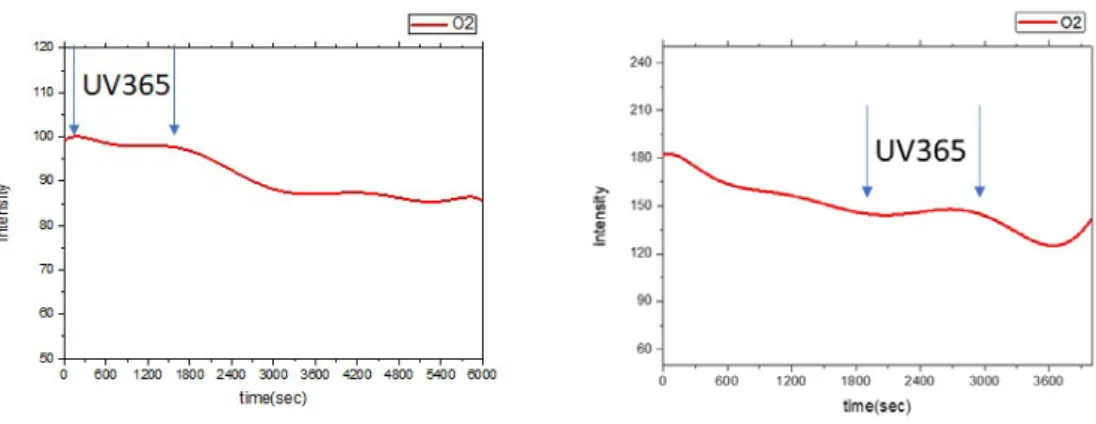
To further realize the influence factors at the early-stage oxidation, UVA (365 nm) light irradiated on the Zn dots under the oxygen pressure of 400 mtorr and the substrate temperature of 200oC. Fig.2 (a) shows the oxidation was suppressed as UVA light irradiated on Zn dots, and the oxidation lasted after moving UVA light. There is little
raise in RSHG intensity during UVA irradiation since some chemical adsorbed oxygen ions were broken down. The frozen behavior happened as UVA irradiation was performed during the early-stage oxidation, as shown in Fig.2(b). To well control UVA irradiation will arrest the oxidation process at any time.
Fig.3 the irradiation of UVA suppresses the oxidation at the early-stage.
Figure 2. RSHG intensity v.s. the time of the oxidation process.
The early-stage oxidation process with time can observe the curvature in Fig.2. O- A sector means the adsorbate on Zn dots were eliminated by laser irradiation. As the
fresh Zn dot A point, high density free electrons on 3m symmetrical Zn atoms exhibit higher polarizability. In the A-B sector, oxygen molecules physically adsorbed on Zn dots and were ionized by the surface electron. The form of Zn-O binding will decrease the RSHG strength till B point which O ion occupied more ratio on the surface of collective Zn-O/Zn dots. In the process of A-B sector, while oxygen ion chemically adsorbed on Zn atom, an electrical field generated from the mono-oxidation layer deduces activation energy to enhance the out-diffusion of Zn ions. Therefore, the top of Zn-O/Zn dots will composite of (i) O ion (Zn-O binding) (ii) out-diffused Zn ion in the A-B process, and B point have larger ratio in (i) O ion (Zn-O binding).
In the B-C sector, the occupation of (ii) on Zn-O/Zn dots gradually increases till the C point where the occupation of (ii) is larger than the one of (i). In this stage, the first oxidation layer is almost formed. The oxidation process of C-D sector is similar as the A-B sector and there is relatively more O ion (Zn-O binding) on Zn-O/Zn dot at D point; The oxidation process of D-E sector is similar as B-C sector and there is relatively more out-diffused Zn ion on Zn-O/Zn dot at E point where the second oxidation layers were nearly formed. However, the oxidation rate will decrease as the number of oxidation layers increases. The number of oxidation layers is strongly dependent on the substrate temperature since Zn ions beneath oxide layer have larger energy to out- diffuse.
Conclusion
The early-stage oxidation of nano metal is fine and hard to observe. The variation of surface dipole on nano metal is an index to distinguish each step of oxidation. RSHG is a way to analyze the evolution of surface polarizability during oxidation. This work presents a direct witness to observe early-stage oxidation step-by step by RSHG method.
The main factors, oxygen pressure and substrate temperature, in the early-stage oxidation were confirmed. Besides, suitable UVA light irradiation can hold and control the oxidation process.
Reference
[1]. J. K. Kim, J. L. Lee, J. W. Lee, H. E. Shin, Y. J. Park, T. Kim, Appl. Phys. Lett. 73 2953 (1998).
[2]. Z. Zhang, Q. Liao, X. Zhang, G. Zhang, P. Li, S. Lu, S. Liu, Y. Zhang, Nanoscale 7, 1796 (2015).
[3]. S. Calderon V., B. Gomes, P. J. Ferreira and S. Carvalho, Nanoscale, 9, 5254
(2017).
[4]. W. I. L. Lawrie, et al., Appl. Phys. Lett., 116, 080501 (2020)
[5]. V.P. Zhdanov and B. Kasemo, Chemical Physics Letters 452, 285 (2008).
[6]. Eli Sutter , Francisco Ivars-Barcelo, and Peter Sutter, Part. Part. Syst. Charact., 31, 879 (2014).
[7]. Li-Chi Kao, Bo-Jia Huang, Yu-En Zheng, Kai-Teng Tu, Shang-Jui Chiu, Ching- Shun Ku, Kuang Yao Lo, Nanotechnology, 29, 035705 (2018).
[8]. E. Sutter and P. Sutter, J. Phys. Chem. C, 116, 20574 (2012).
[9]. V.P. Zhdanov and B. Kasemo, Nano letter, 9, 2172 (2009)
[10]. Albert T.FromholdJr.Regina G.Fromhold, Comprehensive Chemical Kinetics, 21, 1 (1984)
[11] C.H. Lee, R.K. Chang and N. B]oembergen, Phys. Rev. Lett. 18 (1967) 167 [12] HESKETT, L.E. URBACH, K.J. SONG, E.W. PLUMMER and H.L. DAI,
Surface Science, 197, 225 (1988)
[13] Robert M. Corn and Daniel A. Higgins, Chem. Rev. 1994, 94, 107 (1994) [14] E. Leiva and W. Schmickler, Surface Science 291, 226 (1993)
[15] Chun-Chu Liu, Jun-Han Huang, Ching-Shun Ku, Shang-Jui Chiu, Jay Ghatak, Sanjaya Brahma, Chung-Wei Liu, Chuan-Pu Liu, & Kuang-Yao Lo, Scientific Reports 5, 12533 (2015)
[16] Bo-Jia Huang, Li-Chi Kao, Sanjaya Brahma, Yu-En Jeng, Shang-Jui Chiu, Ching-Shun Ku and Kuang-Yao Lo, Journal of Physics D: Applied Physics, 50, 175301(2017)
Publications 2019.8-2020.8
Under preparation
1. S.J. Chiu, L.C. Kao#, C.C. Liu, S. Brahma, K.T. Tu, J.H. Huang, C.P. Liu, H.Y.
Lee, C.S. Ku* and K.Y. Lo*, “Metastable states behave as a buffer layer to control the relaxation of Zn dots on Si(111)” submitted to Nature Comm.(under
preparation)
2. Wei-Ting Chen1, Kuang-Yao Lo1*, Dai-Yan Lu1, Hong-Shing Lin1, Sheng-Jhong Ji1, Duo-Wen Chen2, Jia-Sheng Chang2, Shao-Nai Lin2, Shih-Hao Yang3 and Chi- Yen Huang4, Simon Čopar5, Slobodan Žumer5* and Hong-Ping Lin2*,
“Disordered-layer Surrounding Mesoporous Silica Hollow Sphere in Nematic Liquid Crystals for suppressing aggregation”, submitted to Nature Comm.(under preparation)
108年度專題研究計畫成果彙整表
計畫主持人:羅光耀 計畫編號:108-2112-M-006-015-
計畫名稱:鋅量子點表面對稱性電偶極矩變化與物理吸附,化學反應及熱效應之關係
成果項目 量化 單位
質化
(說明:各成果項目請附佐證資料或細 項說明,如期刊名稱、年份、卷期、起 訖頁數、證號...等)
國
內 學術性論文
期刊論文 0
研討會論文 0 篇
專書 0 本
專書論文 0 章
技術報告 0 篇
其他 0 篇
國
外 學術性論文
期刊論文 2
篇
1. Wei-Ting Chen, Sheng-Chung Ji, Shih-Hsuan Chen, Chia-Yi Huang* and Kuang Yao Lo* (2020, Sep).
Competitive Dye Adsorption- Desorption on the Isotropic
Surface at the Early Stage of the Photoexcitation of Azo Dye-Doped Liquid Crystals. Crystals, 10, 802.
本人為通訊作者.
2. Feng-Ming Chang, Zong-Zhe Wu, Yeh Chen, Ting-Yu Yen, Yu-Hsiang Huang, Li-Yun Chong, Shiu-Ko JangJian, Fu-Ying Lee, Yu-Ming Chang and Kuang-Yao Lo* (2020, Apr). Structural evolution of in situ boron-doped SiGe ultrathin film analyzed by multi-optical methods. Nanotechnology, 31, 275702.
研討會論文 0
專書 0 本
專書論文 0 章
技術報告 0 篇
其他 0 篇
參 與 計 畫 人 力
本國籍
大專生 2
人次
劉驊興,洪楊皓
碩士生 5 林宏信,莊耕碩,顏廷宇,方培丞,陳
彥維,
博士生 2 張峰銘,陳偉庭
博士級研究人員 0
專任人員 0
非本國籍 大專生 0
碩士生 0
博士生 0
博士級研究人員 0
專任人員 0
其他成果
(無法以量化表達之成果如辦理學術活動
、獲得獎項、重要國際合作、研究成果國 際影響力及其他協助產業技術發展之具體 效益事項等,請以文字敘述填列。)