Physical activity levels of school-age children with congenital heart disease in Taiwan
Ching-Chiu Kao, MS, RN
a, Pi-Chen Chang, PhD, RN
b, Ching-Wen Chiu, MS, RN
c, Lee-Pin Wu, MS, RN
a, Jen-Chen Tsai, DNSc, RN
b,⁎
aDepartment of Nursing, Taipei Medical University-Wan Fang Medical Center, Taipei 116, Taiwan
bSchool of Nursing, Taipei Medical University, Taipei 110, Taiwan
cDepartment of Nursing, Cathay General Hospital, Taipei 106, Taiwan Received 4 May 2007; revised 24 December 2007; accepted 30 December 2007
Abstract Congenital heart disease (CHD) is a common childhood health problem. The incidence of CHD is estimated between 8 and 10 per 1,000 children in Taiwan. Evidence indicates that exercise capacity for children with CHD can reach the level of children with no CHD after surgical repair. However, it is necessary to further investigate if their physical activity is comparable to their peers who have no CHD to prevent chronic disease caused by physical inactivity. This study aimed to investigate the differences of physical activity level (PAL) between Taiwanese children with no CHD and children after total correction for CHD. A case control design was used in this study. During the study period, 68 schoolchildren were recruited: 34 children with CHD and 34 age- and gender-matched children who have no CHD. The data collection tools included three-day physical activity record (3DPAR) and RT3 triaxial accelerator. The average total energy expenditure (TEE) measured by both 3DPAR and RT3 triaxial accelerator were lower for boys with CHD than boys with no CHD (t =−3.39, p = .002; t = −3.43, p = .002). PAL was also lower for boys with CHD than for boys with no CHD (t =−2.29, p = .03). Whereas, TEE did not differ between girls with CHD and girls with no CHD (t = 0.58, p = .57; t =−0.27, p = .79). Overall, the level of moderate-to-vigorous physical activity (MVPA) was similar between children with CHD and children with no CHD. These results suggest that boys with CHD engage in less physical activity than do boys with no CHD. Both children with CHD and children with no CHD should be encouraged to engage in more physical activity especially MVPA.
© 2009 Published by Elsevier Inc.
1. Introduction
Congenital heart disease (CHD) is a collective term for structural anomalies of the heart present at birth. The CHD incidence is about 8 to 10 per 1,000 children in Taiwan (Kao, Wang, Pai, & Hwang, 2000). Apart from those having patent ductus arteriosus, children may have atrial and ventricular septal defects which may close spontaneously, but about one third of CHD patients require surgical repair at an opportune moment for prolonged survival (Park, 2002). If surgical corrections were carried out sufficiently early in childhood,
many of those patients can improve exercise tolerance significantly (Dent, 2003). Furthermore, those surgical repairs can help restore cardiorespiratory function and exercise tolerance in children with CHD to the levels of children with no cardiac anatomy problems (Paridon, 1997).
Children with CHD who have received adequate exercise training may improve their maximal oxygen uptake (VO2 max), physiological activity, and physical fitness (Calzolari, Giordano, Di Giacinto, & Turchetta, 2001).
Physical activities encompass sporting events and all other occupational, pastime, and lifestyle activities. Sirard and Pate (2001) further defined children's physical activity as the energy expenditure (EE) in the movements resulting from muscular contractions, including daily life activities such as housework, competitive sports, or pastime activities.
Applied Nursing Research 22 (2009) 191–197
www.elsevier.com/locate/apnr
⁎ Corresponding author. Tel.: +886 2 27395606; fax: +886 2 23772842.
E-mail address:jenchent@tmu.edu.tw(J.-C. Tsai).
0897-1897/$– see front matter © 2009 Published by Elsevier Inc.
doi:10.1016/j.apnr.2007.12.002
Regular exercise can increase body VO2 max, facilitate circulation, improve oxygenation of the blood, lower blood lipid levels, and accelerate metabolic activities (Boreham et al., 2002). Physical inactivity in childhood and adoles- cence is particularly correlated with risk factors for adult cardiovascular diseases and obesity (Twisk, Kemper, & van Mechelen, 2000).
According to Hirth, Reybrouck, Bjarnason-Wehrens, Lawrenz, and Hoffmann (2006), regular exercise at recom- mended levels can be performed and should be encouraged in all patients with CHD. Physical activity has shown beneficial effects on the physical, psychological, and social level in children with CHD (Hirth et al., 2006). Despite this evidence, a large number of children with CHD remain physically inactive because they are overprotected by their parents and their environment (Reybrouck & Mertens, 2005). Abnormalities in cardiac function, restrictions of activities by parents and/or school teachers, as well as failure of some treating doctors to provide adequate exercise recommendations, render some children with CHD fairly inactive after cardiac surgeries (Fredriksen, Ingjer, Nystad, &
Thaulow, 1999). Yet, children with CHD are more likely to endure sickness, physical examinations, surgical procedures, and hospitalizations than are children with no CHD. The resulting sedentary lifestyle leads to diminished physical capacity and places them at risk for early development of illnesses associated with physical inactivity (Hirth et al., 2006). It is therefore extremely important for children with CHD to maintain an active lifestyle.
Children with CHD are currently encouraged to be active in participating in recreational sport activities after their having received corrective cardiac surgery (Hirth et al., 2006;
Lunt, Briffa, Briffa, & Ramsay, 2003). But some scholars found that children with CHD exert lower physical activity levels (PALs) than do normal children (Bar-Mor et al., 2000;
Fredriksen, Ingjer, & Thaulow, 2000). Thus, comparing physical activity between children with no CHD and children with CHD remains an important issue. The aim of this study was to evaluate the patterns of physical activity in Taiwanese children with CHD. We measured patients' total energy expenditure (TEE), EE in moderate-to-vigorous physical activities (MVPA), and their PALs to compare these parameters between children with CHD and children with no CHD.
2. Methods 2.1. Design
In this study, we adopted a cross-sectional descriptive design that used a case control approach for participants' sampling and data collection.
2.2. Participants
We recruited outpatients with CHD at two medical centres in Taipei City. Selection criteria of the children with CHD
included the following: (a) 9 to 12 years old, enrolled in fourth to sixth grade; (b) diagnosed by cardiologists with atrial septal defects, ventricular septal defects, or Tetralogy of Fallot, with no other major illnesses; (c) have received complete surgical repairs more than 1 year; (d) have not received angiography within the last 6 months; (e) not currently receiving any medication related to cardiac diseases; (f) not implanted with a pacemaker or any artificial cardiac rhythm control device; and (g) do not have any other major medical illnesses such as diabetes, asthma, or neuromuscular diseases that impair the child's physical activity.
We recruited children with no CHD for control from the school of the group of children with CHD. Selection criteria of “healthy” children consisted of (a) being individually matched with CHD children in age (no more than 1 year of age difference), gender, and attendance of physical educa- tion (PE) classes during the study period; (b) not having major medical illness such as heart disease, diabetes, asthma, or neuromuscular diseases that impair the child's physical activity; and (c) having no history of hospitaliza- tion within the last 6 months. Both the child participants and their parents gave informed consent to take part in the study.
2.3. Data collection
The purpose and procedure of this study were explained to the parents of each child. All children and their parents completed written informed consent. Permission was received from the medical centers and school of the children before collecting the data. We measured the body mass index (BMI) of each child. We measured TEE of each subject with three-day physical activity record (3DPAR) as well as Triaxial Research Tracker (RT3). Data on basic information sheet and 3DPAR were collected at children's home. Each child wore the RT3 for 3 days starting on Friday.
3. Instruments
3.1. Demographic information
Data collection on child participants included gender, age, school grade, height, weight, BMI, diagnosis, age at first operation, number of operations, and duration of a complete cardiac repair surgery.
4. 3DPAR
We used the 3DPAR, a self-reported instrument in this study to analyze daily TEE of the children. This activity record was originally designed by Bouchard et al. (1983), which was translated into Chinese and modified byHuang and Malina (2002) based on cultural considerations. This Chinese version of 3DPAR has been used by many researchers (Huang & Malina, 2002; Liou & Chiang,
2004). Each participant was advised to record his or her physical activities on Friday, Saturday, and Sunday using the guidelines prescribed by Katzmarzyk, Malina, Song, and Bouchard (1998). We calculated daily TEE of each child subsequently. The physical activities were recorded in 15- minute blocks. Each participant was asked to record all physical activities and classify them according to intensity (ranging from category 1 to 9) and duration of the activity.
The nine activity categories were (a) sleeping, resting, or lying in bed; (b) sedentary activities; (c) low-intensity activity requiring standing; (d) walking slowly; (e) low- intensity manual activity; (f) leisure activities or sports; (g) moderate manual activity; (h) high-intensity but noncompe- titive leisure activity or sports; and (i) highly intensive manual activity or competitive sports. The 3DPAR was completed in children's home under the supervision of their parents. Each record was also checked for clarity and completeness by the researcher.
The frequency of physical activities in each of the nine categories was multiplied by the equivalent EE for each specific category; this was then multiplied by the partici- pant's weight. Then, the absolute daily TEE (kcal/day) was obtained for each participant. The values calculated for Categories 6, 7, 8, and 9 were defined as the EE of MVPA (Bouchard et al., 1983). The test–retest reliability of the original 3DPAR was 0.91 in children and 0.97 in adults (Bouchard et al., 1983). Preliminary trials prior to this study yielded a retest reliability of 0.75 (pb .001). The criterion- related validity of 3DPAR assessed by RT3 was 0.90 (pb .001) in this study.
5. RT3
We instructed each child to wear the RT3 device on his or her hip except when taking a shower for 3 consequent days. The RT3 (Stayhealthy Inc., Monrovia, CA) used in this study is a piezoelectric accelerometer that records motion of the child in three dimensions and provides data of EEs in units (kcal/min). We calculated daily TEE and recorded them for each child. The reliability of RT3 is consistently between 0.92 and 0.98 (Jakicic et al., 1999).
The correlation coefficients between the RT3 and various monitoring devices are 0.88 for the Caltrac Activity Monitor and 0.68 to 0.92 for indirect calorimetry (Welk
& Corbin, 1995).
6. PAL
Daily PAL for the children was calculated from the formula: PAL = TEE/basal metabolic rate (BMR; James, Ferro-Luzzi, & Waterlow, 1988). TEE was measured by RT3. We calculated children's BMR based on the equations suggested bySchofield (1985): BMR = 0.074 weight (kg) + 2.754 megajoule (MJ) per day (males), or BMR = 0.056 weight (kg) + 2.898 MJ per day (females).
7. Statistical analysis
SPSS (Chicago, IL)/Windows 11.0 software was used for data processing. We analyzed the data with frequency, percentage, sample mean, standard deviation, chi-square tests, and independent t tests to compare the background characteristics between children with CHD and children with no CHD.
We used two-way analysis of variance (ANOVA) and independent t tests to examine differences in physical activity between children with CHD and children with no CHD for boys and girls. The differences were considered significant if theα values were smaller than .05.
8. Results
Sixty-eight children were recruited and completed this study: 34 children with CHD and 34 children with no CHD. Within each group there was an equal number (17) of girls and boys. The average age of the children was 10.5 years. Children with CHD have lower mean weight (p = .03) and BMI (p = .04) than do their counterparts.
No significant difference between the two groups was detected in the age distribution and attendance of PE classes (Table 1).
Among the 34 children with CHD, 16 had ventricular septal defects (47.0%), 7 had atrial septal defects (20.6%), and 11 had tetralogy of Fallot (32.4%). The mean age at the time of diagnosis was 7.3 (0–108) months, and all children with tetralogy of Fallot were diagnosed within 3 months following birth. The age of first open heart surgery averaged 31.1 (2–118) months, and the average age of receiving total reparative surgery was 37.4 (8–118)
Table 1
Comparison of study group characteristics (N = 68) Characteristics Children with
CHD group (n = 34)
Children with no CHD group (n = 34)
Total (n = 68)
x2/t p
n (%) n (%) n (%)
Age (years)
9 6 (17.6) 7 (20.6) 13 (19.1)
10 12 (35.3) 11 (32.4) 23 (33.8) 0.24 .81
11 9 (26.5) 10 (29.4) 19 (27.9)
12 7 (20.6) 6 (17.6) 13 (19.1)
Height (cm)
Mean ± SD 142.1 ± 7.9 144.6 ± 8.1 143.4 ± 8.0 1.27 .21 Weight (kg)
Mean ± SD 35.1 ± 8.3 39.6 ± 8.7 37.4 ± 8.8 2.20 .03 BMI (kg/m2)
Mean ± SD 17.3 ± 3.1 18.8 ± 3.1 18.0 ± 3.2 2.06 .04 PE class on a school day
No 20 (58.8) 20 (58.8) 40 (58.8) 0.00 1.00
Yes 14 (41.2) 14 (41.2) 28 (41.2)
Independent t test was applied for height, weight, and BMI; chi-square for others.
months. Most of the patients (79.4%) underwent only one surgery.
9. TEE
The TEE of each child was simultaneously measured by the 3DPAR (TEE3DPAR) and the RT3 (TEE RT3). As a two- way ANOVA revealed an interaction between the CHD and gender in the TEE of the children (F = 5.67, pb .05), the TEE was assessed separately for girls and boys in both the children with CHD group and children with no CHD group.
During the school day, the average TEE3DPARof boys with CHD was significantly lower than that of boys with no CHD (t = −3.65, p = .001), whereas there was no significant difference in TEE3DPAR between girls in the two groups (t = 0.24, p = .82). During weekends, a difference was detected between the two groups of boys, where the average TEE3DPAR of boys with CHD was significantly lower (t = −2.91, p = .007). There was no significant difference between the TEE3DPAR of girls with no CHD and girls with CHD (t = 0.76, p = .45). By averaging the measurements of school days and weekends, the TEE3DPAR
of boys with CHD was significantly lower than that of boys with no CHD (t = −3.39, p = .002), and there was no
significant difference observed between the girls' groups (t = 0.58, p = .57; Table 2).
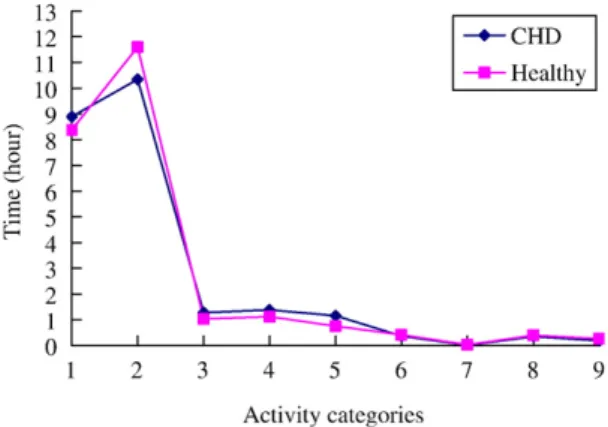
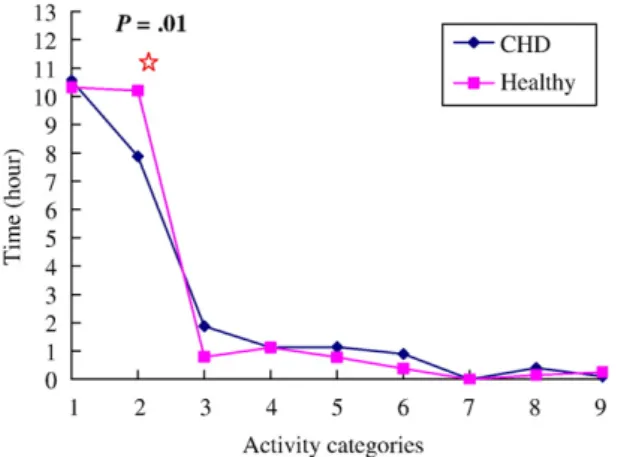
In terms of different physical activity categories, boys with no CHD spend longer performing Category 9 activity compared with boys with CHD (0.34 ± 0.54 vs. 0.04 ± 0.18 hr, t = −2.11, p = .04) on school days (Fig. 1). No difference of duration performing physical activities in each category was observed between boys with CHD and boys with no CHD during weekends (Fig. 2). The two groups of girls did not differ in their physical activity on school days (Fig. 3). But girls with no CHD spend longer per day in Category 2 activity than do their counterparts during weekends (10.21 ± 1.93 vs. 7.88 ± 2.73 hr, t = −2.88, p = .01;Fig. 4).
The daily TEERT3 of boys with CHD was consistently significantly lower than that of boys with no CHD, whether during school days (t =−3.22, p = .003) or weekends (t =
−3.30, p = .002). Meanwhile, no significant difference was found in TEERT3 between the two groups of girls either during school days or weekends (t = −0.18, p = .86). By averaging the measurements of school days and weekends, the TEERT3of boys with CHD was significantly lower than that of boys with no CHD (t =−3.43, p = .002), and there was
Table 2
Comparison of TEE by 3DPAR (N = 68) Children with CHD group (n = 34)
Children with no CHD group (n = 34)
t p
Mean ± SD Mean ± SD School days (kcal/day)
Boys (n = 17) 1344.0 ± 389.6 1871.5 ± 451.9 −3.65 .001 Girls (n = 17) 1547.8 ± 670.4 1502.6 ± 418.9 0.24 .82 Weekends (kcal/day)
Boys (n = 17) 1283.9 ± 358.6 1613.2 ± 299.3 −2.91 .007 Girls (n = 17) 1552.8 ± 567.7 1426.3 ± 386.2 0.76 .45 3-day average (kcal/day)
Boys (n = 17) 1304.0 ± 352.4 1699.3 ± 327.1 −3.39 .002 Girls (n = 17) 1551.2 ± 599.0 1451.8 ± 379.9 0.58 .57
Fig. 1. Activity categories during school days for boys.
Fig. 2. Activity categories during weekends in boys with CHD and boys with no CHD.
Fig. 3. Activity categories during school days for girls.
no significant difference observed between the girls' groups (t =−0.27, p = .79;Table 3).
10. MVPA
The presence of CHD and gender of children did not appear to interact with their MVPA (F = 1.00, p = .33) or the duration of undertaking MVPA (F = 0.62, p = .43), as revealed by two-way ANOVA. No significant difference was found in the 3-day average MVPA and duration of MVPA between the group with CHD and group with no CHD, whether in boys or girls, and during school days or weekends (Table 4).
11. PAL
There was no interaction between the presence of CHD and gender of children with their PAL (F = 0.82, p = .37).
The PAL of children did not differ between the group with CHD and group with no CHD (F = 3.03, p = .09).
However, girls had higher PAL than boys had (F = 35.03, pb .001), and this difference was consistent across school
days, weekends, and during the 3-day average recording period (Table 5).
12. Discussion
Children with CHD's cardiorespiratory function and exercise tolerance can be improved significantly after cardiac surgery (Lunt et al., 2003). Most children remain unrestricted in their physical activities after receiving surgery (Salzer- Muhar et al., 2002). In fact, exercise tolerance can be decreased due to reduced physical activity in some severe children with CHD or increased considerably if adequate physical activity is maintained (Paridon, 1997). The results of this study showed that the daily EE of boys with CHD was significantly lower than that of boys with no CHD, whereas no difference was observed between the girls with CHD and girls with CHD.
In our study, the weight of children with CHD was less than that of the children with no CHD. This may have an impact on the TEE measurements of our participants. Other reasons that can potentially affect the physical activity of children with CHD include parental, school teachers, treating doctors' attitudes toward the illness, and patients' gender differences. Parents of CHD children may be overprotective due to unfamiliarity with appropriate physical exercises, resulting in restricting participation of their children in MVPA and leading to reduced PALs (Bar-Mor et al., 2000).
Some studies showed that because many school teachers are concerned about students with CHD's safety, they often permit these children to attend the PE classes only after receiving a letter from the treating doctor (Olson et al.,
Fig. 4. Activity categories during weekends in girls with CHD and girls with no CHD.
Table 3
Comparison of TEE by RT3 (N = 68) Children with CHD group (n = 34)
Children with no CHD group (n = 34)
t p
Mean ± SD Mean ± SD
School days (kcal/day)
Boys (n = 17) 1657.5 ± 285.2 1968.3 ± 278.5 −3.22 .003 Girls (n = 17) 1759.7 ± 323.5 1801.5 ± 237.2 −0.43 .67 Weekends (kcal/day)
Boys (n = 17) 1544.5 ± 232.2 1823.8 ± 260.8 −3.30 .002 Girls (n = 17) 1669.9 ± 288.7 1685.9 ± 234.1 −0.18 .86 3-day average (kcal/day)
Boys (n = 17) 1582.2 ± 239.7 1872.0 ± 252.7 −3.43 .002 Girls (n = 17) 1699.8 ± 293.3 1724.5 ± 228.1 −0.27 .79
Table 4
Comparison of MVPA between the study groups (N = 68) Children with
CHD group (n = 34)
Children with no CHD group (n = 34)
t p
Mean ± SD Mean ± SD School days (kcal/day)
Boys (n = 17) 205.7 ± 201.1 362.3 ± 435.8 −1.35 .19 Girls (n = 17) 238.3 ± 374.8 232.5 ± 254.1 0.05 .96 Weekends (kcal/day)
Boys (n = 17) 180.1 ± 235.8 154.0 ± 158.5 0.38 .71 Girls (n = 17) 305.2 ± 361.6 182.7 ± 240.0 1.16 .25 3-day average (kcal/day)
Boys (n = 17) 188.6 ± 181.9 223.4 ± 201.6 −0.53 .60 Girls (n = 17) 282.9 ± 357.7 199.3 ± 210.9 0.83 .41
Table 5
Comparison of PALs between boys and girls (N = 68)
Boys (n = 34) Girls (n = 34) t p Mean ± SD Mean ± SD
School days (MJ/day) 1.36 ± 0.10 1.50 ± 0.12 −5.32 .001 Weekends (MJ/day) 1.26 ± 0.11 1.42 ± 0.13 −5.21 .001 3-day average (MJ/day) 1.29 ± 0.09 1.44 ± 0.12 −5.84 .001
2004). Moreover, anxious teachers may also limit these students' activities to participate in the PE classes (Nixon et al., 2001). If the treating doctor cannot give appropriate advice on physical activity and its benefits, parents' and school teachers' attitude may affect the children's illness and their physical fitness indirectly. In addition, future research should include measurements of exercise capacity, knowl- edge, and belief toward physical activity in both groups.
Fredriksen et al. (2000)also proposed that children with chronic illnesses have been found to be less physically active than children with no illnesses, and boys show notable differences particularly in cardiac patients. The forms and types of physical activities are different between boys and girls. Boys tend to undertake MVPA, whereas girls prefer relatively more sedentary activities (Telama & Yang, 2000).
As reflected in the analysis of the different physical activity categories in our study, we found that the boys with no CHD had significantly higher Category 9 physical activity in school compared to boys with CHD. The findings in our study of lower MVPA in boys with CHD during school days were consistent with those in the study byLunt et al. (2003).
But this difference was not observed in girls. During weekends, we found that the girls with no CHD had only higher Category 2 activity compared to the girls with CHD.
In this study, the mean EE of the two groups of girls was not significantly different, but the EE in boys with CHD during school days was significantly lower than that of their counterparts. The boys with CHD may falsely believe that they cannot engage in high-intensity exercises or worry about the possible cyanotic episodes (Lunt et al., 2003). The boys with CHD have been found to have relatively distorted self-perception compared to boys with no CHD, but such differences have not been observed in girls (Salzer-Muhar et al., 2002). The possible explanation for lower self- perception in boys with CHD is that they consider themselves unable, compared to their counterparts, to take part in all physical activities, especially when they are more generally expected to accomplish higher physical and psychological standards than those expected of girls. But these concepts should be further tested in future studies.
Data analysis of our study revealed a tendency for higher physical activity to occur during school days than during weekends in our participants. This finding is similar to those of previous studies (Fredriksen et al., 2000).Fredriksen et al.
(2000) concluded that children spend much time in bed during weekends; despite the fact that sedentary activities are common during school days, levels of physical activity are still higher than those during weekends. Moreover, Chinese students encounter heavy academic pressures, resulting in being less likely to take part in physical activities (Tudor- Lock, Ainsworth, Adair, Du, & Popkin, 2003). Taken together, we suggest that further studies are needed to explore the factors that may potentially influence the form of children's physical activities.
As further explored in this study, we found that the children's physical activity was not correlated to either the
presence of CHD or PE class, and in the boys' or the girls' groups, the total physical activity during school days did not differ significantly, even with attending PE classes. Based on these findings, we suggest that increasing frequency of PE classes in school and giving more opportunities for children to participate in sports or to exercise are needed.
The childrens' expenditure and BMR can be influenced by their body weight. The parameter of PAL (PAL = TEE ÷ BMR) adjusts EE for BMR which may control the impact of body weight. Overall, the PAL of our subjects did not differ between children with CHD and children with no CHD groups. The 3-day average PAL of the girls was higher than that of the boys, partially because the boys have higher BMR than the girls do (1330.6 ± 166.6 vs. 1182.4 ± 108.6 kcal/day, F = 8.46, p = .005). Furthermore, on the basis of the suggestion put forward by Hoos et al. (2003), the PAL of children over age 9 should be higher than 1.63 MJ/day (0.025 × age + 1.40). The 3-day average PAL of our sample was lower than both recommended value and the average of many European countries (Ekelund et al., 2001), and the PAL of boys were lower than that of girls. Based on this result, we suggest that both children with CHD and children with no CHD, especially the boys who need to engage in more physical activity.
Limitations of the study are as follows: (a) Eligible subjects of this study had to be over the age of 9 years and had to receive a complete surgical repair of the heart for more than 1 year. Although a matched sample was used in this study, the samples were restricted to the area of Taiwan.
Further study should include larger representative samples that can be evaluated over extended periods of time; (b) In this study, the 3DPAR and RT3 were simultaneously used as research tools to measure the physical activities of the study participants. A discrepancy among the readings obtained by each measurement tool existed. This may be attributed to two reasons: (a) Some physical activities were not categorized for 3DPAR measurements; and (b) RT3 could not be worn in the water, preventing assessment of any water-related activities (including swimming and showering). However, no swim- ming activity was reported by our study participants.
Children who have inadequate physical activities can have the risk of becoming sedentary adults and of having associated latent lifestyle disease considerably. In this study, both objective and subjective measures were applied to assess physical activity of children with CHD. We found that overall PALs of both children with CHD and children with no CHD are yet to be improved to meet the recommended global standards, particularly in boys. Parents and teachers of boys with CHD may worry about the children's health and probably further limit patients in participating in activities.
Therefore, promoting increased physical activity in this group should be implemented. We should actively engage in promoting awareness and giving education for children with CHD and their parents about adequate sports and exercises as well as encouraging children to participate in a wide range of physical activities. Results of this study may add to the
body of knowledge in the area of pediatric cardiology and the quality of life of school-age children following open heart surgery.
Acknowledgments
This study was supported by a grant from the Taipei Medical University-Wan Fang Medical Center.
References
Bar-Mor, G., Bar-Tal, Y., Krulik, T., & Zeevi, B. (2000). Self-efficacy and physical activity in adolescents with trivial, mild, or moderate congenital cardiac malformations. Cardiology in the Young, 10, 561–566.
Boreham, C., Twisk, J., Neville, C., Savage, M., Murray, L., & Gallagher, A.
(2002). Associations between physical fitness and activity patterns during adolescence and cardiovascular risk factors in young adulthood:
The Northern Ireland Young Hearts Project. International Journal of Sports Medicine, 23, S22–S26.
Bouchard, C., Tremblay, A., Leblanc, C., Lortie, G., Savard, R., & Theriault, G. (1983). A method to assess energy expenditure in children and adults.
American Journal of Clinical Nutrition, 37, 461–467.
Calzolari, A., Giordano, U., Di Giacinto, B., & Turchetta, A. (2001).
Exercise and sports participation after surgery for congenital heart disease: The European perspective. Italian Heart Journal, 2, 736–739.
Dent, J. M. (2003). Congenital heart disease and exercise. Clinics in Sports Medicine, 22, 81–99.
Ekelund, U., Sjostrom, M., Yngve, A., Poortvliet, E., Nilsson, A., Froberg, K, Wedderkopp, N., & Westerterp, K. (2001). Physical activity assessed by activity monitor and doubly labeled water in children. Medicine &
Science in Sports & Exercise, 33, 275–281.
Fredriksen, P. M., Ingjer, F., Nystad, W., & Thaulow, E. (1999). A comparison of VO2 (peak) between patients with congenital heart disease and healthy subjects, all aged 8–17 years. European Journal of Applied Physiology & Occupational Physiology, 80, 409–416.
Fredriksen, P. M., Ingjer, F., & Thaulow, E. (2000). Physical activity in children and adolescents with congenital heart disease. Aspects of measurements with an activity monitor. Cardiology in the Young, 10, 98–106.
Hirth, A., Reybrouck, T., Bjarnason-Wehrens, B., Lawrenz, W., &
Hoffmann, A. (2006). Recommendations for participation in competitive and leisure sports in patients with congenital heart disease: A consensus document. European Journal of Cardiovascular Prevention and Rehabilitation, 13, 293–299.
Hoos, M. B., Gerver, W. J., Kester, A. D., & Westerterp, K. R. (2003).
Physical activity levels in children and adolescents. International Journal of Obesity & Related Metabolic Disorders: Journal of the International Association for the Study of Obesity, 27, 605–609.
Huang, Y. C., & Malina, R. M. (2002). Physical activity and health-related physical fitness in Taiwanese adolescents. Journal of Physiological Anthropology & Applied Human Science, 21, 11–19.
Jakicic, J. M., Winter, C., Lagally, K., Ho, J., Roberson, R. J., & Wing, R. R. (1999). The accuracy of the RriTrac-R3D accelerometer to
estimate energy expenditure. Medicine & Science in Sports &
Exercise, 31, 747–754.
James, W. P. T., Ferro-Luzzi, A., & Waterlow, J. C. (1988). Definition of chronic energy deficiency in adults. Report of a working party of the International Dietary Energy Consultative Group. European Journal of Clinical Nutrition, 42, 969–981.
Kao, Y. L., Wang, R. H., Pai, L., & Hwang, B. T. (2000). Life adjustment of school-aged children with congenital heart disease after corrective surgery. The Journal of Nursing (Chinese), 47, 43–54.
Katzmarzyk, P. T., Malina, R. M., Song, T. M., & Bouchard, C. (1998).
Television viewing, physical activity, and health-related fitness of youth in the Quebec family study. Journal of Adolescent Health, 23, 318–325.
Liou, Y. M., & Chiang, L. C. (2004). Levels of physical activity among school-age children in Taiwan: A comparison with international recommendations. Journal of Nursing Research, 12, 307–316.
Lunt, D., Briffa, T., Briffa, N. K., & Ramsay, J. (2003). Physical activity levels of adolescents with congenital heart disease. Australian Journal of Physiotherapy, 49, 43–50.
Nixon, P. A., Orenstein, D. M., & Kelsey, S. F. (2001). Habitual physical activity in children and adolescents with cystic fibrosis. Medicine &
Science in Sports & Exercise, 33, 30–35.
Olson, A. L., Seidler, A. B., Goodman, D., Gaelic, S., & Nordgren, R.
(2004). School professionals' perceptions about the impact of chronic illness in the classroom. Archives of Pediatrics & Adolescent Medicine, 158, 53–58.
Park, M. K. (2002). Pediatric Cardiology for Practitioners. (4th ed., pp. 98–240). St. Louis: Mosby.
Paridon, S. M. (1997). Congenital heart disease: Cardiac performance and adaptations to exercise. Pediatric Exercise Science, 9, 308–323.
Reybrouck, T., & Mertens, L. (2005). Physical performance and physical activity in grown-up congenital heart disease. European Journal of Cardiovascular Prevention and Rehabilitation, 12, 498–502.
Salzer-Muhar, U., Herle, M., Floquet, P., Freilinger, M., Greber-Platzer, S., Haller, A., Leixnering, W., Marx, M., Wurst, E., & Schlemmer, M.
(2002). Self-concept in male and female adolescents with congenital heart disease. Clinical Pediatrics, 41, 17–24.
Schofield, W. N. (1985). Predicting basal metabolic rate, new standards and review of previous work. Human Nutritio-Clinical Nutrition, 39(Suppl), 5–41.
Sirard, J. R., & Pate, R. R. (2001). Physical activity assessment in children and adolescents. Sports Medicine, 31, 439–454.
Telama, R., & Yang, X. (2000). Decline of physical activity from youth to young adulthood in Finland. Medicine & Science in Sports & Exercise, 32, 1617–1622.
Tudor-Locke, C., Ainsworth, B. E., Adair, L. S., Du, S., & Popkin, B. M.
(2003). Physical activity and inactivity in Chinese school-aged youth:
The China Health and Nutrition Survey. International Journal of Obesity & Related Metabolic Disorders: Journal of the International Association for the Study of Obesity, 27, 1093–1099.
Twisk, J. W., Kemper, H. C., & van Mechelen, W. (2000). Tracking of activity and fitness and the relationship with cardiovascular disease risk factors. Medicine & Science in Sports & Exercise, 32, 1455–1461.
Welk, G. J., & Corbin, C. B. (1995). The validity of the Tritrac-R3D activity monitor for the assessment of physical activity in children. Research Quarterly for Exercise & Sport, 66, 202–209.