Photosynthesis Related Characteristics of Upper and Lower Canopy Leaves of Kandelia obovata, a Mangrove Species in Taiwan
全文
(2) 182. TAIWANIA. parameters in response to light reveal how plants acclimate to variation in light intensity in their environment (Ishida et al., 1999; Ishida et al., 2001). In this study, the Chl fluorescence technique was used to assess the efficiency of photosynthetic energy conversion by the upper and lower canopy leaves of K. obovata. In addition to leaf angle, leaf size and chlorophyll content also affect light interception, and nitrogen content affect photosynthetic activity. Hence, in this study I also measured these photosynthetic related characters. The objective of this study was to understand better the relationship between light-use properties and leaf display of K. obovata in the saline environment. I tested the hypothesis that morphological and physiological differences occur between upper and lower canopy leaves as a result of light environment.. MATERIALS AND METHODS Twenty-four branches, 12 from upper (about 3 m height) and 12 from lower canopy, of K. obovata were collected from the mangrove forest in Waltz-wei Natural Reserve (25˚10’ N, 121˚24’ E), Taipei County in Oct., 1999. As soon as the branches were detached, they were placed in water and brought back to the laboratory. The most recent fully expanded leaves (the third or fourth pair of leaves) from each branch were used for following analyses. Leaf angles from the horizontal were measured by aligning a hand-held clinometer (Suunto Co. PM-5/360 PC) with the plane of the lamina. Twelve leaves, 6 from upper and 6 from lower canopy, were chosen for fluorescence measurements. To measure the fluorescence-PFD (photosynthetic photon flux density) response, leaves were held horizontally in a leaf-clip holder (2030-B, Walz, Effeltrich, Germany) (Biger et al., 1995). The PFD on leaves, provided by a halogen lamp (2050-H, Walz, Effeltrich, Germany), was adjusted from darkness to 1600 μmol m-2 s-1 in steps of 50-200 μmol m-2 s-1. The halogen lamp was equipped with a heat-reflectance filter to reduce heat generated by the lamp. PFD on the leaf was monitored with a microquantum sensor installed on the leaf-clip holder next to the spot where fluorescence is measured. After the leaf was exposed to the desired PFD for 10 min, the Chl a fluorescence of PSII was measured using a portable, pulsed amplitude modulated fluorometer (PAM 2000, Walz, Effeltrich, Germany). The values of Fv/Fm, Fv’/Fm’, the effective quantum yield of PSII. Vol. 51, No. 3. ( Φ II = qP * Fv’/Fm’), and the coefficient of photochemical and non-photochemical quenching [qP = (Fm’-Ft)/(Fm’-Fo’), qN = (Fm-Fm’)/ (Fm-Fo’)], were computed (Schreiber, Schlina and Bilger, 1986), where Fv and Fm are the variable and maximal fluorescence, respectively, in dark adapted state, Fm’ is the maximal fluorescence, Fo’ is minimal fluorescence, Fv’ is the difference between Fm’ and Fo’, and Ft is the steady-state fluorescence in the light adapted state. During the fluorescence measurement, room temperature was maintained at 26℃ while the leaf temperature was not controlled. Following fluorescence measurement, a 2 * 2 cm2 area from each experimental leaf was collected and total leaf Chl concentration was measured by extracting Chl from the leaf segment with 96% ethyl alcohol, and subsequent spectrophotometric (Model V-560, Jasco, Tokyo, Japan) analysis of the extract at wave-lengths of 649 and 665 nm (Wintermans and Mots, 1965). A thin layer of nail polish was applied to the lower surface of a segment of the experimental leaves (n = 6) for estimates of stomatal density. After drying, the nail polish was peeled and scanned at 100 x magnification with a light microscope equipped with a calibrated ocular micrometer. The area of leaves (n = 12, for each canopy layer) was determined with an area meter (LI-3100, Li-Cor, Nebraska), and each leaf was dried at 60℃ for 48 h and then weighed. The specific leaf area (SLA) was calculated as leaf area per unit dry mass. These samples were then ground to a fine powder with a mortar and pestle. Total nitrogen (N) and carbon contents of samples were determined with an elemental analyzer (NA 1500, Fisons, Italy). All statistical tests were performed with the computer software SYSTAT (statistical Solution, Cork, Ireland). Significant differences are reported as P < 0.05.. RESULTS Leaves that had developed in exposed positions (upper canopy) exhibited significantly steeper leaf angles, smaller leaf area, less SLA and higher stomatal density than those in shaded positions (lower canopy) (Table 1). Lower canopy leaves had a 86 % and a 61 % increase in leaf area and SLA, respectively. No significant difference in Chl a/b ratio and nitrogen (N) content per unit leaf dry weight was found between leaves on upper and lower canopy (Table 1). However, upper canopy leaves had significantly higher N content per unit leaf area. In.
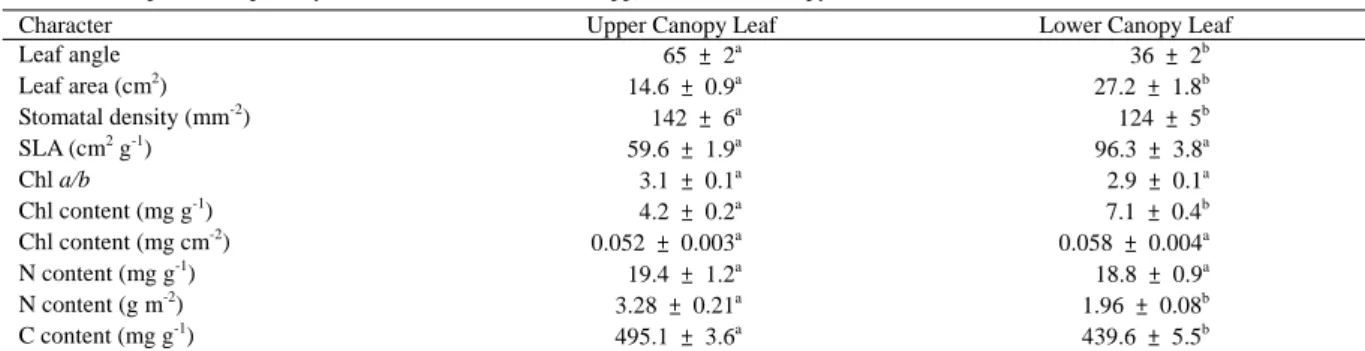
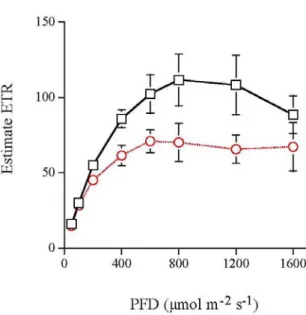
(3) September, 2006. Kao: Photosynthesis of Kandelia obovata. 183. Table 1. A comparison of photosynthesis related characters of upper and lower canopy leaves of K. obovata (mean ± s. e.). Character Upper Canopy Leaf Leaf angle 65 ± 2a Leaf area (cm2) 14.6 ± 0.9a Stomatal density (mm-2) 142 ± 6a SLA (cm2 g-1) 59.6 ± 1.9a Chl a/b 3.1 ± 0.1a Chl content (mg g-1) 4.2 ± 0.2a Chl content (mg cm-2) 0.052 ± 0.003a N content (mg g-1) 19.4 ± 1.2a N content (g m-2) 3.28 ± 0.21a C content (mg g-1) 495.1 ± 3.6a * Means within a row followed by different superscripts are different at P = 0.05 (t-test). contrast, lower canopy leaves had significantly higher Chl content per unit leaf dry weight than upper canopy leaves, however, no significant difference was found in Chl content per unit leaf area between the two layers of leaves (Table 1). Thus, variation in leaf Chl and N contents was mainly caused by variation in SLA. Upper and lower canopy leaves had similar potential quantum yield of 0.81 ± 0.02 and 0.82 ± 0.02 (mean ± s. e., n = 6), respectively, in darkadapted condition. Under illumination, Fv’/Fm’ and qP decreased with increasing PFD (Fig. 1A, B). The reduction rates in Fv’/Fm’ and qP in response to increasing PDF were faster in lower canopy leaves than in higher canopy leaves. Consequently, at PFD > 200 μmol m-2 s-1, upper canopy leaves had significantly higher qP and ΦII ( = (Fv’/Fm’) * qP) than lower canopy leaves compared at the same PFD (Figs. 1B and C). qN increased with increasing PFD and a more rapid rise in qN was measured in lower canopy leaves. Consequently, at PFDs of 200, 400, 600 and 800 μmol m-2 s-1, lower canopy leaves had significantly higher qN than upper canopy leaves. qN saturated at PFDs of 800 and 1600 μmol m-2 s-1 for lower and upper canopy leaves, respectively (Fig. 1B). No significant difference in light saturated qN was found between upper and lower canopy leaves, indicating similar capacity for non-photochemical quenching. Both types of leaves had similar area based chlorophyll content and Chl a/b ratio (Table 1) indicating similar leaf absorptance. Hence, a standard leaf absorptance value of 0.84 was used and the electron transport rate through PSII was estimated (ETR = ΦII * PFD * 0.5 * 0.84) (Genty et al., 1989). Figure 2 shows that the estimate ETR of upper canopy leaves increased with increasing PFD, reached saturation at PFD between 800 and 1200 μmol m-2 s-1, then declined at PFD of 1600 μmol m-2 s-1. The estimate ETR of lower canopy leaves also increased with increasing PFD, reached. Lower Canopy Leaf 36 ± 2b 27.2 ± 1.8b 124 ± 5b 96.3 ± 3.8a 2.9 ± 0.1a 7.1 ± 0.4b 0.058 ± 0.004a 18.8 ± 0.9a 1.96 ± 0.08b 439.6 ± 5.5b. saturation at PFD of 600 μmol m-2 s-1, then remained constant as PFD increased further to 1600 μmol m-2 s-1. The light-saturated ETR was significantly higher in upper canopy leaves than in lower canopy leaves.. DISCUSSION Plants exhibit a wide range of adaptation, including biochemical, physiological and morphological adjustment that enables them to live successfully in different habitats. Results of this study show that morphological and physiological differences occur between upper and lower canopy leaves of K. obovata. The plastic response may confer this plant to grow in the tidal waters. Leaves that had developed in upper canopy exhibited significantly steeper leaf angles, smaller leaf area and higher stomatal density than those in lower canopy (Table 1). Excessive irradiance and high temperature can adversely affect photosynthesis resulting in photoinhibition, particularly if other factors are not optimal. Upper canopy leaves of K. obovata may avoid conditions that predispose them to photoinhibition by reducing leaf size and developing leaves with steeper leaf angle. A steeper leaf angle would decrease the interception of excess light hence reduce leaf temperature, water loss and photoinhibition. In addition, steep leaf angles in the upper canopy leaves enhance light penetration in the canopy (Ehleringer and Werk, 1986; Forseth and Teramura, 1986; Gamon and Peracy, 1990). A reduction in leaf size lowers boundary layer resistance and provides more effective convective heat loss to the surrounding air (Givnish, 1987). In addition, increasing stomatal density would potentially increase transpirational heat dissipation and CO2 uptake. In contrast to upper canopy leaves, leaves on the lower canopy their photosynthetic activity are more limited by light availability. The morphological characters of these leaves, more horizontally positioned, larger leaf area and higher SLA, would enhance their light interception..
(4) 184. TAIWANIA. Vol. 51, No. 3. Fig. 2. Response of electron transport rate through PSII, estimated from chlorophyll fluorescence measurement, of upper canopy (square) and lower canopy (circle) leaves of K. obovata to photosynthetic photon flux density (PFD) at the leaf surface. Bars represent s. e. (n = 6).. Fig. 1. The efficiency of excitation capture by open PS II reaction centers (Fv’/Fm’) (A), photochemical (open symbols) and non-photochemical (closed symbols) quenching coefficients (B), and effective quantum yield of PSII (ΦII) (C) of upper canopy (square) and lower canopy (circle) leaves of K. obovata in response to photosynthetic photon flux density (PFD) at the leaf surface. Bars represent s. e. (n = 6).. No significant difference was found in Chl a/b ratio and Chl content per unit leaf area (Table 1) between upper and lower canopy leaves, which would reflect a similar light absorptance. In contrast, Chl fluorescence measurement revealed that upper canopy leaves had significantly higher qP and ΦII than lower canopy leaves compared at the same PFD (Figs. 1B and C), which indicates that upper canopy leaves adjust their photochemical capacity to maintain more reaction centers in an open (unreduced) state. Consequently, a higher photosaturated ETR was found in upper canopy leaves. The ETR values may be underestimated, since the absorptance value of K. obovata could be higher than the standard value (0.84) used to estimate ETR. Nevertheless, the estimates of ETR are valid for comparison purpose. Positive correlation between photosatureated ETR and light-saturated rates of whole-leaf photosynthetic CO2 uptake (Amax) was found (Krall and Edwards, 1992). Accordingly, fluorescence measurement suggests that upper canopy may have higher Amax than lower canopy leaves. Leaf N content analysis supports this suggestion. A positive correlation between leaf N content and photosynthetic capacity has been reported in a wide variety of plant species (Field and Mooney, 1986). The result that more nitrogen was allocated to upper canopy leaves than to lower canopy leaves (per unit area) (Table 1) suggest that upper canopy leaves had higher photosynthetic capacity. Mooney and Ehleringer (1978) have shown that leaves with high photosynthetic capacities can.
(5) September, 2006. Kao: Photosynthesis of Kandelia obovata. have a high daily carbon in high-light environment. The higher carbon contents measured in upper canopy leaves than in lower canopy leaves (Table 1) may reflect this correlation. Thus, the differential distribution of N between upper and lower canopy leaves may increase the whole canopy carbon gain. In addition, the growth of K. obovata in tidal waters has been shown to be limited by nitrogen (Kao and Chang, 1998; Kao et al., 2001). Accordingly, it is expected that the plant would try to optimize its nitrogen use efficiency in this environment. Variation in foliar N content associate with different light environments results in a higher carbon gain for the whole canopy than for a canopy in which N content is uniformly distributed among leaves (Hirose and Werger, 1987; Elsworth and Reich, 1993, Evens, 1993; Hollinger, 1996). Thus, by allocating different N content to canopy leaves receiving different light regime, K. obovata may also increase photosynthetic N use efficiency for the whole canopy. The result that lower canopy leaves had higher qN than upper canopy leaves at intermediate PFDs suggests that more energy is dissipated through non-photochemical quenching in lower canopy leaves than in higher canopy leaves at intermediate PFDs, due to the lower photochemistry ability of lower canopy leaves (indicating by lower qP and lower Fv’/Fm’). However, both type of leaves have similar light saturated qN indicating similar capacity of non-photochemical quenching ability. The ability of non-photochemistry quenching is related to the pool of xanthophylls (Demmig-Adams and Adams, 1992). Accordingly, the upper and lower canopy leaves of K. obovata may have similar size of xanthophylls pool. Increasing light stress was found to increase the pool size of the xanthophylls cycle (Demmig-Adams and Adams, 1996). Consequently, upper canopy leaves were found to have higher pool size of the xanthophylls cycle than lower canopy leaves in Rhizophora apiculata mangrove forest (Lovelock and Clough, 1992) and in a tropical tree species, Acacia crassicarpa (Liu, et al., 2003). However, for K. obovata a reduction of the amount of solar radiation through the leaf inclination and the higher capacity of photochemical and assimilation activity in upper canopy leaves may reduce the need for increasing the size of xanthophyll pool in its upper canopy leaves. This could potentially save resource allocation to protect photosynthetic apparatus from light stress. In conclusion, the plasticity in morphological and physiological response to variation in light regimes may represent an important mechanism for K. obovata to adapt to the saline and nitrogen. 185. limiting environment. The hypothesis remains to be tested.. LITERATURE CITED Bilger, W., U. Schreiver and M. Bock. 1995. Determination of the quantum efficiency of photosystem II and non-photochemical quenching of chlorophyll fluorescence in the field. Oecologia 102: 425-432. Björkman, O. and B. Demmig-Adams. 1994. Regulation of Photosynthetic Light Energy capture, Conversion, and Dissipation in Leaves of Higher Plants. In: Schulze, E. D. and M. M. Caldwell (eds.), Ecophysiology of Photosynthesis. Springer-Verlag, Berlin, Germany. pp. 17-44. Björkman, O. and S. B. Powles. 1984. Inhibition of photosynthetic reactions under water stress: interaction with light level. Planta 161: 490-504. Demmig-Adams, B. and W. W. Adams III. 1992. Photoprotection and other responses of plants to high light stress. Ann. Rev. Plant Physiol. Plant Mol. Biol. 43: 599-626. Demmig-Adams, B. and W. W. Adams III. 1996. Cholorophyll and carotenoid composition in leaves of Euonymus kiautschovicus acclimated to different degrees of light stress in the field. Aust. J. Plant Physiol. 23: 649-659. Ehleringer, J. R. and K. S. Werk. 1986. Modifications of Solar-radiation Absorption Patterns and Implications for Carbon Gain at the Leaf Level. In: Givnish, T. J. (ed.), On the Economy of Plant Form and Function. Cambridge University Press, Cambridge, USA. pp. 57-82. Ellsworth, D. S. and P. B. Reich. 1993. Canopy structure and vertical patterns of photosynthesis and related leaf traits in a deciduous forest. Oecologia 96: 169-178. Evans, J. R. 1993. Photosynthetic acclimation and nitrogen partitioning within a Lucerne canopy. II. Stability through time and comparison with a theoretical optimum. Aust. J. Plant Physiol. 20: 69-82. Field, C. and H. A. Mooney. 1986. The Photosynthesis-Nitrogen Relationship in Wild plants. In: Givnish, T. J. (ed.), On the Economy of Plant Form and Function. Cambridge University Press, Cambridge, USA. pp. 25-55. Forseth, I. N. and A. H. Teramura. 1986. Kudzu leaf energy budget and calculated transpiration: the influence of leaflet orientation. Ecology 67: 564-571..
(6) 186. TAIWANIA. Gamon, J. A. and R. W. Pearcy. 1990. Photoinhibition in Vitis californica: interactive effects of sunlight, temperature and water status. Plant, Cell Environ. 13: 267-275. Genty, B., J. M. Briantais and N. R. Baker. 1989. The relationship between quantum yield of photosynthetic electron transport and quenching of chlorophyll fluorescence. Biochimica. et. Biophysica. Acta. 990: 87-92. Givnish, T. J. 1986. Comparative studies of leaf form: assessing the relative roles of selective pressures and phylogenetic constrains. New Phytol. 106: 131-160. Hirose, T. and M. J. A. Werger. 1987. Maximizing daily canopy photosynthesis with respect to the leaf nitrogen allocation pattern in the canopy. Oecologia 72: 520-526. Hollinger, D. Y. 1996. Optimality and nitrogen allocation in a tree canopy. Tree Physiol. 16: 627-634. Huang, Y.-H. and S.-C. Chen. 1995. Salt tolerance in seedlings of the mangrove Kandelia candel Druce, Rhizophoraceae. Bot. Bull. Acad. Sin. 36: 25-31. Ishida, A., T. Toma and Marjenah. 1999. Leaf gas exchange and chlorophyll fluorescence in relation to leaf angle, azimuth, and canopy position in the tropical pioneer tree, Macaranga conifera. Tree Physiol. 19: 117-124. Ishida, A., T. Nakano, A. Uemura, N. Yamashita, H. Tanabe and N. Koike. 2001. Light-use properties in two sun-adapted shrubs with contrasting canopy structures. Tree Physiol. 21: 497-504. Kao, W.-Y. and I. N. Forseth. 1992. Diurnal leaf movement, chlorophyll fluorescence and carbon assimilation in soybean grown under different nitrogen and water availability. Plant Cell Environ. 15: 703-710. Kao, W.-Y. and K.-W. Chang. 1998. Stable carbon isotope ratio and nutrient contents of the Kandelia candel mangrove populations of different growth forms. Bot. Bull. Acad. Sin. 39: 39-45. Kao, W.-Y., H.-C. Tsai and T.-T. Tsai. 2001. Effect of NaCl and nitrogen availability on growth and photosynthesis of seedlings of a mangrove species, Kandelia candel (L.) Druce. J. Plant Physiol. 158: 841-846. Krall, J. P. and G. E. Edwards. 1992. Relationship between photosystem II activity and CO2 fixation. Physiol. Plant. 86: 180-187. Krause, G. H. and E. Weis. 1991. Chlorophyll fluorescence and photosynthesis: the basics. Ann. Rev. Plant Physiol. Plant Mol. Biol. 42: 313-349.. Vol. 51, No. 3. Küppers, M. 1989. Ecological significance of above-ground architectural patterns in woody plants: a question of cost-benefit relationships. Trend Ecol. Evol. 4: 375-379. Liu, T.-S. 1982. The mangrove in Taiwan. Quart. J. Chinese For. 15: 9-16. Liu, L.-X., S.-M. Xu and K.-C. Woo. 2003. Influence of leaf angle on photosynthesis and the xanthophylls cycle in the tropical tree species Acacia crassicarpa. Tree Physiol. 23: 1255-1261. Lovelock, C. E. and B. F. Clough. 1992. Influence of solar radiation and leaf angle on leaf xanthophyll concentrations in mangroves. Oecologia 91: 518-525. Mooney, H. A. and J. R. Ehleringer. 1978. The carbon gain benefits of solar tracking in a desert annual. Plant Cell Environ. 1: 307-311. Sheue, C.-R., H.-Y. Liu and J. W. H. Yong. 2003. Kandelia obovata (Rhizophoracear), a new mangrove species from Eastern Asia. Taxon 52: 287-294. Wintermans, J. F. and A. Mots. 1965. Spectrophotometric characteristics of chlorophyll a and b and their pheophytins in ethanol. Biochem. Biophys. Acta. 109: 448-453..
(7) September, 2006. Kao: Photosynthesis of Kandelia obovata. 187. 水筆仔樹林上、下層葉光合作用相關特徵比較 高文媛(1) (收稿日期:2006 年 2 月 2 日;接受日期:2006 年 5 月 12 日). 摘. 要. 水筆仔是北台灣西岸主要紅樹林樹種。水筆仔樹林的上層葉和下層葉可能因為接受 到的光量不同,導致其在形態和生理反應上有所調整。本文比較該樹林上、下層葉的葉 面角度、葉面積大小、氣孔密度、單位重葉面積、葉綠素螢光等與光合作用相關特徵, 以了解水筆仔樹林對光使用特性。結果發現上、下層葉在形態上有顯著差異:上層葉葉 面角度較大、面積較小、氣孔密度較高以及單位重葉面積較小。雖然兩者單位面積葉綠 素含量和葉綠素 a/b 比沒有顯著差異,但上層葉單位面積有顯著較高的氮含量。葉綠素 螢光測量顯示:相較於下層葉,上層葉其電子傳遞鏈速率在較高光量下才會達到飽和且 有顯著較高的光飽和值。這些特徵差異顯示水筆仔為因應不同光環境,在葉形態和生理 上有所調整。 關鍵詞:紅樹林、水筆仔、光合作用、葉綠素螢光。. ___________________________________________________________________________ 1. 國立臺灣大學生命科學系、生態學與演化生物學研究所,106 台北市羅斯福路 4 段 1 號,臺灣。 Tel: 886-2-33662511; Email: wykao@ntu.edu.tw.
(8)
數據
相關文件
The first row shows the eyespot with white inner ring, black middle ring, and yellow outer ring in Bicyclus anynana.. The second row provides the eyespot with black inner ring
The existence of transmission eigenvalues is closely related to the validity of some reconstruction methods for the inverse scattering problems in an inhomogeneous medium such as
The function f (m, n) is introduced as the minimum number of lolis required in a loli field problem. We also obtained a detailed specific result of some numbers and the upper bound of
HPM practice in Taiwan: A case study of HPM Tongxun (HPM Newsletter). These articles have documented the process of development and evolution of HPM practice in Taiwan as well
HPM practice in Taiwan: A case study of HPM Tongxun (HPM Newsletter). These articles have documented the process of development and evolution of HPM practice in Taiwan as well
(Another example of close harmony is the four-bar unaccompanied vocal introduction to “Paperback Writer”, a somewhat later Beatles song.) Overall, Lennon’s and McCartney’s
As for current situation and characteristics of coastal area in Hisn-Chu City, the coefficients of every objective function are derived, and the objective functions of
The high density of mangrove might bring negative impacts which include that tidal flow prevention, landformation and coastal habitat deterioration.. In this study, the