Elemental composition of otoliths as a discriminator of life stage
and growth habitat of the European eel, Anguilla anguilla
W. N. Tzeng
A,D, K. P. Severin
B, C. H. Wang
Aand H. Wickström
CA
Institute of Fisheries Science, College of Life Science, National Taiwan University, Taipei 106, Taiwan.
BDepartment of Geology and Geophysics, University of Alaska Fairbanks, Alaska 99775-0760, USA.
C
National Board of Fisheries, Institute of Freshwater Research, SE-178 93 Drottningholm, Sweden.
DCorresponding author. Email: wnt@ccms.ntu.edu.tw
Abstract. The hypothesis that elemental composition of otoliths of the eel (Anguilla spp.) changes with life stage
and growth habitat was tested in the present study. The minor elements Cl, Na, K, Mg, Ca, Sr and P in otoliths
of European eels (Anguilla anguilla) were examined by using an Electron Probe Microanalyser (EPMA) equipped
with wavelength dispersive spectrometers (Cameca SX-50). Yellow-stage eels were collected from coastal waters
and lakes of Sweden in 1987, 1988, 1991, and 1994, with ages ranging from 5 to 18 years old. Strontium maps
and profiles of Sr : Ca ratio, as well as the elver check in otoliths, were used to classify life history stages of
the eels as leptocephalus, and freshwater- and seawater-resident yellow eels. Canonical score plots of the otolith
elemental compositions of the freshwater-resident yellow eel were completely separated from those of leptocephalus
and seawater-resident yellow eel, but the latter two partially overlapped. Strontium is the primary component in
determining the discrimination, but the nutrient-related (S and P), and the physiologically controlled elements
(Na and Cl), may also play an important role in the discrimination. These results indicate that multiple-elemental
information can provide additional insight into the migratory environmental history of diadromous fishes.
Extra keywords: electron probe microanalysis, microchemistry.
Introduction
The European eel, Anguilla anguilla (L.), is a diadromous
fish, widely distributed in freshwater and marine littoral areas
of North Africa, the Mediterranean Sea, the British Isles,
Iceland, and the western and northern European continent
(Tesch 2003). After spawning in the Sargasso Sea, the
lepto-cephali are transported by the North Equatorial Current, Gulf
Stream and North Atlantic Current to the continental shelf of
northern European countries (Bertin 1956). The larvae
meta-morphose into glass eels in coastal waters. Glass eels become
pigmented elvers when they enter estuaries. Their migration
from the Sargasso Sea to the estuaries requires 6–9 months
(Lecomte-Finiger 1992), or 14–16 months (Wang and Tzeng
2000). Male eels grow in rivers for 3–7 years, whereas female
eels grow from 4 to 15 years in rivers (Vollestad and Jonsson
1986). In late autumn, they transform from the yellow eel
into silver eel stage and start the downstream migration back
to the Sargasso Sea. Recent studies on Sr (strontium) : Ca
(calcium) ratios in otoliths indicate that a part of the eel
popu-lation may skip the freshwater life of the yellow eel phase and
can complete their entire life history in the seawater (Tzeng
et al. 1997, 2000; Tsukamoto et al. 1998; Tsukamoto and
Arai 2001).
Ratios of Sr : Ca in the otolith of anguillid eels
dramati-cally change at metamorphosis from leptocephalus to glass
eel during their migration from the ocean to the river (Otake
et al. 1994; Tzeng and Tsai 1994; Arai et al. 1997). This
drastic change in Sr/Ca ratios was proposed to be related to
metamorphosis rather than the transition of habitat from
sea-water to fresh sea-water (Tzeng 1996). In contrast, the Sr : Ca
ratios in otoliths of yellow eels changed significantly when
the eel migrated between fresh water and seawater (Tzeng
et al. 1997, 2002, 2003a, 2003b; Jessop et al. 2002, 2004;
Kraus and Secor 2003; Limburg et al. 2003; Shiao et al. 2003;
Cairns et al. 2004). These indicated that the changes in otolith
microchemistry throughout their life history of the eel were
more complicated than our current understanding.
There-fore, it is important to examine how elemental composition
of eel otoliths is influenced by both ontogenetic
develop-ments and habitat shift before using the elemental signature
to reconstruct their migratory environmental history.
In the present study, elements in addition to Ca and Sr
were measured to further understand the change in elemental
composition of otoliths in European eels during
metamor-phosis from leptocephalus to glass eel and during migration
of yellow-phase eels between fresh water and seawater.
Materials and methods
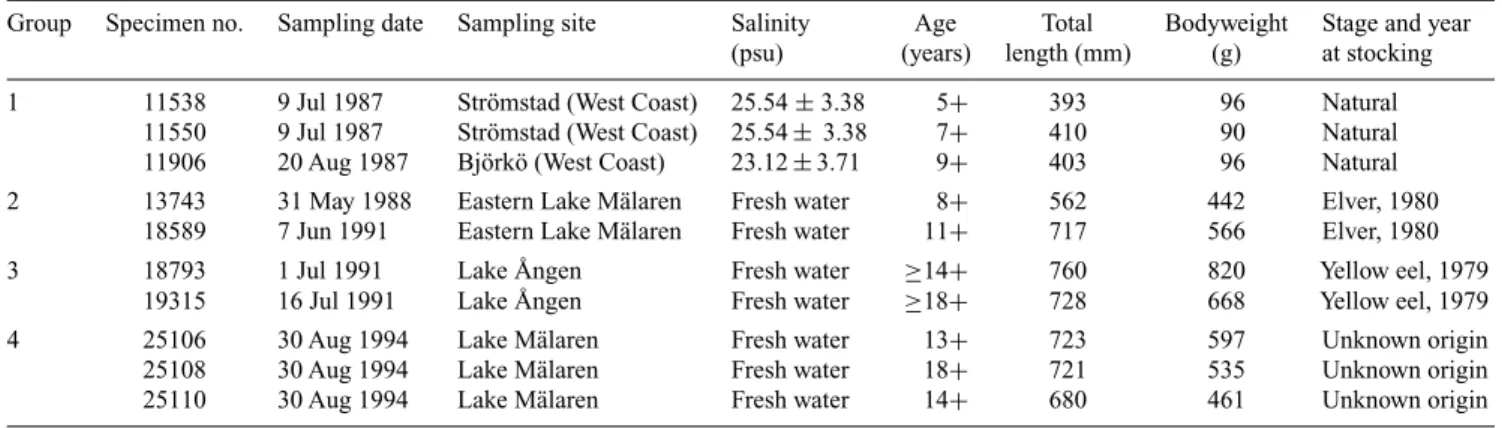
Sampling designsTen otoliths of European eels collected from three freshwater lakes and two brackish estuaries in Sweden were used for microchemical analysis. These samples were classified into four groups according to sampling sites (Tzeng et al. 1997). The origin of groups 1 through 3 was clear: these eels were captured from areas where the restocking programme is well known. The individuals of group 4 were collected from a site some kilometres away from Eastern Lake Mälaren in 1994, and the origin of this group was unknown. They were derived either from a natural population that had migrated from brackish Baltic Sea, or from a stocked population caught from brackish waters on the west coast of Sweden and released at the yellow eel stage at∼40 cm in total length, or from a stocked population released at the elver stage imported from France or the British Isles. The stocking and sampling dates and biological characteristics of the four groups of eels (including sampling date, mean (± s.d.) salinity of sampling sites, stage, age, total length, bodyweight, and the stage and year at stocking) are given in Table 1.
Microchemical analysis
After removal from the eel, the otoliths were cleaned with distilled water, dried in air, embedded in thermo-epoxy (Petropoxy 154; Palouse Petro Products, Pullman, WA) and cured for 1 h at 135◦C. Embedded otoliths were ground from the proximal side of the sagittal plane of the fish until the primordium of the otolith was revealed. For microprobe analysis, the polished otoliths were coated under vacuum with a 30 nm layer of carbon to increase electron conductance.
The elemental composition of the eel otolith was measured with an electron probe microanalyser (EPMA) equipped with wavelength dispersive X-ray spectrometer (Cameca SX-50, Paris, France). Mea-surements were made at∼18 µm intervals along a transect from the primordium to the edge of the otolith for each of the 10 yellow eels in Table 1. The beam condition of the EPMA used in the measurement of these elements was 15 kV, 4 nA, and a 15µm diameter beam. The EPMA has minimum detection limits of a few hundred ppm (Statham 1981, 1982) and is not sensitive enough to detect trace elements. As a result of the detection limit of EPMA, only calcium and seven minor elements (Na, Mg, Cl, P, S, K and Sr) were selected for analysis. The counting time, standard for calibration, detection limit and analytical error for each of the eight elements are listed in Appendix 1.
Data analysis
The element : Ca concentration ratios on each sampling spot of the otoliths were calculated by weight (%) and grouped by stage and habitat use, which determined the life-history stages of leptocephalus of both
Table 1. Life history of the four groups of yellow stage, female European eels used in the present study
Group Specimen no. Sampling date Sampling site Salinity Age Total Bodyweight Stage and year
(psu) (years) length (mm) (g) at stocking
1 11538 9 Jul 1987 Strömstad (West Coast) 25.54± 3.38 5+ 393 96 Natural
11550 9 Jul 1987 Strömstad (West Coast) 25.54± 3.38 7+ 410 90 Natural
11906 20 Aug 1987 Björkö (West Coast) 23.12± 3.71 9+ 403 96 Natural
2 13743 31 May 1988 Eastern Lake Mälaren Fresh water 8+ 562 442 Elver, 1980
18589 7 Jun 1991 Eastern Lake Mälaren Fresh water 11+ 717 566 Elver, 1980
3 18793 1 Jul 1991 Lake Ången Fresh water ≥14+ 760 820 Yellow eel, 1979
19315 16 Jul 1991 Lake Ången Fresh water ≥18+ 728 668 Yellow eel, 1979
4 25106 30 Aug 1994 Lake Mälaren Fresh water 13+ 723 597 Unknown origin
25108 30 Aug 1994 Lake Mälaren Fresh water 18+ 721 535 Unknown origin
25110 30 Aug 1994 Lake Mälaren Fresh water 14+ 680 461 Unknown origin
freshwater- and seawater-resident yellow eels. Leptocephalus and yel-low eel were separated by the elver check on the otolith (Shiao et al. 2003) and yellow eels were further divided into freshwater and seawater residents according to the Sr concentration on the Sr map and the Sr : Ca ratio profile in their otoliths (Tzeng et al. 1997, 2002, 2003a, 2003b). Freshwater-resident yellow eel referred to the individuals with a Sr : Ca ratio less than 4× 10−3in between the elver check and the otolith edge. Seawater-resident yellow eel had Sr : Ca ratios higher than 4× 10−3 indicating that the individuals did not migrate upstream and lived in high salinity seawater during the yellow eel phase. The estuarine-resident yellow eel migrated between fresh water and seawater.
The significant difference of the mean values of the Ca element concentration ratios among stages of the eel (leptocephalus, freshwater-and seawater-resident yellow eels) were tested by non-parametric Tukey-type multiple comparisons. The significance of the correlation among different Ca element ratios on each sampling spots was tested for each life history stages by Pearson product-moment correlation coefficient (Zar 1984). The contribution of the elemental composition to the group-ing of the above mentioned life history stages was analysed by usgroup-ing stepwise Canonical Discriminant Analysis. The classification success of the life history stages by the elemental composition was calculated with a discriminant function analysis. All the statistics were preformed by using the software SPSS (SPSS Inc., Chicago, IL).
Results
Otolith elemental composition changed with life history
stage and habitat use
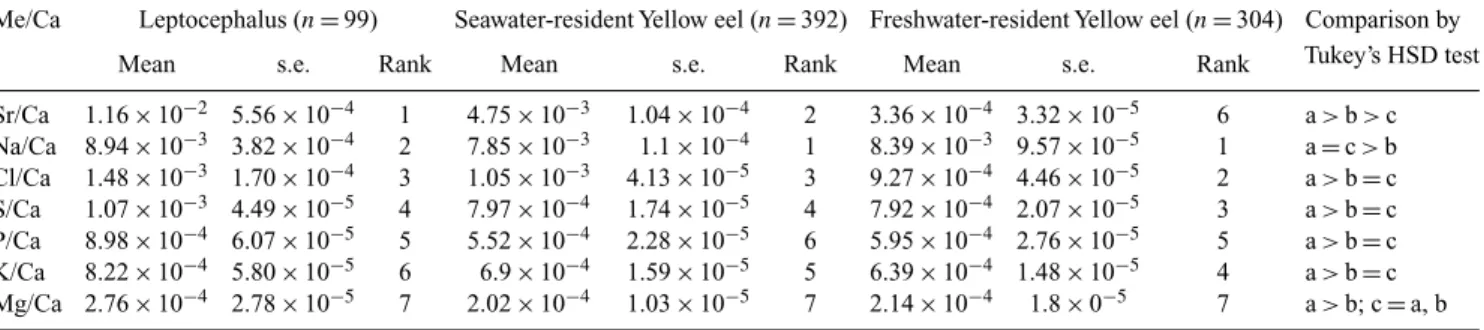
The mean value of the seven elements : Ca ratios within
otoliths of the yellow eel were ranked in descending order by
life history stages and habitat use (Table 2). The Sr : Ca ratios
constituted the first ranking in the elemental composition in
the leptocephalus stage, but dropped to second rank in those
of seawater-resident yellow eel stage and to the sixth rank in
freshwater-resident yellow eel stage. On the contrary, Na : Ca
ratios rose to the first rank in both seawater- and
freshwater-resident yellow eels. This indicated that many elements other
than Sr in otoliths of the eel changed with developmental
stage and habitat use.
Each of the mean (± s.e.) element : Ca ratios in otoliths
were also compared among life history stages and
habi-tat use (Table 2). Tukey’s honestly significantly different
(HSD) tests indicated that Sr : Ca ratios in otoliths of the
eel were the highest at marine leptocephalus stage (1.16
×
10
−2± 5.56 × 10
−4), followed by seawater-resident yellow
eel (4.75
× 10
−3± 1.04 × 10
−4), and it was the lowest in
freshwater-resident yellow eels (3.36
× 10
−4± 3.32 × 10
−5)
(Table 2). Similarly, the ratios of K : Ca, Cl : Ca, P : Ca, and
S : Ca were higher in the leptocephalus stage compared with
the freshwater- and seawater-resident yellow eels (Table 2).
The ratios of Na : Ca and Mg : Ca were higher in
lepto-cephalus than seawater-resident yellow eels. The ratio of
Na : Ca was higher in freshwater than seawater-resident
yel-low eels (Table 2). These indicated that except for Sr : Ca
ratios, the other six element : Ca ratios also changed with life
history stages and habitat use of the yellow eels.
Table 2. Ranking of the seven otolith elements to calcium concentration ratios by life history stages of the eel Ratios were compared among stages
Me/Ca Leptocephalus (n= 99) Seawater-resident Yellow eel (n= 392) Freshwater-resident Yellow eel (n = 304) Comparison by
Mean s.e. Rank Mean s.e. Rank Mean s.e. Rank Tukey’s HSD test
Sr/Ca 1.16× 10−2 5.56× 10−4 1 4.75× 10−3 1.04× 10−4 2 3.36× 10−4 3.32× 10−5 6 a > b > c Na/Ca 8.94× 10−3 3.82× 10−4 2 7.85× 10−3 1.1× 10−4 1 8.39× 10−3 9.57× 10−5 1 a= c > b Cl/Ca 1.48× 10−3 1.70× 10−4 3 1.05× 10−3 4.13× 10−5 3 9.27× 10−4 4.46× 10−5 2 a > b= c S/Ca 1.07× 10−3 4.49× 10−5 4 7.97× 10−4 1.74× 10−5 4 7.92× 10−4 2.07× 10−5 3 a > b= c P/Ca 8.98× 10−4 6.07× 10−5 5 5.52× 10−4 2.28× 10−5 6 5.95× 10−4 2.76× 10−5 5 a > b= c K/Ca 8.22× 10−4 5.80× 10−5 6 6.9× 10−4 1.59× 10−5 5 6.39× 10−4 1.48× 10−5 4 a > b= c Mg/Ca 2.76× 10−4 2.78× 10−5 7 2.02× 10−4 1.03× 10−5 7 2.14× 10−4 1.8× 0−5 7 a > b; c= a, b HSD, Honestly significantly different.
Table 3. Pearson correlation coefficient among seven elements : Ca ratios in weight % within otoliths of the eel by life history stage/habitat use
Sr : Ca K : Ca Cl : Ca P : Ca S : Ca Na : Ca Mg : Ca Leptocephalus Sr : Ca 1 K : Ca 0.15 1 Cl : Ca 0.19 0.62*** 1 P : Ca 0.38*** 0.19 0.05 1 S : Ca 0.31** 0.27** 0.27** 0.16 1 Na : Ca 0.03 0.39*** 0.86*** −0.07 0.14 1 Mg : Ca 0.09 0.21* 0.24* −0.01 0.13 0.22* 1
Seawater-resident yellow eel
Sr : Ca 1 K : Ca 0.24*** 1 Cl : Ca 0.35*** 0.14** 1 P : Ca 0.03 0.05 −0.12* 1 S : Ca −0.11* 0.01 0.01 −0.02 1 Na : Ca 0.32*** 0.14** 0.72*** −0.12* 0.03 1 Mg : Ca −0.04 0.04 0.01 0.06 0.05 –0.03 1
Freshwater-resident yellow eel
Sr : Ca 1 K : Ca 0.12* 1 Cl : Ca 0.03 0.02 1 P : Ca −0.04 0.02 0.13** 1 S : Ca 0.02 0.09 0.20*** 0.07 1 Na : Ca 0.01 −0.04 0.62*** 0.17** 0.25*** 1 Mg : Ca −0.07 0.07 0.06 0.07 0.06 0.02 1 *P < 0.05; **P < 0.01; ***P < 0.001.
Stage/habitat use mediated correlation among
element : Ca ratios in otoliths
The correlations matrix of element : Ca ratios in otoliths of
the eel were different in different life history stages and
habi-tat use (Table 3), for example, Sr : Ca ratios were positively
correlated with K : Ca, Cl : Ca and Na : Ca, and negatively
with S : Ca in seawater-resident yellow eel stage, but
posi-tively correlated with P : Ca and S : Ca in the leptocephalus
stage, and only slightly positively correlated with K : Ca
in freshwater-resident yellow eels. Similarly, the other
ele-ment : Ca ratios also have different combinations of cross
correlation. This may indicate that uptake of elements was
discriminated among different life history stages of the eel.
Classification of the life history stage and habitat
use of the eel by otolith multiple elements
Forward stepwise discriminant function analysis indicated
that the other five element : Ca ratios (except K : Ca and
Mg : Ca) contributed significantly to the discrimination of
the life history stage and habitat use of the eel (Table 4). The
relative contribution of the element : Ca ratio to the groupings
are listed in decreasing order as Sr : Ca, Na : Ca, P : Ca, S : Ca
and Cl : Ca (Table 4).
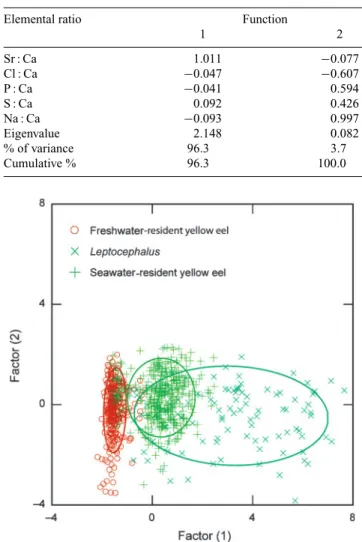
The plot of the first and second components of the
canonical discriminant functions indicated that
freshwater-resident yellow eels can be clearly separated from the
lepto-cephali and seawater-resident yellow eels by the five selected
element : Ca ratios in otoliths of the eel, but the latter two
partially overlapped (Fig. 1). The first component of the
canonical discriminant function contributed 96.3% of the
variance for the grouping of the eels whereas the second
component only contributed 3.7% of the variance. These two
components significantly contributed in the discrimination
(χ
2-square test, P < 0.001). In the first component, the Sr : Ca
ratios contributed the greatest, followed by Na : Ca and S : Ca.
In the second component of the canonical discriminant
func-tion, the Na : Ca ratios were the most important, followed by
Cl : Ca, P : Ca and S : Ca (Table 4). The cross-validated
cor-rect classification percentage in the prediction of the three
different eel groups by the five element : Ca ratios was the
highest in freshwater-resident yellow eels (98.0%), followed
by seawater-resident yellow eel (84.4%), with leptocephalus
being the lowest at 69.7% (Table 5).
Discussion
In earlier studies of the elemental composition of fish otoliths,
it was believed that elemental composition reflects the
ele-mental concentration of ambient water that the fish lives in.
Thus, the Sr : Ca ratio in otoliths was widely used to study the
eel migration between freshwater and marine environments
(e.g. Tzeng et al. 1997, 2000, 2002, 2003a, 2003b; Tsukamoto
and Arai 2001; Jessop et al. 2002, 2004; Shiao et al. 2003).
Table 5. Cross-validated classification of the eel of different stage/habitat use by leave-one-out method using otolith elemental signature (Sr : Ca, Cl : Ca, P : Ca, S : Ca, and
Na : Ca)
Correctly classified percentages in bold Stage/habitat use nA Correct classification (%)
Leptocephalus Yellow eel
Seawater-resident Freshwater-resident
Leptocephalus 99 69.7 24.2 6.1
Yellow stage eel
Seawater-resident 392 2.3 84.4 13.3
Freshwater-resident 304 0.0 2.0 98.0
AThe number of sampling spots for the measurement of elemental composition on the otoliths of the 10 yellow eels in Table 1.
Table 4. Standardised canonical discriminant function coefficients of element : Ca ratios for discriminating the eel among different stage/habitat use as leptocephalus and freshwater- and
seawater-resident yellow eels in Fig. 1
Elemental ratio Function
1 2 Sr : Ca 1.011 −0.077 Cl : Ca −0.047 −0.607 P : Ca −0.041 0.594 S : Ca 0.092 0.426 Na : Ca −0.093 0.997 Eigenvalue 2.148 0.082 % of variance 96.3 3.7 Cumulative % 96.3 100.0
Fig. 1. Anguilla anguilla. Canonical discriminant scores plot of otolith element : Ca ratios for the European eel. The different stages assayed were those present in the adult eel otoliths, and classified by life stage and habitat use as leptocephalus, freshwater- and seawater-resident yellow eels. The elliptical circles indicate 95% confidence limit of each group.
In the present study, we explored elements other than Sr
and found that they also changed with life stage and habitat.
The Sr : Ca ratios ranked first in the leptocephalus stage, but
the top rank changed to Na : Ca in freshwater- and
seawater-resident yellow stage eels. In other words, the ranking of the
relative importance of the element : Ca ratios were different
between leptocephalus and both freshwater- and
seawater-resident yellow stage eels (Table 2). This demonstrated that
the elemental composition in otoliths of the eel significantly
changed with life stage and habitat transition. The
mecha-nism of the change of elemental composition of the otolith
with life stage is not clear in most fishes, but may relate to
the elemental concentration of ambient water and osmotic
pressure regulation of the diadromous eel when they change
from osmoconformer in the leptocephalus stage to
osmoreg-ulation in the yellow stage eel. The body fluid of a
lepto-cephalus is isotonic to seawater and its body tissue is known
to contain extensive amounts of a gelatinous extracellular
matrix composed of sulphated glycosaminoglycans (GAGs),
which have an affinity for alkali elements, particularly Sr
2+(Comper and Laurent 1978; Nishizawa 1978; Hascall and
Hascall 1981; Toole 1981). Sulphated glycosaminoglycans
breakdown during metamorphosis from leptocephalus to
glass eel may possibly reduce the absorption of Sr
2+and other
elements. Thus, the Sr : Ca ratios in otoliths of leptocephali
significantly decreased when they metamorphose into glass
eels (Otake et al. 1994; Arai et al. 1997). However, the effect
of ambient environment on the otolith Sr : Ca ratios of the eel
cannot be excluded because the growth habitat shifted from
high to low saline water when the eel metamorphoses from
leptocephalus to glass eel and Sr concentration is
∼100 fold
higher in seawater than in fresh water (Campana 1999). On the
contrary, the Na : Ca ratios in otoliths of the eel were higher
in freshwater- compared with seawater-resident yellow stage
eels, although Na concentration is higher in seawater than
fresh water. This may indicate that sodium uptake is
physio-logically controlled by the eel. Accordingly, except with the
exception of Sr, the other elements in fish otoliths are also
very important in the linking study between otolith
micro-chemistry and the life and migratory environmental history
of the fish.
This is the first paper to use multiple elements in addition
to Sr to study the life history and migratory environment of
the eel. The eight elements selected in the present study were
reported to have a positive correlation in abundance between
otoliths and ambient water (Goldberg 1965; Thresher 1999).
With the exception of the Sr : Ca and Na : Ca ratios, no
sig-nificant difference was found among leptocephalus and both
freshwater- and seawater-resident yellow eels (Table 2). This
indicates that it is difficult to use a single element : Ca ratios to
discriminate the eel stage and habitat use. However, the cross
correlation matrix of the seven element : Ca ratios in otoliths
was found to be different among stage and habitat use of the
eel (Table 3). This may indicate that the elements incorporated
into otoliths of the eel were conditionally selected. The
canon-ical plot and stepwise canoncanon-ical discriminant analysis also
indicated that along with Sr : Ca, the other four element : Ca
ratios (Cl : Ca, P : Ca, S : Ca and Na : Ca) have obviously
con-tributed to the grouping of the eels (Fig. 1). The classification
success of the different stage and habitat use of the eel reached
70.7–98.0% (Table 5). This suggested that multiple-elemental
information could provide additional insight into
environ-mental life history traits of the facultative catadromous eels.
A total of 31 elements have been detected in fish otoliths to
date (Campana 1999). Future studies are needed to assess the
use of elements that are below the detection limits of EPMA
for more detailed fingerprints regarding eel otoliths.
Acknowledgments
The present study was conducted with financial support from
the National Science Council (contract no. NSC
91–2313-B002–291, awarded to WNT). We thank Ms Y. C. Tsai for
otolith preparation, and Mr Brian M. Jessop, Dr Steven
Cam-pana and the anonymous referee for their helpful comments
on an early draft of this paper.
References
Arai, T., Otake, T., and Tsukamoto, K. (1997). Drastic changes in otolith microstructure and microchemistry accompanying the onset of meta-morphosis in the Japanese eel Anguilla japonica. Marine Ecology Progress Series 161, 17–22.
Bertin, L. (1956). ‘Eels – A Biological Study.’ (Cleaver-Hume Press: London.)
Cairns, D. K., Shiao, J. C., Iizuka, Y., Tzeng, W. N., and MacPherson, C. D. (2004). Movement patterns of American eels in an impounded watercourse, as indicated by otolith microchemistry. North American Journal of Fisheries Management 24, 452–458. doi:10.1577/M03-054.1
Campana, S. E. (1999). Chemistry and composition of fish otoliths: pathways, mechanisms, and applications. Marine Ecology Progress Series 188, 263–297.
Comper, W. D., and Laurent, T. C. (1978). Physiological function of connective tissue polysaccharides. Physiological Reviews 58, 255–315.
Goldberg, E. D. (1965). Minor elements in seawater. In ‘Chemi-cal Oceanography, Vol. 1’. (Eds J. P. Railey and G. Skirrow.) pp. 181–195. (Academic Press: New York.)
Hascall, V. C., and Hascall, G. K. (1981). Proteoglycans. In ‘Cell Biology of Extracellular Matrix’. (Ed. E. D. Hay.) pp. 39–63. (Plenum Press: New York.)
Jessop, B. M., Shiao, J. C., Iizuka,Y., and Tzeng, W. N. (2002). Migratory behaviour and habitat use by American eels Anguilla rostrata as revealed by otolith microchemistry. Marine Ecology Progress Series 233, 217–229.
Jessop, B. M., Shiao, J. C., Iizuka, Y., and Tzeng, W. N. (2004). Variation in the annual growth, by sex and migration history, of silver Amer-ican eels Anguilla rostrata. Marine Ecology Progress Series 272, 231–244.
Kraus, R. T., and Secor, D. H. (2003). Response of otolith Sr : Ca to a manipulated environment in young American eels. American Fisheries Society Symposium 33, 79–85.
Lecomte-Finiger, R. (1992). Growth history and age at recruit-ment of European glass eels (Anguilla anguilla) as revealed
by otolith microstructure. Marine Biology 114, 205–210. doi:10.1007/BF00349520
Limburg, K. E., Wickstrom, H., Svedang, H., Elfman, M., and Kristianson, P. (2003). Do stocked freshwater eel migrate? Evi-dence from the Baltic suggests ‘Yes’. In ‘Biology, Management, and Protection of Catadromous Eels’. (Ed. D. A. Dixon.) pp. 275–284. (American Fisheries Society symposium 33: Bethesda, MD.) Nishizawa, K. (1978). Marine algae from a viewpoint of pharmaceutical
studies. Japanese Journal of Phycology 26, 73–78.
Otake, T., Ishii, T., Nakahara, M., and Nakamura, R. (1994). Drastic changes in otolith strontium/calcium ratios in leptocephali and glass eels of Japanese eel Anguilla japonica. Marine Ecology Progress Series 112, 189–193.
Scott, V. D., Love, G., and Reed, S. J. B. (1995). ‘Quantitative Electron-Probe Analysis.’ (Ellis Horwood Limited: Hertfordshire, UK.) Shiao, J. C., Iizuka, Y., Chang, C. W., and Tzeng, W. N. (2003).
Dis-parities in habitat use and migratory behavior between tropical eel Anguilla marmorata and temperate eel A. japonica in four Taiwanese rivers. Marine Ecology Progress Series 261, 233–242.
Statham, P. J. (1981). X-ray microanalysis with Si (Li) detectors. Journal of Microscopy 123, 1–23.
Statham, P. J. (1982). Confidence in microanalysis; lies, damned lies or statistics. In ‘Proceeding of the 17th Annual Conference of Microbeam Analytic Society’. pp. 1–7. (Microbeam Analytic Society: Washington, DC.)
Tesch, F. W. (2003). ‘The Eel.’ 3rd edn. (Blackwell Publishing: Oxford.) Thresher, R. E. (1999). Elemental composition of otoliths as a stock delineator in fishes. Fisheries Research 43, 165–204. doi:10.1016/S0165-7836(99)00072-7
Toole, B. P. (1981). Glycosaminoglycans in morphogenesis. In ‘Cell Biology of Extracellular Matrix’. (Ed. E. D. Hay.) pp. 259–294. (Plenum Press: New York.)
Tsukamoto, K., and Arai, T. (2001). Facultative catadromy if the eel Anguilla japonica between freshwater and seawater habitats. Marine Ecology Progress Series 220, 265–276.
Tsukamoto, K., Nakai, I., and Tesch, W. V. (1998). Do all freshwater eels migrate? Nature 396, 635–636. doi:10.1038/25264
Tzeng, W. N. (1996). Effects of salinity and ontogenetic movements on strontium:calcium ratios in the otoliths of the Japanese eel, Anguilla
http://www.publish.csiro.au/journals/mfr
japonica. Journal of Experimental Marine Biology and Ecology 199, 111–122. doi:10.1016/0022-0981(95)00185-9
Tzeng, W. N., and Tsai, Y. C. (1994). Changes in otolith microchemistry of the Japanese eel, Anguilla japonica, during its migration from the ocean to the rivers of Taiwan. Journal of Fish Biology 45, 671–683. Tzeng, W. N., Severin, K. P., and Wickström, H. (1997). Use of otolith microchemistry to investigate the environmental history of European eel Anguilla anguilla. Marine Ecology Progress Series 149, 73–81. Tzeng, W. N., Wang, C. H., Wickstrom, H., and Reizenstein, M. (2000). Occurrence of the semi-catadromous European eel Anguilla anguilla in the Baltic Sea. Marine Biology 137, 93–98. doi:10.1007/ S002270000330
Tzeng, W. N., Shiao, J. C., and Iizuka, Y. (2002). Use of otolith Sr : Ca ratios to study the riverine migratory behaviors of Japanese eel Anguilla japonica. Marine Ecology Progress Series 245, 213–221.
Tzeng, W. N., Shiao, J. C., Yamada, Y., and Oka, H. P. (2003a). Life history patterns of Japanese eel Anguilla japonica in Mikawa Bay, Japan. American Fisheries Society Symposium 33, 285–293. Tzeng, W. N., Iizuka, Y., Shiao, J. C., Yamada, Y., and Oka, H. P. (2003b).
Identification and growth rates comparison of divergent migratory contingents of Japanese eel (Anguilla japonica). Aquaculture 216, 77–86. doi:10.1016/S0044-8486(02)00053-4
Vollestad, L. A., and Jonsson, B. (1986). Life-history characteristics of the European eel Anguilla anguilla in the Imsa River, Nor-way. Transactions of the American Fisheries Society 115, 864–871. doi:10.1577/1548-8659(1986)115<864:LCOTEE>2.0.CO;2 Wang, C. H., and Tzeng, W. N. (2000). The timing of metamorphosis
and growth rates of American and European eel leptocephali: A mechanism of larval segregative migration. Fisheries Research 46, 191–205. doi:10.1016/S0165-7836(00)00146-6
Zar, J. H. (1984). ‘Biostatistical Analysis.’ (Prentice Hall International: London.)
Manuscript received 16 July 2004; revised 22 March 2005; and accepted 6 April 2005.
Appendix 1. Electron probe microanalyser counting times (peak and background), standards, detection limits, and analytical errors
Detection limits (wt %) and typical analytical errors (wt %, 1 σ) calculated after Scott et al. (1995)
Element Counting time Standard Detection limit Typical analytical error
(sec) Na 60 Halite (CM Taylor) 0.029 0.023 Mg 60 Osumilite (USNM 143967) 0.019 0.022 P 60 Apatite (Wilberforce) 0.036 0.027 S 60 Gypsum (CM Taylor) 0.023 0.017 Cl 46 Halite (CM Taylor) 0.027 0.015 K 46 Osumilite (USNM 143967) 0.019 0.012 Ca 20 Calcite (NMNH 136321) NA 0.245 Sr 120 Strontianite (Smithsonian R-10065) 0.036 0.019