ELSEVIER S0032-3861 (96)00180-2
Polymer Vol. 37 No. 16, pp. 3493-3499, 1996 Copyright © 1996 Elsevier Science Ltd Printed in Great Britain. All rights reserved 0032-3861/96/$15.00 + 0.00
A study of the relationship between the
electroluminescence characteristics and
compositions of PPV-PVA-based polymers
Win-Pin Chang and Wha-Tzong Whang*
Institute of Materials Science and Engineering, National Chiao Tung University, Hsinchu 30049, Taiwan, China
(Received 12 October 1995; revised 20 November 1995)
A series of poly(p-phenylene vinylene) (PPV)-poly(vinyl alcohol) (PVA)-based polymers were prepared from the PPV precursor and PVA aqueous solution mixtures. The luminescence characteristics of the PPV PVA-based polymers were dependent on their composition and the following treating conditions. The emitted light of the PPV-PVA-based light-emitting diodes (LEDs) shifted from yellow-green (550 nm) to blue (485 nm) as the PVA component increased. The infra-red (i.r.) absorption spectra showed that some part of the PPV precursor could react with PVA to form C-O-C linkages and interrupted the conjugation of PPV polymer chains. This resulted in decrease of the conjugated chain length, as evidenced in ultraviolet visible u.v.-vis, absorption spectra. The relative photoluminescence (PL) intensity increases with decreasing the conjugated chain length. The relative electroluminescence (EL) decreases with the increase of the PVA component in the polymers. However, both PL and EL intensity normalized with PPV molar ratio of PPV- PVA-based polymers were higher than that of pure PPV. The relative low threshold voltage at 4-6 V was found in these PPV-PVA-based polymer LEDs with A1 (negative) and indium tin oxide ITO (positive) as the two electrodes. Copyright © 1996 Elsevier Science Ltd.
(Keywords: electroluminescence; PPV-PVA-based polymer; light emission efficiency; threshold voltage)
I N T R O D U C T I O N
Conjugated polymers are currently of interest as materials for application in the electronic and opto- electronic fields, especially for electroluminescence devices. Conjugated polymers, with their 7r-Tr* energy gap in the range of 1.5-3.0eV, may emit light in the visible spectrum as a result of electronic excitation and recombination. The first reported polymer light-emitting diode (LED) was obtained from poly(p-phenylene vinylene) (PPV) by Burroughes et al. in 19901. Upon applying a bias on the sandwich configuration of A1/ PPV/AI20 3 it emitted yellow-green light. Recently, many research groups 2-9 have prepared polymer LEDs based on a variety of conjugated polymers with light emission in the whole visible spectrum, i.e. red, yellow, green, and blue light. Emission of blue light has attracted much more attention in this field, because conventional inorganic LEDs still have problems in the fabrication process for blue light emission.
It is relatively easy to tailor-make the molecular structure of organic materials, in comparison with inorganic material. The organic conducting polymer provides a promising LED material for scientists to design a practical blue LED. However, because this is a new field in organic materials, there are only a few published blue-light polymer LEDs including poly(p-phenylene) 1°, poly(alkylfluorene) 5, PPV-based * To w h o m correspondence should be addressed
copolymer 4, and poly(p-phenylphenylene vinylene)/ poly(N-vinylcarbazole) blends 7, etc.
In this study, we try to use common and easily obtainable polymers to prepare blue and green LEDs. We investigated a range of PPV-PVA-based polymers as an emissive layer. These P P V - P V A polymers are prepared by coating the mixture of PPV precursor with PVA in the presence of cosolvent water on indium-tin oxide (ITO) glass followed by the heat treatment in a high vacuum oven. By adjusting the weight ratios of PPV to PVA, a series of P P V - P V A polymers can be obtained with different 7r-conjugated chain lengths. Therefore, we can tune the light emission from yellow-green to blue. The performance of this series of PPV-PVA-based LEDs is also characterized in this article. Additionally, the mixture of PPV-PVA is not a physical blending, but includes formation of chemical bonding in between these two different polymers as demonstrated in the results. Therefore, the processing conditions also affect the luminescence characteristics of the PPV-PVA-based polymers.
E X P E R I M E N T A L
The sulfonium precursor route for preparing P P V The PPV precursor was prepared by addition of 20 ml of 0.22 M N a O H aqueous solution into 20ml of 0.2 M p-xylylenebis(tetrahydrothiophenium chloride) (aqueous solution) with 80 ml pentane. Both solutions were first
Study of PPV-PVA-based polymers." 14/. -P. Chang and W.- T. Whang
Table 1 The denotation and composition of PPV PVA-based polymers
Composition PPV precursor (0.%) PVA (4%)
Energy
Sample molar molar gap a
denotation wt% ~ ratio %" wt% ratio % (eV)
PPV 100 100 0 0 2.34
PPV PVA1 38.5 10.8 61.5 89.2 2.39 PPV-PVA2 11.1 2.4 88.9 97.6 2.51 PPV PVA3 3.0 0.6 97.0 99.4 2.60 "Energy gap was determined by the onset of u.v.-vis, absorption b Based on the total weights of PPV precursor and PVA
c Based on the total moles of repeat unit of PPV precursor and PVA
1"
gi e ~ .< Figure I (a) 400O t q T I t I I 3500 3000 2500 2000 1500 1000 500 W a v e n u m b e r s ( c m "1)1.r. absorption spectra of (a) PPV and (b) PPV-PVA3 polymers
cooled to 0 - 5 ° C in an ice bath. The reaction proceeded for 1 h and then was terminated by the addition o f 0.1 M HCI aqueous solution to neutralize the reaction solution. After the pentane was decanted off, the PPV precursor aqueous solution was dialysed (molecular weight cut-off o f 6000) against deionized water for several days. The PPV film was obtained by spin-coating the PPV precursor solution on an ITO glass and then heating in a vacuum oven at 220°C for 2 h.
The preparation of PPV-PVA-based polymers
PPV precursor aqueous solution (0.5%) was homo- geneously mixed with predissolved PVA (Mw = 72 000) (aqueous solution, 4.0%) in various weight ratios, as listed in Table 1. The P P V - P V A - b a s e d films were obtained by spin-coating these solutions onto the ITO glass and, with or without standing in a high vacuum oven at room temperature for 2 h, followed by heating at 70°C for 2 h, and finally at 200°C for 2 h.
The preparation of the LED device
PPV and P P V - P V A films ( 8 0 - 1 0 0 n m ) on ITO glass were coated with A1 metal (5000A, area 7 m m 2) by
thermal evaporation in vacuum (4 × l0 6 torr) to give A1/polymer/ITO sandwich devices. These sandwich devices were then annealed in a high vacuum oven at 160°C for 3 h.
Characterization
The thickness of PPV and P P V - P V A films was measured with a Dektak 3030 surface profilometer. Infra-red (i.r.) absorption spectra o f PPV and P P V - P V A films were taken using a Bio-rad FTS-165 Fourier transform infra-red (FTi.r.) spectrometer. Ultraviolet visible (u.v. vis.) absorption spectra of PPV and P P V - PVA films were measured using a Beckman 7400 spectrometer. A Jasco FR-770 spectrometer was used to obtain photoluminescence spectra of PPV and P P V - PVA films with the excitation wavelength at 365 nm, and also electroluminescence spectra of the Al/polymer/ITO sandwich devices. The I(current)-V(voltage) curves of these sandwich devices were measured with a pro- grammable Keithley 237 electrometer. The electro- luminescence intensities of these sandwich devices were recorded using a photodiode detector connected with a Newport (Model 1815-C) Power Meter. All character- izations were done in the laboratory environment.
R E S U L T S A N D D I S C U S S I O N
The chemical structures of PPV and PPV PVA polymers were characterized with an FTi.r. spectrometer, shown in
Figures la and lb respectively. The denotation and
composition o f P P V - P V A polymers were listed in
Table 1. The absorption band near 963cm -l was due
to C - H out-of-plane bending o f trans configurations of the vinylene group. The band near 3024cm -] was assigned to the trans-vinylene C - H stretching mode. The band near 555 cm 1 was due to the phenylene out- of-plane ring bending mode. The absorption bands near 831cm -] and 1512cm -I were assigned to p-phenylene C - H out-of-plane bending and C - C ring stretching respectively. These absorption bands were seen both in PPV and P P V - P V A polymers. In Figure lb, the absorption bands of P P V - P V A polymer near
l 1
2 9 5 0 c m - and 2917cm are due to the aliphatic C H stretching mode and at 3367cm i due to the - O H stretching mode o f PVA units. An extra absorption band at 1100 cm-l assigned to C O C asymmetric stretching mode was observed in P P V - P V A polymer. The forma- tion of C - O - C ether linkage may result from the reaction of hydroxyl groups o f PVA with the ethylene radical o f the PPV precursor during the elimination of sulfonium groups from PPV precursors. The chemical reaction was illustrated in Figure 2.
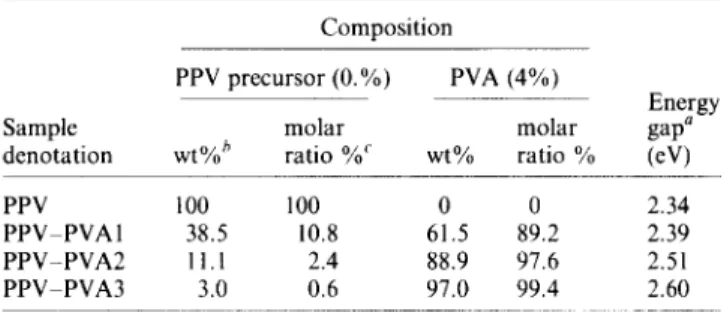
U.v.-vis. absorption spectra of PPV and PPV-PVA polymers are depicted in Figure 3. The onset of u.v. vis. absorption of these P P V - P V A polymers shifted to a higher energy level as the PVA content increased. It meant that these P P V - P V A polymers with a higher PVA content had a shorter conjugation length. The pure PPV showed the best conjugation. The formation of C - O - C linkages shown in the i.r. spectra o f these P P V - P V A polymers resulted in the interruption of 7r-conjugated system of PPV structure. The result o f the u.v.-vis. spectra coincided with the observation in the i.r. absorption spectra. The energy gaps of PPV and various P P V - P V A polymers were listed in Table 1.
Study of PPV-PVA-based polymers." 14/. -P. Chang and W.- T. Whang CH 2 - CH - - 7 PPV PreGursor + OH PVA Figure 2 - -° / I ' = " / o
I
OH OH 0 OHSchematic representation of chemical reaction between PPV precursor and PVA
U 2 4 ~ 494 518 (d) (c) (b) (a)
30O 4O0 5OO 6O0 700 800
Wavelength (am)
Figure 3 U.v.-vis. absorption spectra of(a) PPV; (b) PPV-PVA1; (c) PPV-PVA2; and (d) PPV-PVA3 polymers
Figure 4 illustrated the PL spectra of PPV, and those
P P V - P V A polymers with the room-temperature- standing step, 70°C low-temperature treatment, and final heating at 200°C. The PPV emitted yellow-green light with an emission maximum at 550 nm. However, by introducing PVA into PPV segments, there was a remarkable blue shift in the PL spectra. P P V - P V A 1
rill
J
4oo 500 6o0 70o
Wavelength (am)
Figure 4 PL spectra of(a) PPV; (b) PPV-PVA1; (c) PPV-PVA2; and (d) PPV PVA3 polymers
emitted green light with two emission peaks at 507 nm and 530 nm. The green-blue light emission from P P V - PVA2 was observed with an emission maximum at 4 9 7 n m and a shoulder at 525nm. The P P V - P V A 3 emitted blue light with an emission maximum at 485 nm and a shoulder at 515 nm. Therefore, the significant blue shifts in PL spectra were associated with the PVA content and, in turn, with the shorter conjugated chain length. However, when the P P V - P V A films were prepared in the different treatment procedure, i.e. the spin-coated films of PPV p r e c u r s o r - P V A mixtures were
Study of PPV-PVA-based polymers: W.-P. Chang and W. - T. Whang
!wo 600
Wavelength (am)
700
Figure 5 PL spectra of PPV-PVA2 with different treatment proce- dures: (a) standing at room temperature for 2 h, at 70°C for 2 h, and finally at 200°C for 2 h, and (b) directly heating at 200°C for 2 h
. PPV . PPV-PVAI l PPV-PvA2 m PPV-PvA3 I l 0 20 40 60 80 100 Composition (PVA wt%) A PPV 0 PPV-PvAl . PW-WA2 m Pw-w.43 I l 0 0 20 40 60 80 100 Composition (PVA wt%)
Figure 6 (a) Dependence of relative PL intensity on PVA contents. (b) Dependence of relative PL intensity normalized with PPV molar ratios on PVA contents
directly heated in a vacuum oven at 200°C for 2 h without the room-temperature-standing step. The lumi- nescence characteristics of these resultant PPV-PVA films showed a red shift. As seen in Figure 5, the PPV-
PVA2 emitted light with emission maxima at 497nm shifting to 503 nm and the shoulder at 525 nm shifting to 530nm. The red shift of PPV-PVA2 obtained by treating directly at 200°C for 2 h resulted from longer conjugation of the PPV backbone. This experimental result showed that the PPV precursor in the PPV precursor-PVA mixture preferred to eliminate the sulfonium groups to form phenylene vinylene segments rather than to react with PVA to form C-O-C linkages at higher temperatures. Therefore, the multistep process for preparing PPV-PVA films in this study, initially standing at room temperature for 2 h and then following with heat treatment at 70°C provided an opportunity for the reaction between the PPV precursor and PVA. These types of films were finally treated at 200°C to insure the completion of the reaction, and to remove the labile sulfonium group and volatile components completely. It is clear that the treating process affects the luminescence characteristics of the PPV-PVA polymers.
The dependence of relative PL intensity on PVA contents is seen in Figure 6a. The PL intensity increased with the PVA content because the extent of r-conjugated chain length decreased. The highest PL intensity was obtained from PPV-PVA3 and the PPV showed the lowest PL intensity. This result was consistent with the observations of PPV-based LEDs reported by other groups419. As has been reported4, the excitons in the longer delocalized r-electron system would have had more rapid motion to the quenching sites that led to non- radiative decay. The dependence of relative PL intensity normalized with PPV molar ratios (based on the PPV units in the PPV-PVA polymer) on the PVA contents is shown in Figure 6b. It is clear that the addition of PVA into PPV may tremendously improve the relative PL intensity per PPV unit of PPV-PVA-based polymers (more than two orders).
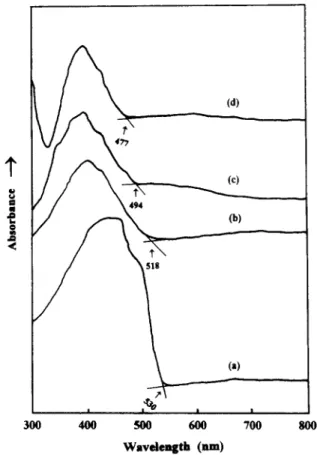
Figure 7 shows the typical I-V characteristics of Al/
polymer/IT0 sandwich devices. In these LED sandwich devices, Al is used as a negative electrode (electron- injecting electrode), IT0 as a positive electrode (hole- injecting electrode). When an applied bias on these LED devices exceeds their threshold voltage, light emission occurs with their own wavelength. It is worth noting that the threshold voltage of these PPV-PVA LEDs were about 447V lower than that of PPV LED, as well as many single-layer polymer LEDs with the same Al and IT0 as the two electrodes. The magnitudes of current from the I- I’ curves shown in Figure 7 reduced as the PVA content increased. It would be a combination effect of the shorter conjugated segments of PPV with a wider band gap and the more non-conjugated PVA content impeding the transport of charge carriers.
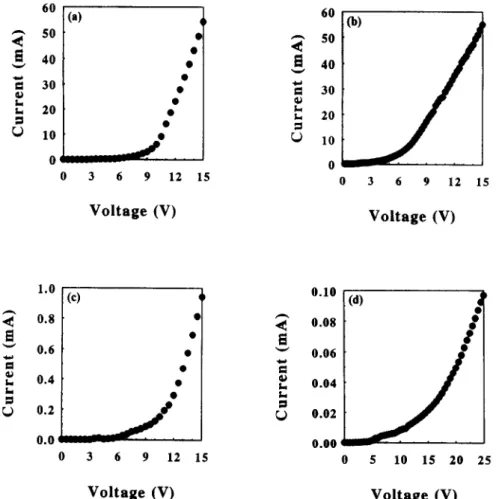
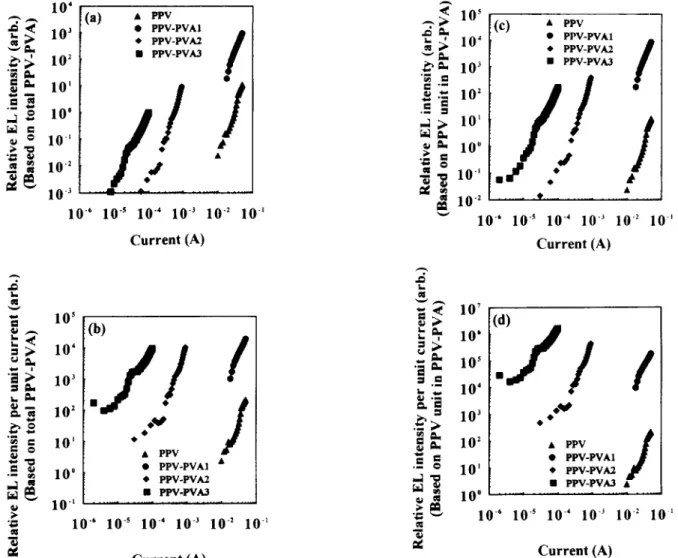
Figure 8a depicts the dependence of relative EL intensity
on the current for these LED devices. The highest EL emission intensity was obtained from PPV-PVAl, in which the PVA content was low. However, as the non- conjugated segments increased, from PPV-PVAl to PPV-PVA3, the light emitted became weaker. Figure 86 shows the dependence of relative EL intensities per unit current on the applied current. Based on the same applied unit current, the light emission intensities of these PPV- PVA LEDs were higher than that of PPV LED (about two orders). However, the light emission intensities per unit current, from PPV-PVAl to PPV-PVA3, also decreased with the increase of non-conjugated segments. The
6O ~" 50 ¢I 40 N 3o ~. 20 ~ lO (a) Co) 0 3 4
,_.~
6 9 12 15 V o l t a g e (V) 6O 4O 30 2oStudy of PPV-PVA-based polymers." W.-P. Chang and W. - T. Whang
r 0 3 6 9 12 15 V o l t a g e (V) 1,0 (c) q 0.8 • 0.6 / 0.4 0.0 0 3 6 9 12 15
°1°l,,,
0.06 0.04 ~) 0.02 0.00 0 5 10 15 20 25 V o l t a g e (V)Figure 7 I V curves of (a) PPV: (b) PPV-PVAI; (c) PPV-PVA2; and (d) PPV-PVA3 LEDs
V o l t a g e (V)
difference was less than that in
Figure 8a.
The reduction in light emission was due to the decrease of the PPV content, i.e. the lower effective emissive chromophore unit, resulted in lessening the light emission intensity.Figure 8c
displayed the dependence of relative EL intensity normalized with PPV molar ratios (based on the PPV units in the PPV- PVA polymer) on the applied current. As we excluded the effect of the non-conjugated PVA, the relative EL intensities per PPV unit of these PPV-PVA polymers followed the same order as that inFigure 8a:
PPV- PVA1 > PPV-PVA2 > PPV-PVAY The higher the PVA content, the lower the EL intensity per PPV unit. This phenomenon is different from that in PL, as shown inFigure 6b.
No interchain and/or intrachain motion of carriers are necessary in the PL process. But in the EL process, the two opposite carriers were injected from two counter electrodes, and led to the formation of singlet excitons in the PPV-PVA polymer layer through the interchain and/or intrachain motion. As shown in u.v.-vis. absorption spectra, PPV-PVA polymers with higher PVA content gave a shorter PPV conjugated length. The shorter PPV-conjugated length in the PPV backbone means more non-conjugated segments and higher band gaps. It may lower the carrier mobility in the PPV chain and lead to the formation of singlet excitons being more difficult. Even- tually the EL intensity was lessened 9. Therefore, the EL intensity decreased with the increase of the PVA content. The relative EL intensities per PPV unit of these PPV- PVA LEDs were superior to those of pure PPV LED, but the superiority was much less than in PL. It was due to the combination of two opposite effects in the EL process: ashorter conjugated length of PPV enhancing light emission efficiency and lessening the singlet exciton formation.
Figure 8d
shows the dependence of relative EL intensity normalized with PPV molar ratio per unit current on the applied current. Based on the same PPV chromophore unit and the applied unit current, the highest light emission efficiency was obtained from PPV-PVA3, followed by the PPV-PVA2 and then PPV-PVA1, and the pure PPV showed the lowest light emission efficiency. The light emission efficiencies of these PPV-PVA LEDs were higher than that of PPV LED (about three orders).The EL spectra of these LED devices, in
Figure 9,
were similar to their PL spectra. The PPV emitted yellow- green light with a maximum peak at 550 nm. The PPV- PVA1 emitted green light was an emission maximum at 507 nm and a second maximum near 530 nm. The PPV- PVA2 and PPV-PVA3 emitted green-blue light with an emission peak at 497 nm, and blue light with an emission peak at 485 nm respectively. The PPV-PVA-based poly- mers provide a new way to prepare a blue light LED. CONCLUSIONSWe have prepared a series of PPV-PVA-based polymers as light emission materials. These PPV-PVA-based polymers, with different conjugation, were obtained by spin-coating the mixtures of PPV precursors with controlled fractions of PVA in cosolvent water, followed by heat treatment in a high vacuum oven. The treatment conditions also affected the luminescence characteristics of these PPV-PVA-based films. The
Study of PPV-PVA-based polymers. W.-P. Chang and W.- T. Whang
~ " ~ 10' (a) . PPv ~-~" 10"~1( c • PPV1 0
_!
• , PPV-PVA2 ~ ~ 1 0 ' [ * P P V - P V A 2 / [ ~,. 1 0 2 • PPV-PVA3 D ' . ~ , 10 ~ [ • PPV-PVA3I
,.~ ~ 1 0 ° .-~ ~ 10' ;~'¢° 1 0 " ~ ~ 1 0 o * • . ~ . . 10" J ' * * l O . i ¢g 1 0 " 2 ... . . . _.. ~ 102 - . 1 0 " 1 0 "s 1 0 " 1 0 -~ 1 0 "~ 1 0 " 1 0 " 1 0 "s 1 0 " 1 0 "~ 1 0 ~ 1 0 "C u r r e n t (A) Current (A)
,-7. l O s i 1 0 ~ ~ 102
"~ lo'
~ ' ~ 1 0 o 1 0 . . . "~ 1 0 " 1 0 -s 1 0 - ' 1 0 -~ 1 0 -~ 10-' C u r r e n t (A) B ~ ~ 107 = "" 104 = 1 0 ~ .~;~ ** ,d ed 10' 10' 10 °• PPV
/
•PPV-PVAI
*' PPV-PVA2 • PPV-PVA,3 10-' 10 -~ 10-' 10 ~ 10: 10' C u r r e n t (A)Figure
8 (a) Relative EL intensity vs. current for PPV and P P V - P V A s LEDs. (b) Relative EL intensity per unit current vs. current for PPV and PPV PVAs LEDs. (c) Relative EL intensity normalized with PPV molar ratios vs. current for PPV and P P V - P V A s LEDs. (d) Relative EL intensity normalized with PPV molar ratios per unit current vs. current for PPV and PPV PVAs LEDs(d). I (b) i I i 400 500 600 700 Wavelength (rim)
Figure
9 EL spectra of (a) PPV; (b) P P V - P V A 1 ; (c) PPV PVA2; and (d) P P V - P V A 3 ; L E D sresultant PPV-PVA-based LED devices emitted light from green-yellow (550 nm) to blue (485 nm) region.
I.r. spectra showed that PPV PVA-based polymers contained C - O - C linkages which interrupted the con- jugated chain length of PPV. This resulted in the energy gap of PPV PVA-based polymers shifting to a higher energy level as the PVA fractions increased. The relative PL inten- sity increased with the PVA content for having a shorter conjugated length. The threshold voltages of these PPV- PVA-based LEDs were about 4-6V lower than many reported polymer LEDs, especially using A1 and ITO as electrodes. The relative EL intensity of PPV-PVA1 showed the highest luminescence intensity in this series of polymer LEDs. The EL intensity strongly depended on the tran- sport feasibility of the carriers from opposite electrodes into the light emission layer to from singlet excitons in PPV backbone. The relative EL intensities of PPV-PVA-based polymer LEDs were primarily determined by the two opposite effects of shorter conjugated length in PPV: enhancing the light emission efficiency, but impeding the carrier transportation, and lessening the singlet exciton formation.
ACKNOWLEDGEMENT
The authors would like to express their appreciation to the National Science Council of the Republic of China
Study of PPV-PVA-based polymers." W. -P. Chang and W. - T. Whang
f o r t h e f i n a n c i a l s u p p o r t o f this s t u d y u n d e r t h e g r a n t N S C - 8 4 - 0 4 0 5 - E - 0 0 9 - 0 0 4 .
R E F E R E N C E S
1 Burroughes, J. H., Bradley, D. D. C., Brown, A. R., Marks, R. N., Mackay, K., Friend, R. H., Burn, P. L. and Holmes, A. B. Nature 1990, 347, 539
2 Braun, D. and Heeger, A. J. Appl. Phys. Lett. 1991, 58, 1982 3 Woo, H. S., Graham, S. C., Haliday, D. A., Bradley, D. D. C.,
Friend, R. H., Burn, P. L. and Holmes, A. B. Phys. Rev. B 1992, 46, 7379
4 Zhang, C., Braun, D. and Heeger, A. J. J. Appl. Phys. 1993, 73, 5177
5 Uchida, M., Ohmori, Y., Morishima, C. and Yoshino, K. Synth. Met. 1993, 55-57, 4168
6 Yang, Z., Sokolik, I. and Karasz, F. E. Macromolecules 1993, 26, 1188
7 Zhang, C., von Seggern, H., Pakbel, K., Krabel, B., Schmidt, H.-W. and Heeger, A. J. Synth. Met. 1994, 62, 35
8 Berggren, M., Gustafsson, G., Ingan/is, O., Andersson, M. R., Wennerstr6m, O. and Hjertberg, T. Adv. Mater. 1994, 6, 488 9 Braun, D., Stating, E. G. J., Demandt, R. C. J. E., Rikken, G. L.
J., Kessener, Y. A. R. R. and Venhuizen, A. H. J. Synth. Met. 1994, 66, 75
10 Grem, G., Leditzky, G., Ullrich, B. and Leising, G. Adv. Mater. 1992, 4, 36