Abstract.
Background: The effects of baicalein on SCC-4
human tongue cancer cells were examined to better understand
its effect on apoptosis and associated possible signal pathways
in vitro. Materials and Methods: Apoptosis induction, reactive
oxygen species (ROS), cytoplasmic Ca
2+, mitochondrial
membrane potential (MMP) and caspase-3 activity were
analyzed using the flow cytometric assay. Apoptosis-associated
proteins, such as p53, BAX, BCL-2, cytochrome c, caspase-3
and -9, EndoG and AIF were determined by Western blotting.
Results: Our results showed that baicalein promoted the levels
of p53, BAX, cytochrome c, capase-3 and -9 and reduced the
level of BCL-2, which were associated with the induction of
apoptotic cell death of SCC-4 cells. A release of cytochrome c
from mitochondria into cytosol was demonstrated and an
activation of caspase-3, which led to the occurrence of
apoptosis in SCC-4 cells treated with baicalein as determined
by Western blot. In order to understand the role of Ca
2+in the
induction of apoptosis, cells were pre-treated with BAPTA
(intracellular calcium chelator) and baicalein. It was shown
that the MMP was restored, and the level of cytoplasmic Ca
2+suppressed, the proportion of cells undergoing apoptosis was
also markedly diminished. Our data suggest that cellular Ca
2+modulates baicalein-induced cell death via a Ca
2+-dependent
mitochondrial death pathway in SCC-4 cells.
Many studies have demonstrated that flavonoids from natural
plants exhibit a variety of biological activities, such as the
anti-inflammatory, antioxidant, antitumor and antiviral actions (1).
Baicalein (5,6,7-trihydroxyflavone) is a flavonid derived from
the root of Scutellaria baicalensis Georgi, a medicinal plant
traditionally used in Chinese herbal medicine (2). It was
reported that baicalein ameliorated all the considered
inflammatory symptoms in dextran sulfate sodium-induced
colitis in mice in vivo experiments (3). Many experiments also
showed that baicalein is a free-radical scavenger, an
antioxidant (4-7) and exhibits a cytoprotective effects (8-11).
Baicalein was also protective against benzo[·]pyrene and
aflatoxin B1-induced genotoxicity (12). Baicalein has been
reported to be an antiinflammatory agent (13) and an
inhibitor of prostaglandin E2 (14). Recently, it was reported
that baicalein inhibited hydrogen peroxide-induced apoptosis
via ROS-dependent heme oxygenase 1 gene expression (15).
It is well-known that the best strategy for killing cancer
cells is to induce cancer cell apoptosis. Antitumor agents can
trigger apoptosis and then lead to rapid elimination of tumor
cells (16, 17). Interference with the apoptotic process is
considered a crucial part of cancer prevention and therapy
(18). Another important factor is the interactions among
BCL-2 family proteins (pro-apoptotic and anti-apoptotic
proteins) which are also involved in cell death or survival (19,
20). Although many reports have demonstrated that baicalein
induced apoptosis in human cancer cells including breast
cancer (21), hepatoblastoma (22), prostate cancer (23) and
gastric cancer cells (24), there is no available information to
address the mechanism of baicalein-induced apoptosis in
SCC-4 human tongue cancer cells. The aim of the present
study was to investigate the effects and the role of Ca
2+and
caspase-3 in the induction of apoptosis by baicalein in SCC-4
human tongue cancer cells.
Correspondence to: Jing-Gung Chung, Ph.D., Department of
Microbiology, School of Medicine, China Medical University, No 91, Hsueh-Shih Road, Taichung 404, Taiwan, R.O.C. Tel: +88 642205 3366, Fax: +88 642205 3764, e-mail: jgchung@mail.cmu.edu.tw
Key Words: Baicalein, reactive oxygen species, cytochrome c,
cytoplasmic Ca2+, caspase-3, mitochondrial death pathway, apoptosis, SCC-4 cells.
Baicalein Induces Apoptosis in SCC-4 Human Tongue Cancer
Cells via a Ca
2+
-dependent Mitochondrial Pathway
YUH-TZY LIN
1, JAI-SING YANG
2, HUI-JU LIN
3,4, TZU-WEI TAN
2, NOU-YING TANG
5,
JO-HUA CHAING
8, YUNG-HSIEN CHANG
6, HSU-FENG LU
7and JING-GUNG CHUNG
8,91
Department of Nursing and Management, Jen-Teh Junior College of Medicine, Taiwan;
Departments of
2Pharmacology and
8Biological Science and Technology,
5School of Chinese Medicine,
6
Graduate Institute of Integration Chinese and Western Medicine, China Medical University, Taichung, Taiwan;
3Department of Ophthalmology, China Medical University Hospital, Taichung, Taiwan;
4
Department of Life Science, Tunghai University, Taichung, Taiwan;
7
Department of Clinical Pathology, Cheng Hsin Rehabilitation Medical Center, Taipei, Taiwan;
9Department of Biotechnology, Asia University, Wufeng, Taichung County 41354, Taiwan, R.O.C.
USA). Caspase-3 activity assay kits were bought from Boehringer Mannheim (Mannhein, Germany).
SCC-4 human tongue cancer cell line. The SCC-4 cell line was
purchased from the Food Industry Research and Development Institute (Hsinchu, Taiwan, ROC). SCC-4 cells have been cultured for several generations and have been checked for viability as described previously. The cells were placed into 75 cm3tissue culture flasks and grown at 37ÆC under a humidified 5% CO2and 95% air at one atmosphere in DMEM supplemented with 10% FBS, 1% penicillin-streptomycin (10 ng/ml penicillin and 10 ng/ml streptomycin) and 1% glutamine (25).
Baicalein effect on caspase-3 activity. The SCC-4 cells were plated
in 6-well plates at a density of 5x105cells/well and grown for 24 hours. Then cells were grown in 25 and 75 ÌM baicalein, with DMSO (solvent) alone for the control regimen, at 37ÆC in a humidified 5% CO2 for 24 hours. The caspase-3 activity was analyzed by flow cytometry (Becton Dickinson FACS Calibur) as described elsewhere (26-28).
Baicalein effect on reactive oxygen species (ROS). The SCC-4 cells were
treated with or without 0, 25 or 75 ÌM of baicalein (or pre-treated with 10 ÌM caspase inhibitor Ac-DEVD-CHO for 3 hours) for 24 hours to detect the changes of ROS. The cells were harvested and washed twice, re-suspended in 500 Ìl of 2,7-dichlorodihydrofluorescein diacetate (10 ÌM) (DCFH-DA, Sigma) and incubated at 37ÆC for 30 min, before being analyzed by flow cytometry (26-28).
Baicalein effect on Ca2+concentrations. The SCC-4 cells were treated
with or without 0, 25 or 75 ÌM of baicalein for 24 hours then to detect the changes in Ca2+concentrations, and then they were harvested and washed twice for re-suspension in Indo 1/AM (3 Ìg/ml) (Calbiochem, La Jolla, CA, USA), incubated at 37ÆC for 30 min, and then analyzed by flow cytometry (26-28).
Baicalein effect on mitochondrial membrane potential (MMP).
Cells were treated with 75 ÌM baicalein for 24 hours. The cells were harvested and washed twice, re-suspended in 500 Ìl of DiOC6 (4 mol/L), incubated at 37ÆC for 30 min, and then analyzed by flow cytometry (26-28).
Effect of baicalein on cytoplasmic Ca2+, MMP, and proportion of apoptosis in human tongue cancer SCC-4 cells pretreated with BAPTA. The level of Ca2+, MMP and apoptosis of the SCC-4 cells was determined by flow cytometry (Becton Dickinson FACS Calibur) using the Indo 1/AM (Calbiochem). Cells were pre-treated with or without 10 ÌM BAPTA-AM (intracellular calcium chelator) for 3 hours then with 25 and 75 ÌM baicalein for 24 hours. The cells were harvested and washed twice, for apoptosis
analysis and for re-suspension in Indo 1/AM (3 Ìg/ml), incubated at 37ÆC for 30 min, and then analyzed by flow cytometry (26-28) for Ca2+concentration, MMP and apoptosis.
Effect of baicalein on the expressions of p53, BAX, BCL-2, cytochrome c, caspase-3 and -9, EndoG and AIF. The total proteins were
collected from SCC-4 cells treated with or without 0, 25 or 75 ÌM of baicalein for 48 hours. Subsequently, p53, BAX, BCL-2, cytochrome
c, caspase-3 and -9, EndoG and AIF were measured by sodium
dodecylsulfate polyacrylamide gel electrophoresis (SDS-PAGE) and Western blot. The levels of cytochrome c in the mitochondria and in the cytosol were also determined. The SCC-4 cells after treatment with or without 75 ÌM baicalein were harvested and disrupted. They were then centrifuged to obtain cytosolic and mitochondria fractions and underwent further examination in regards to the level of cytochrome c by Western blot as described elsewhere (28, 29).
Statistical analysis. Statistical calculations of the data were
performed using an unpaired Student’s t-test and Tukey’s test. Statistical significance was set at p<0.05.
Results
Induction of apoptosis by baicalein. After SCC-4 cells were
treated with 75 ÌM baicalein for 0, 6, 12, 24, 48 and 72
hours, apoptosis was detected by PI staining method and
then analyzed by flow cytometry. As shown in Figure 1,
baicalein induced apoptosis in a time-dependent manner.
Effect of baicalein on caspase-3 activity. Caspase-3 activity
increased in baicalein-treated SCC-4 cells and increasing
baicalein dose led to increasing caspase-3 activity, as shown
in Table I.
Effect of baicalein on ROS. The increase of the levels of
ROS was detected in 24 hours after cells being treated with
Figure 1. Baicalein induced apoptosis in SCC-4 human tongue cancercells. PI staining analysis for the effects of baicalein on human tongue cancer SCC-4 cell’s apoptosis. The SCC-4 cells were incubated with 75 ÌM baicalein for 6, 12, 24, 48 and 72 h and apoptosis was determined by flow cytometric analysis as described in Materials and Methods. Data represents mean±S.D. of three experiments (*p<0.05).
baicalein and increasing dose led to an increase in ROS
production (Table I).
Effect of baicalein on the levels of cytoplasmic Ca
2+from
human tongue cancer SCC-4 cells. The increase of the levels
of cytoplasmic Ca
2+was detected 24 hours after treatment
with baicalein, dose-dependently (Table I).
Effect of baicalein on the MMP levels. Mitochondria
membrane potential (MMP) declined in SCC-4 cells treated
with 25 and 75 ÌM baicalein (Table I).
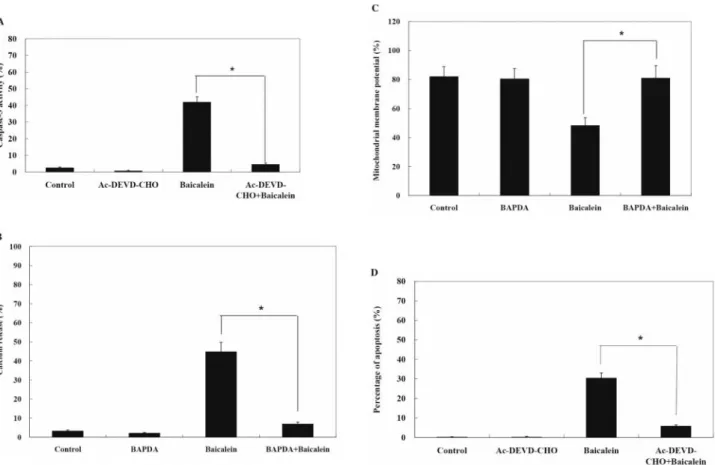
Effect of Ac-DEVD-CHO on baicalein promoted caspase-3
activity in human tongue cancer SCC-4 cells. SCC-4 cells
were pretreated with or without Ac-DEVD-CHO (inhibitor
of caspase-3) followed by treatment with 75 ÌM baicalein
for 24 h. Caspase-3 activity was increased in
baicalein-treated cells of SCC-4, but decreased in caspase-3 inhibitor
(Ac-DEVD-CHO) pretreatment in baicalein-treated
SCC-4 cells, as shown in Figure 2A.
Effects of BAPTA on baicalein-induced changes in levels of
cytoplasmic Ca
2+, MMP and apoptosis of SCC-4 human tongue
cancer cells. The changes of the levels of cytoplasmic Ca
2+,
Figure 2. Caspase-3 inhibitor inhibited baicalein induced apoptosis in human tongue cancer SCC-4 cells. The SCC-4 cells were pre-treated with BAPTAfor 3 h then treated with 75 ÌM baicalein before cells were harvested for caspase-3 activity, Ca2+concentration, MMP levels and apoptosis determinations as described in Materials and Methods. Data represents mean±S.D. of three experiments. *p<0.05. (Panel A: caspase-3 activity; panel B: Ca2+ concentration; panel C: MMP levels; and panel D: apoptosis).
Table I. Flow cytometric analysis of intracellular ROS and Ca2+levels, MMP and caspase-3 activity in SCC-4 cells treated with baicalein.
Baicalein (ÌM) % of control
ROS Ca2+ MMP Caspase-3
0 1.2±0.6 4.8±1.2 96.0±6.8 2.9±0.8
25 19.6±2.9b 32.4±2.8b 78.4±7.2b 26.6±3.4b 75 54.8±6.8a 61.2±5.2a 54.2±4.8a 64.2±7.4a The SCC-4 cells (5x105 cells/ml) were treated with various concentrations of baicalein. The zero concentration was defined as control. The stained cells were determined by flow cytometry as described in the Materials and Methods section. Values are means±SD (n=3). Dates not sharing the same letter are significantly different by Tukey’s test (*p<0.05).
MMP and apoptosis in baicalein-treated SCC-4 cells were
greatly affected by pretreatment with BAPTA. The decline of
MMP induced by baicalein was reversed by BAPTA. After
cells were pretreated with BAPTA, the increase in cytoplasmic
Ca
2+was suppressed and the proportion of apoptosis was also
markedly diminished (Figure 2B, C and D).
(Figure 4).
Discussion
Although many experiments have shown that baicalein
induced apoptosis in human cancer cell lines there is no
available information regarding the effects of baicalein on
SCC-4 human tongue cancer cells. In this study, we
demonstrated that baicalein induced apoptosis in SCC-4
cells via a Ca
2+-associated mitochondrial and
caspase-3-dependent pathway. Our results from Western blotting
demonstrated that baicalein promoted production of p53,
BAX, cytochrome c, EndoG and AIF and reduced the levels
of BCL-2 which led to the disruption of mitochondrial
membrane potential (MMP) and the release of cytochrome
c from the mitochondria to the cytosol. This finding also
points out that baicalein may be useful in clinical trials in
the future for tongue cancer patients.
Our data indicated that baicalein-induced apoptosis also
involved a decrease of the MMP, which is in agreement with
the reports showing that apoptosis is accompanied by a loss
of MMP (30, 31) and exposure of phosphatidylserine at the
surface of the cell (32-35). It is well-known that
mitochondrial alterations constitute a critical event of the
apoptotic cascade (36). Our results also showed that
baicalein promoted cytochrome c release (Figure 4) which
is in agreement with other reports which indicated that the
reduction of MMP is an early reversible step of apoptosis,
followed by cytochrome c release in many cell types based
on the DiOC
6uptake as shown by flow cytometric analysis
(37). Therefore, baicalein induced apoptosis in SCC-4 cells
through a mitochondria-dependent pathway.
Many investigators have demonstrated that baicalein
induced apoptosis in human cancer cells and the
apoptogenic action of baicalein was associated with
caspase activation, mitochondrial dysfunction and
modulation of BCL-2 family proteins (21-24). However,
information regarding the role of Ca
2+in the induction of
apoptosis caused by baicalein is scarce. Based on our
results, baicalein elevated cytoplasmic Ca
2+which is
involved in the induction of apoptosis (based on
pretreatment with BAPTA (Ca
2+chelator) in
baicalein-treated SCC-4 cells), MMP was restored, the level of
cytoplasmic Ca
2+was reduced and the proportion of
Figure 3. Western blotting showing that baicalein affects apoptosis-associatedproteins in SCC-4 human tongue cancer cells. Representative Western blot showing changes on the levels of p53, BAX, BCL-2, cytochrome c, caspase-9, caspase-3, EndoG and AIF in human tongue cancer SCC-4 cells after treated with baicalein. The SCC-4 cells (5x106/ml) were treated with 0, 25 and 75 ÌM baicalein for 48 h, then total protein was prepared and determined as described in Materials and Methods, then followed by evaluation of the protein levels (Panel A: p53, BAX and BCL-2; panel B: caspase-9, caspase-3, EndoG and AIF). Expressions were estimated by Western blotting as described in Materials and Methods.
apoptosis was also markedly diminished. This conclusion
is confirmed by other reports demonstrating that under
normal conditions, the Ca
2+influx increases and cells die
(38). Furthermore, other investigators also showed that
Co
2+blocks Ca
2+influx, resulting in the protection of the
cells despite high ROS levels (21). Our results also showed
baicalein induced ROS production (Table I). It is
well-known that in normal amounts ROS is involved in
maintaining human physiological functions, however
overproduction of ROS can be detrimental and has been
shown to participate in the etiology of several human
diseases such as cancer, inflammation and diabetes.
It is also reported that flavonols may interact directly with
a Ca
2+channel which leads to prevention of its opening
that is responsible for the final demise of the cell (39).
Whether or not they may prevent the signaling mechanism
between high ROS levels and the opening of the Ca
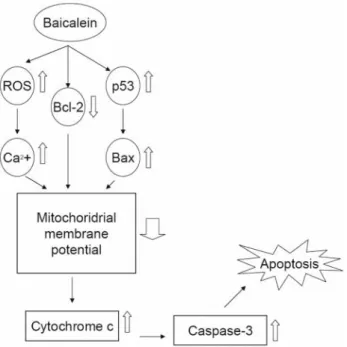
2+channel needs further investigation. We summarize the
possible signal pathway (Figure 5) demonstrating that
baicalein induced apoptosis through a mitochondrial and
caspase-3-dependent pathway and that AIF and EndoG are
involved in these events.
Acknowledgements
This work was supported by grant CMU95-127 from China Medical University, Taichung, Taiwan, R.O.C.
References
1 Middleton E Jr: Some biological properties of plant flavonoids. Ann Allergy 61: 53-57, 1988.
2 Kim YO, Leem K, Park J, Lee P, Ahn DK, Lee BC, Park HK, Suk K, Kim SY and Kim H: Cytoprotective effect of Scutellaria
baicalensis in CA1 hippocampal neurons of rats after global
cerebral ischemia. J Ethnopharmacol 77: 183-188, 2001. 3 Hong T, Jin GB, Cho S and Cyong JC: Evaluation of the
anti-inflammatory effect of baicalein on dextran sulfate sodium-induced colitis in mice. Planta Med 68: 268-271, 2002.
4 Hamada H, Hiramatsu M, Edamatsu R and Mori A: Free radical scavenging action of baicalein. Arch Biochem Biophys
306: 261-266, 1993.
5 Gao Z, Huang K, Yang X and Xu H: Free radical scavenging and antioxidant activities of flavonoids extracted from the radix of Scutellaria baicalensis Georgi. Biochim Biophys Acta 147: 2643-2650, 1999.
6 Shieh DE, Liu LT and Lin CC: Antioxidant and free radical scavenging effects of baicalein, baicalin and wogonin. Anticancer Res 20: 2861-2865, 2000.
7 Chen ZY, Su YL, Bi YR, Tsang SY and Huang Y: Effect of baicalein and acetone extract of Scutellaria baicalensis on canola oil oxidation. J Am Oil Chem Soc 77: 73-78, 2000.
Figure 4. Representative Western blot showing changes on the levels of cytochrome c in SCC-4 human tongue cancer cells after being treated with
baicalein. The SCC-4 cells (5x106/ml) were treated with 75 ÌM baicalein for 48 h before mitochromia and/or cytosolic fraction and total protein were prepared and determined. The evaluation of the protein levels (cytochrome c from total, mitochondria and cytosol fraction) were estimated using Western blotting as described in Materials and Methods.
Figure 5. Proposed model of baicalein mechanism of action for apoptosis in
SCC-4 human tongue cancer cells. Baicalein increased the production of BAX, cytochrome C, ROS and Ca2+and decreased MMP levels led to cytochrome c release and caspase-3 activation before causing apoptosis in SCC-3 cells.
11. Choi J, Conrad CC, Malakowsky CA, Talent JM, Yuan CS and Gracy RW: Flavones from Scutellaria baicalensis Georgi attenuate apoptosis and protein oxidation in neuronal cell lines. Biochim Biophys Acta 1571: 201-210, 2002.
12 Ueng YF, Shyu CC, Liu TY, Oda Y, Lin YL, Liao JF and Chen CF: Protective effects of baicalein and wogonin against benzo[a]pyrene- and aflatoxin B(1)-induced genotoxicities. Biochem Pharmacol 62: 1653-1660, 2001.
13 Lin CC and Shieh DE: The anti-inflammatory activity of
Scutellaria rivularis extracts and its active components, baicalin,
baicalein and wogonin. Am J Chin Med 241: 31-36, 1996. 14 Nakahata N, Kutsuwa M, Kyo R, Kubo M, Hayashi K and
Ohizumi Y: Analysis of inhibitory effects of scutellariae radix and baicalein on prostaglandin E2 production in rat C6 glioma cells. Am J Chin Med 263: 311-323, 1999.
15 Lin HY, Shen SC, Lin CW, Yang LY and Chen YC: Baicalein inhibition of hydrogen peroxide-induced apoptosis via ROS-dependent heme oxygenase 1 gene expression. Biochim Biophys Acta, 2007
16 Li Y, Maher P and Schubert D: Requirement for cGMP in nerve cell death caused by glutathione depletion. J Cell Biol
139: 1317-1324, 1997.
17 Woynarowska BA and Woynarowska JM: Preferential targeting of apoptosis in tumor versus normal cells. Biochem Biophys Acta 1587: 309-317, 2002.
18 Lowe SW and Lin AW: Apoptosis in cancer. Carcinogenesis 21: 485-495, 2000.
19 Gross A, McDonnell JM and Korsmeyer SJ: Bcl-2 family members and the mitochondria in apoptosis. Genes Dev 13: 1899-1911, 1999.
20 Reed JC: Bcl-2 family proteins. Oncogene 17: 3225-3236, 1998. 21 Tong W, Ding X and Adrian T: The mechanisms of lipoxygenase inhibitor-induced apoptosis in human breast cancer cells. Biochem Biophys Res 296: 942-948, 2002.
22 Chang WH, Chen CH, Gau RJ, Lin CC, Tsai CL, Tsai K and Lu FJ: Effect of baicalein on apoptosis of the human Hep G2 cell line was induced by mitochondrial dysfunction. Planta Med
68: 302-306, 2002.
23 Pidgeon GP, Kandouz M, Meram A and Honn KV: Mechanisms controlling cell cycle arrest and induction of apoptosis after 12-lipoxygenase inhibition in prostate cancer cells. Cancer Res 62: 2721-2727, 2002.
24 Wong BC, Wang WP, Cho CH, Fan XM, Lin MC, Kung HF and Lam SK: 12-Lipoxygenase inhibition induced apoptosis in human gastric cancer cells. Carcinogenesis 22: 1349-1354, 2001. 25. Lu HF, Hsueh SC, Ho YT, Kao MC, Yang JS, Chiu TH, Huamg SY, Lin CC and Chung JG: ROS mediates baicalin-induced apoptosis in human promyelocytic leukemia HL-60 cells through the expression of the Gadd153 and mitochondrial-dependent pathway. Anticancer Res 27(1A): 117-125, 2007.
carboxylate (JOT01007) induces apoptosis in human cervical cancer Ca Ski cells. In Vivo 21: 397-406, 2007.
29. Kim HR, Kim EJ, Yang SH, Jeong ET, Park C, Lee JH, Youn MJ, So HS and Park R: Trichostatin A induces apoptosis in lung cancer cells via simultaneous activation of the death receptor-mediated and mitochondrial pathway. Exp Mol Medicine 36: 616-624, 2006.
30 Lin SY, Jang JH and Surh YJ: Induction of cyclooxygenase-2 and peroxisome proliferators-activated receptor-gamma during nitric oxide-induced apoptotic PC12 cell death. Ann NY Acad Sci 1010: 648-658, 2003.
31 Kluza J, Clark AM and Bailly C: Apoptosis induced by the alkaloid sampangine in HL-60 leukemia cells: correlation between the effects on the cell cycle progression and changes of mitochondrial potential. Ann NY Acad Sci 1010: 331-334, 2003. 32 Mathur A, Hong Y, Kemp BK, Barrientos AA and Erusalimsky JD: Evaluation of fluorescent dyes for the detection of mitochondrial membrane potential changes in cultured cardiomyocytes. Cardiovasc Res 46: 24-27, 2000.
33 Isenberg JS and Klaunig JE: Role of the mitochondrial membrane permeability transition (MPT) in rotenone-induced apoptosis in liver cells. Toxicol Sci 53: 340-351, 2000.
34 Koopman G, Reutelingsperger CP, Kuijten GA, Keehnen RM, Pals ST and van Oers MH: Annexin V for flow cytometric detection of phosphatidylserine expression on B cells undergoing apoptosis. Blood 84: 1415-1420, 1994.
35 Zang G, Gurtu V, Kain SR and Yan G: Early detection of apoptosis using a fluorescent conjugate of annexin V. Biotechniques 23: 525-531, 1997.
36 Hajra KM and Liu JR: Apoptosome dysfunction in human cancer. Apoptosis 9: 691-704, 2004.
37 Ozgen U, Savasan S, Buck S and Ravindranath Y: Comparison of DiOC6 uptake and annexin V labeling for quantification of apoptosis in leukemia cells and non-malignant T lymphocytes from children. Cytometry 42: 74-78, 2000.
38 Esposti MD, Hatzinisiriou I, McLennan H and Ralph S: Bcl-2 and mitochondrial oxygen radicals. New approaches with reactive oxygen species-sensitive probes. J Biol Chem 274: 29831-29837, 1999.
39 Li Y, Maher P and Schubert DA: Role for 12-lipoxygenase in nerve cell death caused by glutathione depletion. Neuron 19: 453-463, 1997.