Physicochemical Characterization of Lens Crystallins from
the Carp and Biochemical Comparison with Other
Vertebrate and Invertebrate Crystallins
Shyh-Homg CfflOU,1 Wen-Chang CHANG, Fu-Ming PAN,
Tschining CHANG, and Tung-Bin LO
Institute of Biochemical Sciences, National Taiwan University and Institute of Biological Chemistry, Academia Sinica, P.O. Box 23-106, Taipei 107, Taiwan
Received for publication, October 20, 1986
j
Lens crystallins were isolated from the homogenate of carp (Cyprinus carpio) eye lenses by gel permeation chromatography and characterized by gel electrophoresis,
A immunodiffusion, amino acid analysis, circular dichroism, and protein sequence
analysis. Three well-defined fractions corresponding to a//3-, £-, and y-crystallins were obtained in relative weight percentages of 26, 22, and 52 %. The native molec-ular masses of the purified fractions were determined to be 410, 60, and 20 kDa, respectively. The polypeptide compositions as determined by SDS gel electropho-resis revealed the substantial presence of 0-crystallin polypeptides in the a-crystallin fraction; this is also evident in the fractionation of amphibian crystallins but is not common in the case of higher classes of vertebrates. The circular dichroism spectra indicate a predominant /J-sheet structure in all three fractions, albeit with some contribution of a-helical structure in the y-crystallin, the amino acid composition of which bears a resemblance to that of squid crystallin. Sequence comparison of carp y-crystallin with frog and calf y-crystallins indicates a high degree of homology in their N-terminal segments despite the dissimilarity of amino acid compositions and weak immunological cross-reactivity.
The lens crystallins of vertebrates form a complex crystallin classes encountered in the vertebrate (9). group of highly conserved structural proteins with Before the successful gene sequence analysis of distant evolutionary relationships (7). They have squid crystallin (unpublished data), we think it been examined by recombinant-DNA techniques, worthwhile to present a detailed characterization notably detailed DNA-sequencing studies of crys- of the lens crystallins from the most primitive tallins from rat (2), mouse (5), frog (4), and chicken class of vertebrates, i.e. the fish, in order to provide (5). Another crystallin of interest is that of the a link between the cephalopod and the vertebrate, squid {6-8), an invertebrate which contains a single Furthermore, owing to the limitations of the ana-type of crystallin in contrast to the complexity of lytical methods involved, previous studies on the lens proteins of fishes have emphasized
the total lens extracts of different species of fishes and those of mammalian lenses (10-14). However, there are few reports of well-defined separation and characterization of fish crystallins with regard to their physicochemical properties. In this com-munication we have extended our preliminary re-port (15) to provide a detailed biochemical char-acterization of carp crystallins which forms the basis of our current work on the genomic analysis of crystallin genes from the common carp, Cyprinus
carpio.
MATERIALS AND METHODS
Isolation of Lens Crystallins—Carp (C. carpio),
bullfrog (Rana catesbeiand), pig (Sus scrofa var.
domestica), and squid (Sepia esculenta) lenses were
obtained from local meat companies and the spe-cies identification was done at the Department of Zoology, National Taiwan University. The de-capsulated lenses were homogenized in 10-20 ml of 0.05 M Tris-Na bisulfite buffer, pH 7.5, containing 5 raM EDTA as described before (7, 16, IT). The supernatant from 27,000 x g centrifugation was adjusted to give a protein concentration of about 20-30 mg/ml and a 5.0 ml aliquot was applied to Fractogel TSK HW-55 (Superfine Grade, Merck), equilibrated with 0.1 M ammonium bicarbonate, pH 7.7, containing 5 raM EDTA. Native molec-ular masses of the eluted fractions were estimated on the same column (2.5x115 cm) using the fol-lowing standard proteins: thyroglobulin (670 kDa), catalase (240 kDa), transferrin (80 kDa), ovalbumin (45 kDa), and soybean trypsin inhibitor (20 kDa). The y-crystallin fraction from the gel filtration column was further separated into four subfrac-tions on a TSK CM-650 (S) cation exchange col-umn with a linear gradient of 0.05-0.25 M ammo-nium acetate in the presence of 0.1% 2-mercapto-ethanol, pH 5.9. The most basic peak (the last one, i.e. y-IV) together with the unfractionated y-crystallin were taken for amino acid analysis and peptide mapping.
Gel Electrophoresis—Discontinuous native gel
(3% stacking/8% resolving gel) electrophoresis in Tris-glycine buffer was done according to Davis
(18). SDS-polyacrylamide slab gel (5% stacking/
14% resolving gel) was as described (19) with some modifications.
Amino Acid Analysis—The amino acid
com-positions were determined with an LKB-4150 amino acid analyzer using a single-column system. The dialyzed and lyophilized protein samples were hydrolyzed at 110°C in evacuated tubes with con-stant-boiling 6 N HC1 (Pierce Chemical Company, U.S.A.) for 24 h. Half-cystine was determined separately after performic acid oxidation. Tryp-tophan was not determined. The procedure of Marchalonis and Weltman (20) was used to analyze and compare the relatedness of amino acid com-positions using the equation SAQ=Y1(Xij — Xtj)1,
where the subscripts i and k identify the particular protein pairs being compared, and Xj is the mole content of a given amino acid of type /. The summation is carried out over the 17 types of amino acids typically determined on the 6 N HCI hydrolysates of crystallin samples.
Peptide Mapping—Peptide mapping of the
iso-lated crystallins with trypsin was done on a Mil-lipore-Waters HPLC system using a reversed-phase column (3.9x300 mm, SynChropak RP-P, C-18, 6.5 /im beads) with the solvent systems described in the figure legends. The crystallins (about 1.0 mg/ml each in 1 % ammonium bicarbonate buffer, pH 8.2) were digested with TPCK-trypsin (Worth-ington) at an enzyme/substrate ratio of 1 : 100 at room temperature for 4 h and overnight with a second digestion of the same protein solutions under the same conditions. The digested solutions were lyophilized before analyses.
Immunodiffusion—Ouchterlony's method (21)
was followed with minor modifications. The anti-sera were used without dilution and the final con-centration of each antigen was about 1-5 /ig//d.
Circular Dichroism (CD)—The circular
di-chroic spectra of crystallins were taken on a Jasco J-20 spectropolarimeter thermostated at 20°C, under a nitrogen flow. The instrument was cali-brated with an aqueous solution of (+)-10-cam-phorsulfonic acid. The dialyzed and lyophilized crystallins were dissolved in 0.05 M Tris buffer, pH 7.8, at a concentration of 0.5-1.2 mg/ml, de-pending on the samples. All protein solutions were filtered through an Acrodisc (Gelman) mem-brane (0.2 /<m) before spectral analyses. The ellipticity data were converted to mean-residue-weight ellipticity using a mean residue mean-residue-weight of 115 for all crystallins. Analysis of CD in terms of the fractions of the structural elements, i.e. helix, beta form, beta-turn and unordered form,
J-was carried out according to the procedure of Chang et al. (22). A nonlinear least-squares curve fitting of CD spectra in the 205-240 nm region at 2 nm intervals was used to find the best estimate for the percent contribution of each structural element in the studied crystallins.
Protein Sequence Analysis—The N-termina! sequences of y-III and y-TV of the last two frac-tions from the TSK CM-650 (S) column were determined by automated Edman degradation with a gas-phase protein sequencer (model 470A, Ap-plied Biosystems). The lyophilized samples were dissolved in 200 /<1 of 0.1 % SDS and 10 /il aliquots containing about 1-5 nmol of y-crystallins were used for sequence determinations. The squid crystallin isolated as described before (7) was similarly processed for Edman protein sequencing. The phenylthiohydantoin (PTH) derivative of cys-teine residues was determined in the case of iodo-acetamide-modified crystallin samples.
Protein Concentration Determinations—The protein concentrations of crystallin solutions were determined by amino acid analysis. Due to the inherent interference of Na bisulfite and Tris buffer with the color development of the classical Lowry assay (23), the previous CD study of bovine crys-tallins (760 showed a strong tendency to under-estimate ellipticities arising from overestimation of the protein concentrations. In this study we first determined the protein content of each crystallin solution by means of amino acid analysis and the modified Lowry assay of Wahl et al. (24). When-ever inconsistency arose between the two methods (especially in the samples of squid crystallin), we used the values from amino acid analysis. The absorption coefficient (1 mg/ml, at 280 nm) was then determined for each purified crystallin. Sub-sequently, the protein contents of crystallin samples were estimated from their absorbances at 280 nm.
RESULTS
Characterization of carp crystallins and compari-son with squid and frog crystallins.
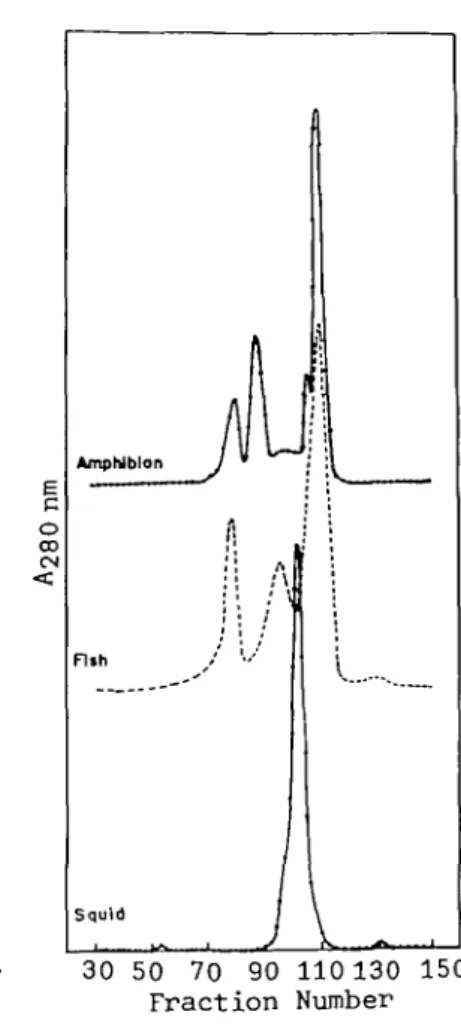
Figure 1 shows a typical elution pattern of lens extracts from three different species belonging to two classes of vertebrates and one class of inver-tebrate. Three well-defined peaks were obtained for the carp lens in contrast to one for the squid (invertebrate) and four for the frog (amphibian).
30 50 70 90 110 130 150 Fraction Number
Fig. 1. Comparative gel permeation chromatography on Fractogel TSK HW-55(S) of lens extracts from the lenses of three species (vertebrates and invertebrate). Conditions were as described in " MATERIALS AND METHODS." The column eluates (3.5 ml/tube per 4.1 min) were monitored for absorbance at 280 nm. The 3 peaks in the middle of the figure correspond to three crystallin fractions characterized in Table I. Rechromatography of the peaks on the same column to remove some cross-contaminating fractions gave sufficient purity for amino acid analysis and SDS gel electrophoresis. The absorbances at 280 nm are rela-tive concentrations in arbitrary units. The small broad peak after the y-crystallin peak in the chromatogram of fish lens is non-protein components having low molecular masses. The shoulder peak near the major frog y-crystallin is a novel crystallin, as described before (17).
It is of interest to find that the first peak of the carp elution pattern contains a-crystallin as well as the aggregated form of /?-crystallin (Fig. 2); this is also the case for the fractionation of amphibian lenses (17) but is not common in the higher classes of vertebrates, i.e. reptiles, birds, and mammals (25).
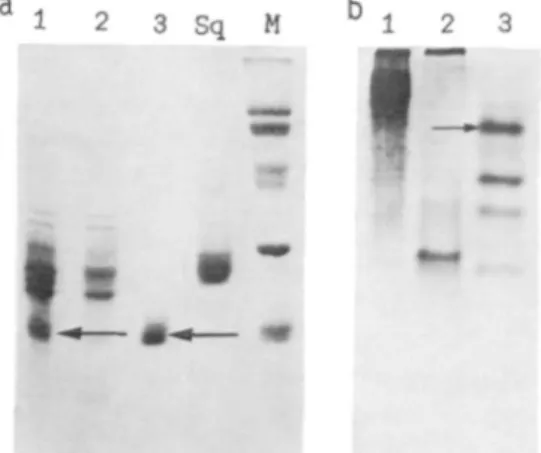
The electrophoretic patterns of the three iso-lated carp crystallins were compared with that of squid crystallin, with a molecular mass of 29 kDa, which is the only class of crystallin detected in the squid lens (7, 8). Rechromatography of the gel permeation peaks on the same column to remove some cross-contaminating fractions was sufficient to provide over 95% pure proteins as indicated in the SDS gels shown in Fig. 2a. However, charge heterogeneity could be detected under non-denaturing conditions, as indicated in Fig. 2b. It is evident that a- and /3-crystallins usually exist as polydisperse aggregated systems under nondenatur-ing electrophoretic conditions with smeared pat-terns and aggregated materials present at the top of the resolving gel.
It is noteworthy that our fractionation of lens crystallins by means of TSK. HW-55 gel is com-parable to that obtained by Bindels et al. (26) by high-resolution HPLC, although we have a lower estimate of native molecular mass in the ajp frac-tion than was obtained in their size determinafrac-tion
i 2 3 S q M 1
I
Fig. 2. Gel electrophoresis of the isolated crystallins under denaturing (SDS-PAGE) (a) and nondenaturing (b) conditions in the presence of 5 mM dithiothreitol. Lanes 1-3 correspond to three crystallin fractions of carp in Fig. 1. Lane Sq is the single crystallin from the squid. Lane M shows the standard proteins used as molecular mass markers: transferrin (80kDa), bo-vine serum albumin (66 kDa), ovalbumin (45 kDa), carbonic anhydrase (30 kDa), and soybean trypsin in-hibitor (20.1 kDa). The gels were stained with Co-omassie blue. The arrows in (a) indicate the subunit positions for a- and y-crystallins with a molecular mass of about 20 kDa. The small arrow in (b) shows the y-IV crystallin which has also been purified on the ion exchange column and compared with the unfractionated y-crystallin in Table II.
TABLE I. Physicochemical parameters and secondary structures of carp and squid crystallins. The methods used for determination of these parameters are described in " MATERIALS AND METHODS." The values reported for % secondary structure represent the estimates from nonlinear least-squares curve fitting of CD spectra ± (extent of uncertainty in the fitting procedure).
Sq-Crystallin Carp-a//3 Carp-/? Carp-y 1. Relative weight (%)
2. Absorption coefficient (1 mg/ml, 280 nm) 3. Native molecular mass (kDa)
4. Subunit molecular mass (kDa)
5. °/o Secondary structure Helix Beta-sheet Beta-turns Unordered form >95 1.77 55 29 27 29±3 14±1 2O±2 37±4 26 1.65 410 20 23-35 13±3 34+9 24±5 29±6 22 2.48 60 26 23 26-35 12±4 42±7 20±3 26±7 52 2.14 20 20 15 + 3 39+11 25 + 10 21±1
of dogfish crystallins by a laser light-scattering method. Our superior and reproducible resolu-tion of the crystallins allowed us to make a sys-tematic and detailed comparison between the crys-tallins of different classes. The well-defined and characteristic distribution of subunit compositions for each isolated fraction (Fig. 2) justified the use of size-exclusion gel (27, 28) in place of ion ex-change chromatography (29) in the general char-acterization and classification of lens proteins.
We have further separated the carp y-crystallin (peak 3 of Fig. 1) into 4 charge isomers on a TSK CM-650 cation exchange column (data not shown), corresponding to the 4 major bands in gel electro-phoresis. We designated the most basic protein (the last peak in the CM-650 chromatography and the first band of gel electrophoresis) as y-IV and compared it with the unfractionated y-crystallin with regard to amino acid composition (Table II). No substantial difference could be found with the exception that y-IV appeared to have higher
con-Fig. 3. Immunodiffusion analyses of the antisera against carp a//? (A), carp P (B), and carp y ( Q crystal-lins to various antigens. The antisera were placed in the center well and the antigens of the surrounding wells (numbered 1 to 6) are as follows: 1, total carp lens crystallins; 2, carp a/0; 3, carp f); 4, carp y; 5, total porcine crystallins; 6, squid crystallin. Note the spur line below well 2 (Fig. 3A) corresponding to the precipitating line of a-cry stall in in addition to a fused line of /J-crystallin below well 3. The antisera against carp crystallins had a very weak reaction with total pig antigens (well 5, not visible here) and no cross-reaction at all with that of squid crystallin.
tents of lysine/arginine, which is consistent with the result of electrophoresis. Hence the physico-chemical characterization was carried out on the unfractionated y-crystallin because it is represen-tative of its four closely-related subfractions.
Physicochemical Parameters and Biochemical Comparison between Different Crystallins—Table I
summarizes the physicochemical properties of carp crystallins characterized in this study. Carp a/0 fraction (peak 1 of Fig. 1) is very heterogeneous as evidenced by the smeared zone in Fig. 2b. Carp /?-crystallin shows a more defined pattern with the mobility of the major band being similar to that of y-I subfraction of carp y-crystallin (the most
• r
Fig. 4. Tryptic peptide maps of carp y (A) and frog
y (B) crystallins in HPLC. Solvent A : 0.07%
trifluoro-acetic acid (TFA) in water. Solvent B : 0.07% TFA in acetonitrile. A linear gradient from 0 to 50% solvent B at a flow rate of 1 ml/min was run for the entire 50 min. Peptide peaks were monitored at 214 tun. The peptide map of squid crystallin showed a similarly com-plex pattern, yet was distinguishable from the two maps here (data not shown).
acidic band of Fig. 2b) except that a large pro-portion of the protein was precipitated at the top of the resolving gel during electrophoresis. Sur-prisingly, carp y-crystallins also showed 4 major bands in nondenaturing gel electrophoresis, which is very similar to the case of calf y-crystallins {30). The native and subunit molecular masses of each fraction as determined by gel permeation and SDS-gel electrophoresis are listed in Table I.
Immunodiffusion experiments (Fig. 3) showed two antigens in carp ajp and one in carp /S fractions (neither of them cross-reacted with carp y). The antisera against each isolated fraction had a very weak reaction with the total pig lens antigens and no cross-reaction at all with that of squid crystallin (wells 5 and 6 in Fig. 3). This is consistent with the previous serological studies which showed no cross-reactivity of lens homogenates between ceph-alopods and most vertebrate species {31-33).
Peptide mapping (Fig. 4) of the tryptic digests
of carp y- and frog y-crystallins by HPLC showed distinct differences in the peptide maps of these two proteins, which are also reflected in their amino acid compositions (Table II).
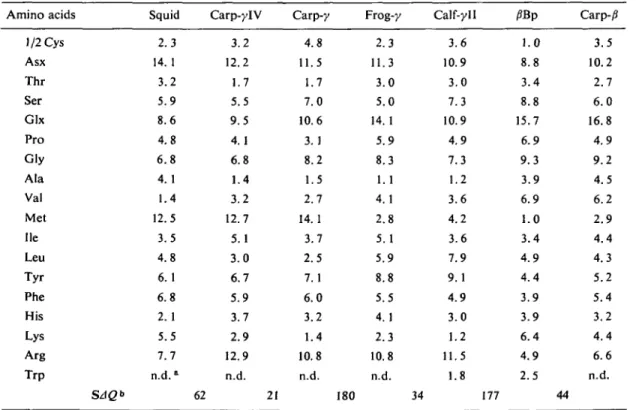
The amino acid compositions of carp y-IV and carp y are shown in Table II and compared with those of squid, calf y-II and the major jSBp polypeptide of calf j?-crystallins (34, 35). Since carp a/jS fraction is very heterogeneous, it is ex-cluded from the comparison. The salient feature of carp y-crystallins is that it contains a very high percentage of methionine (14mol%), like squid crystallin and haddock y-crystallin (36). This may have some bearing on their unique structural prop-erties as compared to those of mammalian crys-tallins, since the methionine contents of most globular proteins are less than 4% (37).
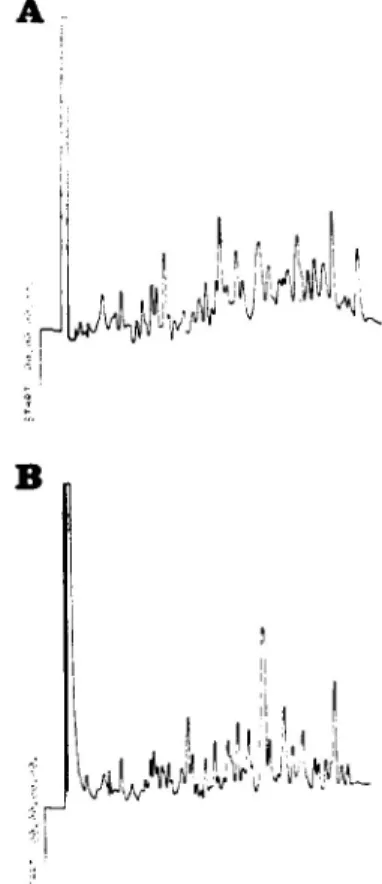
Circular Dichroic Study—CD spectra in the far
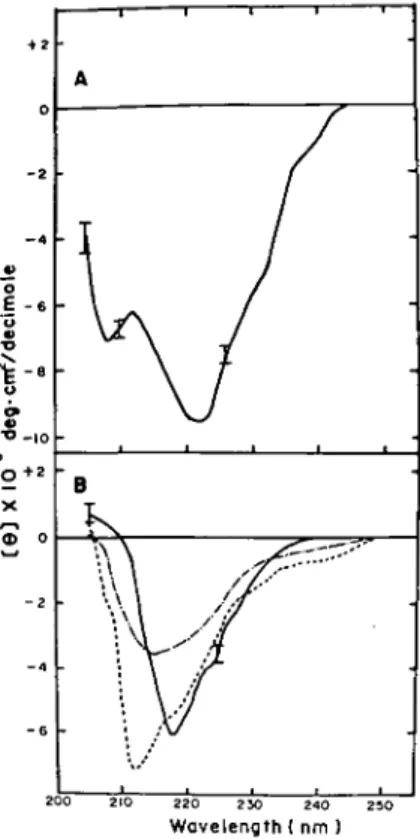
UV region for the three carp fractions and squid crystallin are shown in Fig. 5. All carp crystallins
TABLE II. Pair-wise comparisons of amino acid compositions between crystallins. All data are taken from this study except those for calf-yll (34) and j?Bp (35).
Amino acids 1/2 Cys Asx Thr Ser Glx Pro Gly Ala Val Met He Leu Tyr Phe His Lys Arg Trp SAQ* Squid 2.3 14.1 3.2 5.9 8.6 4.8 6.8 4.1 1.4 12.5 3.5 4.8 6.1 6.8 2.1 5.5 7.7 n.d.a Carp-ylV 3.2 12.2 1.7 5.5 9.5 4.1 6.8 1.4 3.2 12.7 5.1 3.0 6.7 5.9 3.7 2.9 12.9 n.d. 62 21 Carp-y 4.8 11.5 1.7 7.0 10.6 3.1 8.2 1.5 2.7 14.1 3.7 2.5 7. 1 6.0 3.2 1.4 10.8 n.d. 180 Frog-y 2.3 11.3 3.0 5.0 14.1 5.9 8.3 1.1 4.1 2.8 5.1 5.9 8.8 5.5 4.1 2.3 10.8 n.d. Calf-yll 3.6 10.9 3.0 7.3 10.9 4.9 7.3 1.2 3.6 4.2 3.6 7.9 9.1 4.9 3.0 1.2 11.5 1.8 34 177 /?Bp 1.0 8.8 3.4 8.8 15.7 6.9 9.3 3.9 6.9 1.0 3.4 4.9 4.4 3.9 3.9 6.4 4.9 2.5 Carp-j3 3.5 10.2 2.7 6.0 16.8 4.9 9.2 4.5 6.2 2.9 4.4 4.3 5.2 5.4 3.2 4.4 6.6 n.d. 44
" n.d., not determined. b SAQ represents the pair-wise comparison of amino acid contents of the adjacent crystallins
£ - < o * - . o '0+2 X 200 210 220 230 240 250 Wavelength! nm )
Fig. 5. CD spectra of squid (A) and carp (B) crystal-lins in the far UV region. Conditions are as described in " MATERIALS AND METHODS." [0] is the mean residue ellipticity in degrees •cm2/decimole based
on a mean residue weight of 115 for all crystallins. In (B) carp a/fi ( ), carp p ( ), and carp y ( ) correspond to the three re-chromatographed peaks of Fig. 1. The error bars in (A) and (B) represent the noise levels of the instrument, increasing with decrease of wavelength and not acceptable for interpretation be-low 205 nm.
showed predominant /S-sheet structure, in contrast to squid crystallin, which showed a relatively high proportion of a-helical structure. The estimated fractions of the four basic structural elements are shown in Table I. We have also compared the CD spectra of the subfractions of carp y-crystallin with that of native unfractionated y-crystallin (data not shown); essentially no qualitative difference cound be found in the peptide region though there were small quantitative differences in the magnitude of ellipticities.
N-Terminal Sequence Analysis—Two subfrac-tions, y-III and y-TV, obtained from the
cation-5 10 1cation-5 20 2cation-5 P-H-Y-T-L-Y-Y-F-H-G-R-G-R-A-E-I-L-R-H-L-F-A-A-A-G (Squid)
Y^ll-H-H-|D-c4 (Carp Y"'"> K-H K-H F-B-G L-H F-Sl-G L-S R-G F-8-G H-C Y-E I-H-H T-H-S A (Carp Y-IV>
A-D-L (Frog Y-2> P-H-L (Calf y - I l )
Fig. 6. Comparison of N-terminal sequences of carp y- and squid crystallins with those of frog and calf y-crystallins. The sequences for squid and carp crys-tallins were taken from this study and those for frog and calf from Refs. 4, 51. The sequence of the frog y-2 reported in Ref. 4 lacks the first 5 N-terminal residues and that for calf y-II (51) is different from the previous one determined by Croft (34) at six positions. Identical residues in the sequences are boxed. Dashed lines indi-cate the unidentified amino acids in the automatic pro-tein sequencing. Amino acid residues are represented by one-letter symbols.
exchange chromatography of crude y-crystallins, constitute about 70 % of the crude gamma-crystal-lins. The result of N-terminal sequence determi-nations of carp-y and squid crystallins by Edman degradation is shown in Fig. 6 for comparison with the reported sequences of other vertebrates.
DISCUSSION
According to the prevalent view (8, 38), the devel-opment of eye lens crystallins in the animal king-dom is a classical example of convergent evolu-tion. A biochemical comparison is given here with regard to lens crystallins isolated from verte-brate and inverteverte-brate species to draw some sup-port for the divergently evolutionary aspect of lens crystallins. We feel it is imperative to have a detailed characterization of crystallins from more primitive species of vertebrates in order to shed some light on this evolutionarily important issue. To our knowledge, this is the first report of a systematic study of fish crystallins with regard to well-defined physicochemical properties and phylo-genetic comparison with other crystallins. As opposed to the previous report of Cobb et al. (14), who did not detect a-crystallin in the electropho-retic analyses of various fish species, we have clearly identified a-crystallin in the first fraction of gel permeation chromatography, albeit with some contamination of carp /3-crystallin. Immunodif-fusion and gel electrophoresis confirmed that it is
distinct from carp /?- and y-crystallins and implied its presence in the vertebrate lens long before the appearance of the amphibian class.
The gross CD patterns of the carp crystallins were very similar to those found for the bovine crystallins (16, 39), with the main characteristic of beta-sheet structure. It was surprising to find that all vertebrate crystallins with the exception of <5-and e-crystallins of the bird (40, 41) showed essen-tially similar secondary structure irrespective of their differences in amino acid contents, immuno-logical properties and subunit compositions. This would indicate that subunit interactions of crys-tallins have no significant effect on the evolution of well-conserved secondary structure. Recently, X-ray analysis of yll-crystallin of bovine lens showed that this protein may possess the highest internal symmetry of any protein studied by X-ray diffraction (42). It will be of interest to make an X-ray structure analysis of fish y-crystallin, which shows quite different biochemical properties from y-crystallin of mammalian species.
Pair-wise comparisons of amino acid contents between these crystallins based on statistical anal-yses (20, 25, 43) give a crude, yet valuable measure of the relatedness of different families of proteins in the absence of complete sequence data (values of SAQ in Table II). It is very unexpected to find (25) that the recently described novel crys-tallins from the lamprey (44), frog (17, 45), and duck (17, 41) are all interrelated to each other and to the well-characterized 5-crystallin (40) of the bird with regard to their amino acid compositions. The mutual comparisons of different crystallins seem to indicate some phylogenetic relatedness be-tween these apparently unrelated crystallins. Sequ-ence homology has even been found between /?- and y-crystallins with very different amino acid com-positions (SAQ= 177, Table II) (46).
The interesting comparison of /3- and y-crys-tallins based on a combination of X-ray diffraction data and protein sequences has prompted the hypothesis that £• and y-crystallins probably form a single superfamily of Pjy crystallins (47). In addition, Wistow et al. (48) and Crabbe (49) re-cently reported that sequence homologies had been found between a bacterial protein plus an oncogene protein and /J/y crystallins. Alpha crystallin was also shown to be homologous to small heat-shock proteins in terms of sequences (50). The existence
of sequence homology generally implies that the protein pairs being compared probably originated from a common ancestral precursor (divergent evolution) while analogous tertiary structures with no apparent sequence homology would suggest otherwise (convergent evolution).
Comparison of the amino-terminal sequences of crystallins from five different species (Fig. 6) encompassing vertebrate and invertebrate lenses revealed a high degree of sequence homology present in the y-crystallins of vertebrates, yet with-out apparent homology between carp and squid crystallins. This seems to be in sharp contrast to the results of the comparison of amino acid com-positions. At the present time, we can only sug-gest that the evolution of y-crystallins in the verte-brate probably followed a divergent course. The decision between the two different views regarding the evolution of invertebrate and vertebrate crys-tallins must await the complete sequences of these crystallins. Recently we have isolated poly(A)-containing mRNAs from carp lenses and charac-terized their complementary DNA (cDNA) coding for the major y-crystallin (15 and unpublished data). The recombinant-DNA technique should allow analysis of the complete sequence of carp y-crystallin in the near future (manuscript in prep-aration). The sequence comparison at both gene and protein levels should cast some light on crys-tallin evolution. The preliminary N-terminal anal-ysis of purified a- and /3-crystallins on a gas-phase protein sequencer has indicated a blocked N-terminal residue in these crystallins, which has prevented the direct sequence comparison of the three major classes of carp crystallins at the pres-ent time.
In conclusion, carp crystallins have been iso-lated and characterized with special regard to native and subunit structures, immunological prop-erties, protein conformation, amino acid composi-tions, and protein partial sequences. The detailed genomic analyses of these proteins coupled with that of squid crystallin should provide a good testing ground for the two opposing hypotheses concerning the evolution of crystallins in the animal kingdom.
We acknowledge the generous support of the National Science Council, Taipei, Taiwan. The technical assis-tance of Mr. C.-H. Lo in the analysis of CD spectra by computer curve fitting is greatly appreciated.
25.
REFERENCES 2 6'
1. De Jong, W.W. (1981) In Molecular and Cellular Biology of the Eye Lens (Bloemendal, H., ed.) pp. 27. 221-278, Wiley, New York
2. Moormann, R.J.M., Den Dunncn, J.T., Bloemen- 28. dal, H., & Schoenmakers, J.G.G. (1982) Proc. Natl. 29. Acad. Sci. U.S. 79, 6876-6880 30. 3. Lok, S., Tsui, L.-C., Shinohara, T., Piatigorsky, J., 31.
Gold, R., & Breitman, M. (1984) Nucleic Acids Res. 12, 4517^t529 32. 4. Tomarev, S.I., Zinovieva, R.D., Chalovka, P.,
Rrayev, A.S., Skryabin, K.G., & Gausse, G.G., Jr. 33. (1984) Gene 27, 301-308
5. Yasuda, K., Kondoh, H., Okada, T.S., Nakajima, 34. N., & Shimura, Y. (1982) Nucleic Acids Res. 10, 35. 2879-2891
6. Chiou, S.-H. & Bunn, H.F. (1981) Invest. Ophthal- 36. mol. Vis. Sci. Suppl. 20, 138
7. Chiou, S.-H. (1984) / . Biochem. 95, 75-82 37. 8. Siezen, R.J. & Shaw, D.C. (1982) Biochlm. Biophys.
Ada 704, 304-320
9. Bloemendal, H. (1982) CRC Crit. Rev. Biochem. 12, 38. 1-38 39. 10. Manski, W. & Halbert, S.P. (1964) Protides Biol. 40.
Fluids, Proc. Colloq. 12, 117-134
11. Rabaey, M. (1964) Protides Biol. Fluids, Proc. 41. Colloq. 12, 273-277
12. Bon, W.F., RuttenbeTg, G., Swanborn, P.L., Sil-levis, S.W.W., & van der Ploeg, P.H.W. (1966) Exp. 42. Eye Res. 5, 58-62
13. Swanborn, P.L. (1966) Exp. Eye Res. 5, 302-308 14. Cobb, B.F., III, Carter, L., & Koenig, V.L. (1968) 43.
Comp. Biochem. Physiol. 24, 817-826
15. Chiou, S.-H., Chang, T., Chang, W.-C, Kuo, J., & 44. Lo, T.-B. (1986) Biochim. Biophys. Ada 871, 324-328 16. Chiou, S.-H., Azari, P., Himmel, M.E., & Squire, 45.
P.G. (1979) Int. J. Pept. Protein Res. 13, 409-417 17. Chiou, S.-H., Chang, W.-C, Kuo, J., Pan, F.-M.,
& Lo, T.-B. (1986) FEBS Lett. 196, 219-222 46. 18. Davis, B.J. (1964) Ann. NY Acad. Sci. 121, 404-427 19. Laemmli, U.K. (1970) Nature 227, 680-685 47. 20. Marchalonis, J.J. & Weltman, J.K. (1971) Comp.
Biochem. Physiol. 38B, 609-625
21. Ouchterlony, 0. (1958) in Progress in Allergy Vol. 48. V (Kallos, P., ed.) pp. 1-78, Karger, Basel
22. Chang, C.T., Wu, C.-S.C, & Yang, J.T. (1978) 49. Anal. Biochem. 91, 13-31 50. 23. Lowry, O.H., Rosebrough, N.J., Farr, A.L., &
Randall, R.J. (1951) / . Biol. Chem. 193, 265-275 51. 24. Wahl, R., Geissler, W., & Maasch, H.J. (1985)
Hoppe-Seyler's Z. Biol. Chem. 366, 979-984
Chiou, S.-H. (1986) FEBS Lett. 201, 69-73 Bindels, J.G., Bessems, G.J.J., de Man, B.M., & Hoenders, H.J. (1983) Comp. Biochem. Physiol.
76B, 47-55
Bloemendal, H. & Herbrink, P. (1974) Ophthalmic Res. 6, 81-92
Bloemendal, H. (1977) Science 197, 127-138 Spector, A. (1964) Invest. Ophthalmol. 3, 182-193 Bjdrk, I. (1964) Exp. Eye Res. 3, 254-261
Papaconstantinou, J. (1959) Biol. Bull. 117, 422-423
Halbert, S.P. & Manski, P. (1963) Prog. Allergy 7, 107-186
Bon, W.F., Dohrn, A., & Batink, H. (1967) Bio-chim. Biophys. Ada 140, 312-318
Croft, L.R. (1972) Biochem. J. 128, 961-970 Driessen, H.P.C., Herbrink, P., Bloemendal, H., & De Jong, W. (1981) Eur. J. Biochem. 121, 83-91 Croft, L.R. (1973) Biochim. Biophys. Ada 295, 174-177
Dayhoff, M.O. (1969) in Atlas of Protein Sequence and Structure Vol. 4, National Biomedical Research Foundation, Silver Spring, Md.
Packard, A. (1972) Biol. Rev. 47, 241-304 Horwitz, J. (1976) Exp. Eye Res. 23, 471-481 Piatigorsky, J., Zelenka, P., & Simpson, R.T. (1974) Exp. Eye Res. 18, 435-446
Stapel, S.O., Zweers, A., Dodemont, H.J., Kan, J.H., & De Jong, W.W. (1985) Eur. J. Biochem.
147, 129-136
Blundell, T., Lindley, P., Miller, L., Moss, D., Slingsby, C , Tickle, I., Turnell, B., & Wistow, G. (1981) Nature 289, 771-777
Cornish-Bowden, A. (1980) Biochem. J. 191, 349-354
Stapel, S.O. & De Jong, W.W. (1983) FEBS Lett.
162, 305-309
Tomarev, S.I., Zinovieva, R.D., Dolgilevich, S.M., Luchin, S.V., Krayev, A.S., Skryabin, K.G., & Gause, G.G., Jr. (1984) FEBS Lett. 171, 297-302 Driessen, H.P.C., Herbrink, P., Bloemendal, H., & De Jong, W.W. (1980) Exp. Eye Res. 31, 243-246 Wistow, G., Slingsby, C, Blundell, T., Driessen, H., De Jong, W., & Bloemendal, H. (1981) FEBS Lett. 133, 9-16
Wistow, G., Summers, L., & Blundell, T. (1985) Nature 315, 771-773
Crabbe, M.J.C. (1985) FEBS Lett. 181, 157-159 Ingolia, T.D. & Craig, E.A. (1982) Proc. Natl. Acad. Sci. U.S. 79, 2360-2364
Summers, L.J., Slingsby, C , Blundell, T.L., den Dunnen, J.T., Moormann, R.J.M., & Schoen-makers, J.G.G. (1986) Exp. Eye Res. 43, 77-92