A new species of vespertilionid bat from Taiwan and a revision
of the taxonomic status of Arielulus and Thainycteris
(Chiroptera: Vespertilionidae)
GaÂbor Csorba1and Ling-Ling Lee21Department of Zoology, Hungarian Natural History Museum, H-1088 Budapest, Baross u. 13 Hungary 2Department of Zoology, National Taiwan University, Taipei, Taiwan, R.O.C.
(Accepted 7 October1998) Abstract
On morphological and chromosomal grounds Pipistrellus (Arielulus) subgenus is elevated to generic rank and the recently described genus Thainycteris has been synonymized with Arielulus. Besides this taxonomic revision, a new species of bat Arielulus torquatus is described from the central mountains of Taiwan which differs from its congeners chie¯y by its size and colouration.
Key words: taxonomy, Arielulus, Thainycteris, new species, Taiwan
INTRODUCTION
The phenetically similar, very diverse and widely distrib-uted genera of Pipistrellus and Eptesicus have resulted in a long lasting debate concerning the taxonomic distinct-ness and systematic relations of the different named forms. By means of traditional taxonomic comparisons of the two taxa no reliable differences were found other than the presence or absence of the ®rst upper premolar (Koopman, 1975) but the taxonomic value of this character is highly dubious (see Heller & Volleth, 1984). Therefore, it was supposed that the generic separation was provisional and probably both traditionally ac-cepted genera are of polyphyletic origin (Koopman, 1975; Volleth & Tidemann, 1991). The ®rst work using the results of cytological investigations and of compara-tive bacular morphology to clarify the phylogenetic separation between Pipistrellus and Eptesicus was pub-lished by Heller & Volleth (1984) who characterized the genus Eptesicus by its distinct karyotype (2n = 50, fun-damental number = 48) and the small, not stick-like baculum. The genus Pipistrellus has great chromosomal variability with 2n = 44 or less, and an elongate, narrow baculum is typical. Later, P. savii, one of the most controversial species was placed by Horacek & Hanak (1986) in the separate genus Hypsugo on bacular and on other osteological grounds, which was con®rmed on the basis of dental characteristics (Menu, 1987) and by means of isozyme electrophoresis (Ruedi & Arlettaz, 1991). Hill & Harrison (1987) provided a large-scale comparison of the os penis of all related Vespertilio-ninae genera, and erected a new subgenus Arielulus within the genus Pipistrellus. As a result of this work
and numerous subsequent karyological studies, several former Eptesicus species were included in different sub-genera of Pipistrellus (Hill & Harrison, 1987; Koopman, 1994) and further data were provided for the clearer separation of Pipistrellus and Eptesicus on a much wider basis (McBee, Schlitter & Robbins, 1987; Morales et al., 1991; Rautenbach, Bronner & Schlitter, 1993). Beside the Hypsugo, two other subgenera of Pipistrellus have been elevated to generic level, namely Falsistrellus (Kitchener, Caputi & Jones, 1986) and Vespadelus (Volleth & Tidemann, 1991).
The latest described subgenus P. (Arielulus) com-prises three species which are identi®ed by their small Y-shaped baculum and greatly reduced second incisor and PM2(Corbet & Hill, 1992). Pipistrellus circumdatus,
the type species of this new subgenus was originally described from Java by Temminck (1840) and further specimens were obtained from Myanmar, Malaysia (Hill, 1972), Yunnan (as P. circumdatus drungicus Wang, 1982, in Corbet & Hill, 1992), India (Das et al., 1995), Nepal (Bates & Harrison, 1997) and Thailand (specimens in Senckenberg Museum, Frankfurt). From Malaysia, Hill (1972) described a new species P. socie-tatis and discussed its very close af®nities to P. circumdatus. Also from Malaysia, Heller & Volleth (1984) recorded a series of ®ve specimens of the former species and considered P. societatis to be a lowland subspecies of P. circumdatus. They transferred both taxa to Eptesicus on bacular and karyological grounds which, however, has not been accepted by subsequent authors (Hill & Francis, 1984; Koopman, 1994). The third hitherto known species P. cuprosus was named by Hill & Francis (1984) from Borneo. This species is more
closely allied to P. societatis than to P. circumdatus. However, the unique combination of several different characters (cranial, dental, bacular, and karyological) not found in other Pipistrellus subgenera or in Eptesicus suggests that the recently accepted taxonomic position of P. (Arielulus) is not appropriate.
During a series of intensive bat surveys carried out in Taiwan in recent years, three Arielulus specimens were collected. Their distinctive coloration, measurements, craniodental, and other morphological characteristics were very different from any bat species recorded in Taiwan in the past. Based on comparative studies with other similar species and basic karyological work, they are described and reported here as a new species.
Meanwhile, a newly described genus and species Thainycteris aureocollaris Kock & Storch, 1996 was also placed close to Eptesicus and Vespertilio; its coloration and the given craniodental characteristics are very similar to those of the new Arielulus species from Taiwan. Therefore, this taxon is also taken into con-sideration.
MATERIALS AND METHODS
Abbreviations used for institutions in this paper are: NTU ± National Taiwan University, Taipei; BM(NH) ± The Natural History Museum, London, formerly the British Museum (Natural History); HNHM ± Hun-garian Natural History Museum, Budapest; ZMMU ± Zoological Museum of Moscow State University, Moscow; NNM ± Nationaal Natuurhistorisch Museum, Leiden; SMF ± Senckenberg Museum, Frankfurt.
Besides the 3 known specimens of the new species described in detail below the following comparative specimens were examined: Pipistrellus circumdatus ± Indonesia: Java (NNM 35433 [holotype], 35434 [para-type], 14898±9, 15314, 30965±6, BM(NH) 73.1805, 7.11.401); Malaysia: Pahang (BM(NH) 73.618); Nepal: Mechi (HNHM 98.5.23), Gandaki (ZMMU 164469, 164472±3); Thailand: Chiang Mai (SMF 75344±8). Pipistrellus societatis ± Malaysia: Pahang (BM(NH) 67.1605 [holotype], 81.1802), Selangor (SMF 60079±82, 69336±7). Pipistrellus cuprosus ± Malaysia: Borneo (BM(NH) 83.351 [holotype], 84.1989). Thainycteris aur-eocollaris ± Thailand: Chiang Mai (SMF 75443 [holotype], 84361±4 [paratypes]).
The forearm measurements were taken from dry and alcoholic museum specimens to the nearest 0.1 mm. Cranial and dental measurements were collected by digital caliper of 0.01 mm accuracy using a binocular microscope and included: greatest length of skull, from front of incisors to occiput; condylocanine length, from front of canines to back of condyles; palatal bridge length, from the posterior edge of palatal emargination to the end of palatinum excluding post-palatal spine; width across anteorbital foramina; lachrymal width, between the outermost points of lachrymal bones; width across supraorbital processes; zygomatic width, the
distance between the outermost points of zygomatic plates; interorbital width, the least width of the inter-orbital constriction; braincase width, measured above the processus zygomaticus temporalis; mastoid width, between mastoid knobs; width across canines, the dis-tance between the ectocingula of canines; width across M3±M3, measured over crowns; upper toothrow length,
the crown length of C-M3; mandible length, the distance
from the most posterior portion of the articular process to the most anterior edge of the alveolus of I1; lower
toothrow length, the crown length of C-M3.
The karyotype was obtained by preparing metaphase spreads from bone marrow cells of 1 yeast-stimulated individual (Lee & Elder, 1980) and stained with 5% Giemsa stain. The diploid number (2n) was determined by examining 10 cells which showed complete and visible metaphase spreads.
RESULTS
Accepting the diagnostic value of karyological data by which the distinct and invariable Eptesicus is separable from the heterogeneous Pipistrellus, the same separation is found in P. (Arielulus) which also possesses 50 chromosomes (FN = 48) (Heller & Volleth, 1984). Arielulus also differs from Pipistrellus by its myotodont M1±M2. However, the generic distinctness from
Eptesicus is supported by the very broad and short rostrum, more in¯ated braincase, tricuspid ®rst upper incisor and the very small baculum typically curved when viewed laterally, with two widely separated basal lobes and short, narrow shaft (instead of being ¯attened, triangular and the base only slightly lobed) (Hill & Harrison, 1987). Therefore, we propose the elevation of Arielulus to generic level.
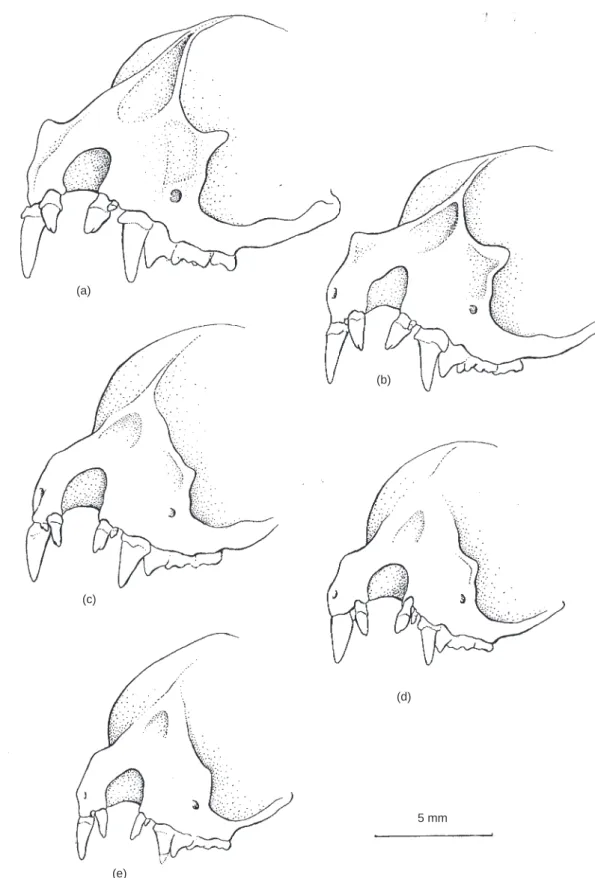
The diagnosis given by Kock & Storch (1996) for the new taxon Thainycteris seems to be suf®cient for the separation at generic level from Eptesicus and Pipistrellus but the cranial and dental characters are essentially the same as those typical for Arielulus. The ®rst detailed description of the type species A. circumdatus (based on specimens from Java and Myanmar) was given by Hill (1972) and included the following features: broad rostrum; prominent supra-orbital ridges terminating in small tubercles; deeply excavated frontal depression. These features are even more de®nite in the most northern (Nepalese) represen-tatives of the species where some specimens (e.g. ZMMU 164472) have similarly strong supraorbital and lachrymal processes to those of Thainycteris (Fig. 1). In addition, the dental characteristics of Thainycteris (large and tricuspid ®rst incisor, very small second incisor, rudimentary or missing PM2, not reduced M3 and
myotodont lower molars) are also typical for all the known Arielulus species. As a consequence, the genus Thainycteris Kock & Storch, 1996 is here regarded as the junior subjective synonym of Arielulus Hill & Harrison, 1987.
(a) (b) (c) (d) (e) 5 mm
Fig. 1. Front view of rostral part of skull of: (a) A. aureocollaris (holotype, SMF 75443); (b) A. torquatus n. sp. (holotype, NTU 019); (c) A. circumdatus (NNM 15314); (d) A. societatis (holotype, BM(NH) 67.1605); (e) A. cuprosus (holotype, BM(NH) 83.351).
ARIELULUS TORQUATUS N. SP. Holotype
Adult male, skin and skull; NTU FB 019. Collected by S. S. Tsao and C. S. Ding on 4 June 1992.
Type locality
Wu-ling Farm, Taichung County, Taiwan, 1800 m elevation, 24 824'N, 121 818'E.
Paratypes
Adult female, skin, NTU FB030, Tsui Fong Lake, Ilan County, 1840 m, collected by S. H. Yang and P. J. Jiang on 31 October 1996; adult female, skull, HNHM 98.23.1., Rei-suei logging road, Hualien County, 1500 m, collected by S. H. Yang and P. J. Jiang on 17 July 1996.
Etymology
The speci®c name is from the Latin word torquis for
necklace, which refers to the bright collar of the new species.
Diagnosis
The blackish dorsal pelage over the entire surface is conspicuously tipped with bronze colour; the similarly dark ventral hairs silvery-grey tipped. A bright ochrac-eous collar spreads across the throat. Forearm length under 46 mm. Supraorbital and lachrymal processes of the skull very strong; length of upper toothrow < 6.6 mm, lower toothrow < 7 mm.
Description
A medium-sized species of the genus, length of forearm 43.6±45.5 mm (Table 1). Fur black, terminal part of individual hairs on the dorsal surface bronze coloured, that of the venter tipped with silvery-grey. A well-de®ned ochraceous collar runs from the base of ears across throat, paralleled with a narrow greyish-white band spreading between shoulders. Ears broadly trian-gular, blackish and not marked with white or yellowish edge; tragus short, curved, anterior border concave, posterior border strongly convex. Muzzle short, broad
Table 1. External and craniodental measurements of Arielulus species (in mm). Sample sizes are in parentheses
A. aureocollaris A. torquatus n. sp. A. circumdatus A. societatis A. cuprosus
Forearm length 47.5±51.8 43.6±45.5 38.6±43.6 35.95±40.7 34.8±36.4
(5) (2) (14) (7) (2)
Greatest length of skull 17.55±17.66 16.25±16.86 15.15±16.78 14.68±15.50 13.88±14.04
(2) (2) (9) (8) (2)
Condylocanine length 16.22±16.28 15.19±15.81 14.01±15.46 13.59±14.25 12.26±12.70
(2) (2) (11) (8) (2)
Palatal bridge length 6.02±6.43 6.03±6.41 5.95±6.94 4.97±5.46 4.78±4.83
(2) (2) (14) (8) (2)
Width across anteorbital 6.31±6.80 5.88±6.27 5.19±5.80 5.05±5.43 4.81±4.96
foramens (5) (2) (12) (8) (2)
Lachrymal width 8.66±8.70 7.72±8.18 6.63±7.69 6.33±6.69 6.02±6.09
(2) (2) (12) (8) (2)
Width across supraorbital 8.69±9.15 7.57±7.74 6.16±7.15 5.93±6.33 5.57±5.77
processes (2) (2) (12) (8) (2) Zygomatic width 13.34±13.50 12.43±12.57 10.69±11.62 10.31±11.21 9.60±9.98 (2) (2) (13) (7) (2) Interobital width 4.85±5.11 4.36±4.44 4.28±4.64 3.89±4.37 4.12±4.14 (2) (2) (17) (8) (2) Braincase width 9.23±9.64 8.38±8.54 7.66±8.50 7.50±7.95 7.46±7.75 (2) (2) (11) (8) (2) Mastoid width 10.24±10.29 9.05±9.36 8.45±9.16 8.06±8.65 7.54±8.03 (2) (2) (13) (8) (2)
Width across canines 6.04±6.37 5.26±5.52 4.94±5.65 4.54±4.98 4.30±4.34
(5) (2) (17) (7) (2)
Width across M3±M3 8.25±8.57 7.78±8.19 7.07±7.70 6.56±7.14 6.38±6.40
(5) (2) (17) (8) (2)
Upper toothrow length 6.70±7.06 6.44±6.58 5.87±6.50 5.31±5.72 4.85±4.94
(5) (2) (17) (8) (2)
Mandible length 13.20±14.43 12.39±12.77 10.97±12.62 10.71±11.21 9.85±10.11
(5) (2) (18) (8) (2)
Lower toothrow length 7.25±7.78 6.86±6.93 6.22±6.86 5.71±6.08 5.15±5.31
black and naked except the furred proximal half of dorsal surface of uropatagium; plagiopatagium inserts at the base of ®fth toe. Calcar extending along more than half of uropatagial margin; last caudal vertebra free from uropatagium.
Skull massive with short and broad rostrum and in¯ated braincase (Figs 1 & 2). Sagittal crest low but supraorbital ridges very strong terminating in promi-nent processes; lachrymal processes also well developed. Frontal (interorbital) depression deeply excavated. Narial emargination V-shaped; palatal emargination wide and relatively shallow, posterior border equal to the middle of canines. Basial pits well-de®ned. Mandible heavily built, mandibular symphysis enlarged ventrally. Coronoid process broadly triangular, angular process curved upwards.
First upper incisor large, and beside the apical cusps bearing a prominent lingual cusp on the cingulum; second incisor small, just reaching beyond the cingulum of I1. First upper premolar (PM2) rudimentary (in the
paratype HNHM 98.23.1. missing from the left toothrow) and completely hidden into recess between C1
and PM3. Paracone and metacone of M1and M2
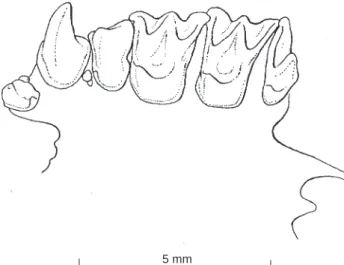
well-developed; postprotocrista and metaloph low and gra-dually sloping towards the base of metacone. M3
unreduced, third commisure and metacone present (Fig. 3). First lower incisor with four lobes, I2 and I3
trilobated. Both lower premolars (PM2and PM4)
well-developed (PM2 more than one-third crown area of
PM4) with distinct main cusp and additional small
cingular cusps and situated in the main axis of the toothrow. M1and M2myotodont, M3unreduced.
Karyotype
Due to the small sample size only one adult female (paratype NTU FB30) was examined for its karyotype, and the diploid chromosome number was determined to be 50.
Comparisons with other species
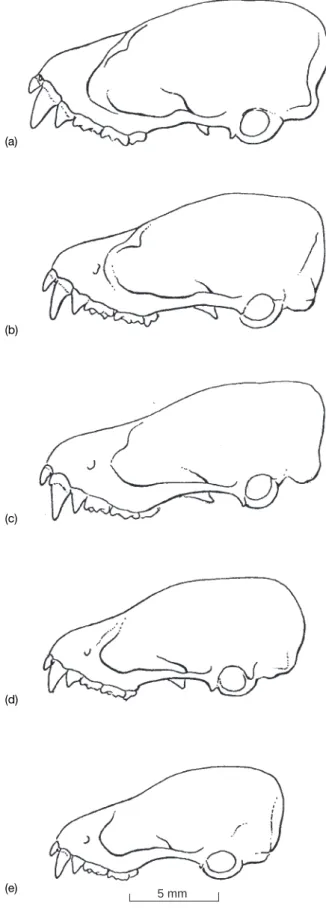
The new species A. torquatus is distinguishable from both A. cuprosus and A. societatis by its overall greater size, more robust skull with protruding supraorbital process and the conspicuous neck collars. A. circum-datus is similar in size and in some skull characteristics (e.g. well-de®ned frontal depression and distinct sagittal crest) but has wider narial emargination, weaker supra-orbital ridges, less-developed suprasupra-orbital process and different coloration with no collar-like markings. In general the dentition, shape, and proportions of skull of A. torquatus is the same as in A. aureocollaris but differs in the following: smaller in every measurement (except the palatal bridge length); narial emargination V-shaped; lachrymal width exceeds the supraorbital width; and palatal bridge relatively longer (Table 1). a
b
c
d
e 5 mm
Fig. 2. Lateral view of skulls of: (a) A. aureocollaris (holo-type, SMF 75443); (b) A torquatus n. sp. (holo(holo-type, NTU 019); (c) A. circumdatus (BM(NH) 73.1805); (d) A. societatis (holotype, BM(NH) 67.1605); (e) A. cuprosus (holotype, BM(NH) 83.351). (a) (b) (c) (d) (e)
A. torquatus is further distinguishable from the former species by its distinctive coloration and the blackish dorsal fur being tipped with bronze (hairs above with silver tips in A. aureocollaris).
DISCUSSION
Although, on the one hand Heller & Volleth (1984) transferred P. (A.) circumdatus and P. (A.) societatis to Eptesicus, and on the other hand retained Arielulus as a subgenus of Pipistrellus (Corbet & Hill, 1992; Koopman, 1994) the following unique character combi-nation distinguishes Arielulus from all living genera: distinctive coloration; short and wide rostrum; high and globular braincase; tricuspid I1; greatly reduced I2;
small (often missing) PM2; myotodont M
1and M2; very
small Y-shaped baculum; and the chromosome number 2n = 50. Based on cytological, dental and bacular char-acteristics the genus is allied to the Vespertilionini tribe (including Eptesicus and Vespertilio).
The investigation of craniodental features (stated as diagnostic by Kock & Storch, 1996) of the recently described Thainycteris aureocollaris showed that this taxon is also referable to Arielulus. It is here assigned as Arielulus aureocollaris.
The new species A. torquatus can be distinguished from the other species in the genus by its size, shape of skull, and coloration. Based on these characters its closest relative is A. aureocollaris (from which it is primarily distinguishable by measurements only) and both species have close af®nities to A. circumdatus, the type species of the genus. It is noteworthy that these three species are known only from remote mountain areas of the Indomalayan Region while the two other species, A. societatis and A. cuprosus, are distributed in lowlands.
Acknowledgements
We would like to express our sincere thanks to Y. P. Fang, E. S. S. Tsao, T. S. Ding, S. H. Yang and P. J. Jiang of Department of Zoology, National Taiwan University, for assisting in both ®eld and laboratory work. Our special thanks are due to Dieter Kock and Gerhard Storch, Senckenberg Museum Frankfurt; Aleksei Borissenko and Sergey Kruskop, Zoological Museum of Moscow State University, Moscow for their hospitality and useful comments during the preparation of the manuscript and to David Harrison, Harrison Zoological Museum, Sevenoaks and George TopaÂl, Hungarian Natural History Museum, Budapest for their helpful review comments. We are grateful to Paula Jenkins, The Natural History Museum, London and Chris Smeenk, Nationaal Natuurhistorisch Museum, Leiden for their kind help during our investigation in the collection under their care. We are also indebted to PeÂter Ujhelyi for the drawings. The work of GCS was supported by the Hungarian National Scienti®c Fund (OTKA) grant no. F 17700. The work of LLL was supported by the Council of Agriculture, R.O.C., grant no. 86 Conservation-12 (10).
REFERENCES
Bates, P. J. J. & Harrison, D. L. (1997). Bats of the Indian subcontinent. Sevenoaks: Harrison Zoological Museum Publi-cation.
Corbet, G. B. & Hill, J. E. (1992). The mammals of the Indoma-layan region: a systematic review. London: Natural History Museum Publications, Oxford University Press.
Das, P. K., Ghose, R. K., Chakraborty, T. K., Bhattacharyya, T. P. & Ghosh, M. K. (1995). Mammalia. State Fauna Series 4: Fauna of Meghalaya. Part 1: 23±128. Calcutta: Zoological Survey of India.
Heller, K.-G. & Volleth, M. (1984). Taxonomic position of `Pipistrellus societatis' Hill, 1972 and the karyological charac-teristics of the genus Eptesicus (Chiroptera: Vespertilionidae). Z. zool. Syst. Evolut.-forsch. 22: 65±77.
Hill, J. E. (1972). The Gunong Benom Expedition 1967. 4. New records of Malayan bats, with taxonomic notes and the descrip-tion of a new Pipistrellus. Bull. Br. Mus. nat. Hist. (Zool.) 23: 23±42.
Hill, J. E & Francis, C. M. (1984). New bats (Mammalia: Chiroptera) and new records of bats from Borneo and Malaya. Bull. Br. Mus. nat. Hist. (Zool.) 47: 305±329.
Hill, J. E. & Harrison, D. L. (1987). The baculum in the Vespertilioninae (Chiroptera: Vespertilionidae) with a sys-tematic review, a synopsis of Pipistrellus and Eptesicus, and the descriptions of a new genus and subgenus. Bull. Br. Mus. nat. Hist. (Zool.) 52: 225±305.
Horacek, I. & Hanak, V. (1986). Generic status of Pipistrellus savii and comments on classi®cation of the genus Pipistrellus (Chiroptera, Vespertilionidae). Myotis 23±24: 9±16.
Kitchener, D. J., Caputi, N. & Jones, B. (1986). Revision of Australo-Papuan Pipistrellus and of Falsistrellus (Microchirop-tera: Vespertilionidae). Rec. West. Aust. Mus. 12: 435±496. Kock, D. & Storch, G. (1996). Thainycteris aureocollaris, a
remarkable new genus and species of vespertilioninae bats from SE-Asia. Senckenberg. Biol. 76: 1±6.
5 mm
Fig. 3. Occlusal view of left upper toothrow of Arielulus torquatus n. sp. (holotype, NTU FB 019).
Hist. 154: 354±444.
Koopman, K. F. (1994). Chiroptera: systematics. Handbook of zoology. Mammalia, part 60. Berlin: Walter de Gruyter. Lee, M. R. & Elder, F. F. (1980). Yeast stimulation of bone
marrow mitosis for cytogenetic investigation. Cytogenet. Cell Genet. 26: 36±40.
McBee, K., Schlitter, D. A. & Robbins, R. L. (1987). Systematics of African bats of the genus Eptesicus (Mammalia: Vespertilio-nidae). 2. Karyotypes of African species and their generic relationships. Ann. Carnegie Mus. 56: 213±222.
Menu, H. (1987). Morphotypes dentaries actuels et fossiles des chiropteÁres vespertilionineÂs. 2eÁme partie: implications systeÂma-tiques et phylogeÂneÂsysteÂma-tiques. Palaeovertebrata 17: 77±150. Morales, J. C., Ballinger, S. W., Bickham, J. W., Greenbaum, I. F.
& Schlitter, D. A. (1991). Genetic relationships among eight
nidae). J. Mammal. 72: 286±291.
Rautenbach, I. L., Bronner, G. N. & Schlitter, D. A. (1993). Karyotypic data and attendant systematic implications for the bats of southern Africa. Koedoe 36: 87±104.
Ruedi, M. & Arlettaz, R. (1991). Biochemical systematics of the Savi s bat (Hypsugo savii) (Chiroptera: Vespertilionidae). Z. zool. Syst. Evolut.-forsch. 29: 115±122.
Temminck, C. J. (1840). Monographies de Mammalogie, ou de-scription de quelques genres de mammiferes, dont les especes ont eÂte observeÂes dans les diffeÂrens museÂes de l Europe. Leiden: Vander Hoek.
Volleth, M. & Tidemann, C. R. (1991). The origin of the Austra-lian Vespertilioninae bats, as indicated by chromosomal studies. Z. SaÈugetirkd. 56: 321±330.