An aberrant autocrine activation of the platelet-derived
growth factor a-receptor in follicular and papillary thyroid
carcinoma cell lines
*
Kuei-Tien Chen
a,c, Jen-Der Lin
b, Miaw-Jene Liou
b, Hsiao-Fen Weng
b,
C.Allen Chang
c,**, Err-Cheng Chan
a,*
a
School of Medical Technology, Chang Gung University, Taoyuan, Taiwan
b
Division of Endocrinology and Metabolism, Chang Gung Memorial Hospital, Taoyuan, Taiwan
c
The Institute of Biological Science and Technology, National Chiao Tung University, Hsinchu, Taiwan Received 6 June 2004; received in revised form 30 January 2005; accepted 31 January 2005
Abstract
Platelet-derived growth factor receptor (PDGFR) can bind to its ligand and consequently possess a kinase activity, and which is associated with the carcinogenesis of different cell types, including astrocytomas, oligodendrogliomas, and glioblastoma. In a cDNA microarray analysis, we observe the over-expressed mRNA of both PDGF-A and PDGF-a receptor in thyroid carcinoma cells. And the elevated protein expressions of PDGF-A and PDGF-a receptor in thyroid carcinoma cells were also confirmed by a Western blot analysis. The phosphorylation of PDGF-a receptor evaluated by an antibody against Tyr 720-phosphate was found in thyroid carcinoma cells. The tyrosine kinase activity of PDGF-a receptor was inhibited by tyrphostin AG1295 and showed a dose-dependent inhibition for the proliferation of thyroid carcinoma cells. These findings imply that autocrine activation of PDGF-a receptor plays a crucial role in the carcinogenesis of thyroid cells.
q 2005 Elsevier Ireland Ltd. All rights reserved.
Keywords: Platelet-derived growth factor-A; Platelet-derived growth factor a-receptor; Autocrine activation; Thyroid carcinoma cell line
1. Introduction
Platelet-derived growth factor (PDGF) consists of A- and B-polypeptide subunits and arranges as PDGF-AA, PDGF-AB, and PDGF-BB. These isoforms have different specificities and affinities to the PDGF-a and -b receptors. The PDGFR-a has a high affinity for all isoforms, while the PDGFR-b has a high affinity only for PDGF-BB. Both a-receptor and b-receptor belong www.elsevier.com/locate/canlet
0304-3835/$ - see front matter q 2005 Elsevier Ireland Ltd. All rights reserved. doi:10.1016/j.canlet.2005.01.039
*
This study is supported by a grant CMRP894 from Chang Gung Memorial Hospital.
* Corresponding authors. Tel.: C886 3 2118800x5220; fax: C 886 3 2118741.
**Tel.: C886 3 5712121x56903; fax: C886 3 5729288. E-mail addresses: changca@cc.nctu.edu.tw (C.A. Chang), chanec@mail.cgu.edu.tw (E.-C. Chan).
to the same family of receptor proteins as the c-fms[1] and c-kit[2,3]proto-oncogene families, and possess activity of tyrosine kinase after stimulated by ligands binding. Expressions of PDGF and PDGFRs have crucial functions during the embryogenesis, in particular for the development of connective tissue of the kidneys, blood vessels, lungs, and the central nervous system[4–6]. In addition, PDGF and cognate receptors are also important during the formation of connective tissue of wound healing in the adult[7]. Besides the normal functions of PDGF and cognate receptor, other reports indicated that the excess activity of PDGFR caused by abnormal binding of cognate ligands and consequently stimulating the signaling pathway was associated with different disorders, such as glioblastoma and sarcoma [8]. Ectopic autocrine stimulation caused by abnormal expression of PDGF and cognate receptor was also associated with atherosclerosis and various fibrotic conditions, including lung fibrosis, kidney fibrosis, liver cirrhosis, and myelofibrosis[9–11].
Thyroid carcinomas are classified pathologically as papillary, follicular, or anaplastic carcinoma of thyroid follicular epithelial cell origin and as medul-lary carcinoma of parafollicular cell origin. PDGFRs mainly exist in mesenchyme-derived and glia-derived cells, but not in normal epithelial cells[12,13]. With regard to the PDGF and cognate receptor expression in thyroid cells, previous findings show that normal thyroid cells possess receptors for epidermal growth factor (EGF), though all lack PDGF binding sites[12]. However, the presence of PDGFR-b was found in human anaplastic thyroid carcinoma cell line C643 [14]. In another study, the expression of PDGFR-a and -b were found in human anaplastic thyroid carcinoma cell line HTh74 [15]. Those findings indicate that the expression of PDGFRs provides the cells a new growth stimulation route and play a crucial role in the carcinogenesis of thyroid cells. Another possibility, the expression of PDGFRs may only be the remnants of immature progenitor cells.
In the previous study, we found that mRNA of PDGF-A and PDGFR-a were highly expressed in thyroid carcinomas but not in nodular hyperplasia cells by a cDNA microarray technique. These results cause the motive to investigate whether autocrine activation caused by abnormal expression of PDGF and cognate receptor exists in thyroid cells, and whether
play a crucial role in carcinogenesis. In the present study, we examined the expression of PDGF-A and PDGFR-a, phosphorylation activation of PDGFR-a, and whether PDGF-related autocrine activation is a critical event in cell proliferation of thyroid carcinoma. Information from the present study led to the better understanding of the involvement of PDGF-related autocrine activation in thyroid carcinogenesis.
2. Materials and methods
2.1. Tissue samples and cell cultures
Benign and malignant tissue samples were obtained during surgical resection of thyroid hyper-plastic nodules and follicular thyroid carcinoma from the Department of Pathology, Chang Gung Memorial Hospital, Taiwan. The tissue specimens were frozen in liquid nitrogen and then stored at K70 8C until RNA or protein extraction.
In order to provide materials for the thyroid cancer study, Lin et al.[16]have established various thyroid cell lines, including CGTH W-1 (derived from metastatic follicular thyroid carcinoma) and CGTH W-3 (derived from papillary thyroid carcinoma), which were obtained from the Division of Endocrin-ology and Metabolism, Chang Gung Memorial Hospital, Taiwan. Monolayer cultures of CGTH W-1 and CGTH W-3 were grown in RPMI medium W-1640 (GIBCO, Invitrogen Corporation, NY) supplemented with 10% fetal calf serum, 2 g/L sodium bicarbonate (SIGMA, Sigma-Aldrich, MO), 1% (v/v) non-essen-tial amino acid (GIBCO), 1 mM sodium pyruvate (GIBCO), 100 U/mL penicillin G sodium, and 100 mg/mL streptomycin (GIBCO).
2.2. Total RNA isolation
Total RNA was obtained by extracting tissues and cell lines in Trizol reagent (INVITROGEN Life Technologies, Invitrogen Corporation, CA) according to the manufacturer’s instructions. Thyroid tissues (w100 mg each) and thyroid carcinoma cell lines (w5!106cells) were homogenized in Trizol solution (1 mL). Homogenates were incubated for 5 min at 25 8C, and then 0.2 volume of chloroform was added to the homogenates. The inorganic phase was
separated by centrifugation at 12,000!g for 20 min at 4 8C after vigorous agitation for 5 min. RNA was then precipitated in the presence of 0.5 volume of isopropanol. RNA pellets were washed with 70% ice-cold ethanol and then dissolved in RNase-free water. Total RNA concentration was assessed with UV spectrophotometer (Gene Quant II, Pharmacia Biotech, Sweden) at 260 nm. RNA quality was confirmed and visualized as 18S and 28S rRNA bands in the agarose gel without a smearing pattern. 2.3. Synthesis and hybridization of cDNA probe
cDNA probe preparation and membrane hybridiz-ation were performed according to the manufacturer’s instructions for Atlase human cancer cDNA expression array (CLONTECH, CLONTECH Lab-oratories, Inc., CA). Briefly, 1 mg polyA RNA was reverse-transcribed into cDNA by MMLV reverse transcriptase in the presence of CDS primer mix and a-32P-dATP (3000 Ci/mmol, Amersham Biosciences, Amersham Biosciences Ltd, Hong Kong). Labeled cDNA was purified from unincorporated nucleotides using a CHROMA SPIN-200 column (CLONTECH). The human cDNA expression arrays were prehy-bridized at 68 8C for 30 min in ExpressHyb solution (CLONETECH) to which 0.1 mg/mL salmon sperm DNA (Gibco BRL, Invitrogen Corporation, NY) had been added. The cDNA probes were then hybridized to the arrays at 68 8C overnight. The membranes were washed 4 times with 2! SSC solution containing 1% sodium dodecyl sulfate (SDS) and twice with 0.1! SSC solution containing 0.5% SDS for 30 min at 68 8C in all cases and then exposed to a phosphor screen. The images and quantitative data of the gene expression levels were analyzed with a Phosphoima-ger (ImageQuaNT, Molecular Dynamics, CA). 2.4. Quantitative PCR analysis
Total RNA from thyroid carcinoma cell lines or thyroid tissues derived from nodular hyperplasia was extracted by using Trizol reagent (INVITROGEN) according to the manufacturer’s instructions. For reverse transcription, equal amount of total RNA (2 mg) were performed in a 25-mL reaction mixture containing 1! reverse transcriptase reaction buffer (Promega, Promega Corporation, WI), 200 mM dNTPs,
10 ng oligo (dT)15 primer, 8 mM dithiothreithol, 40
units Rnasin (Promega), and 100 units MMLV reverse transcriptase (Promega). The mixture was incubated at 42 8C for 50 min, heated to 70 8C for 10 min, and then chilled on ice. The GeneAmp 5700 sequence detection system (Applied Biosystems, Applied Biosystems, CA) was used to amplify both target genes (PDGF-A and PDGFR-a) and internal control (b-actin). The reaction master mix was prepared according to the manufac-ture’s protocol to give final concentration of 1! SYBR Green PCR buffer, 3 mM MgCl2, dNTP blend (0.2 mM
dATP, 0.2 mM dCTP, 0.2 mM dGTP, 0.4 mM dUTP), 0.025 units AmpliTaq Gold DNA polymerase, 0.01 units AmpErase uracil-N-glycosylase, and 300 nM primers. The specific primers for PCR were as follows:
50-CACGC CACTA AGCAT GTGCC-30 and
50-ATGAC CGTTC CTGGT CTTGC AG-30 for
PDGF-A, GenBank accession number: X06374; 50-TGAAG AAAAC AACAG CGGCC-30 and 50
-CGTCA TTCCT AGAGG TACAA AGGCT-30 for
PDGFR-a, GenBank accession number: M21574; 50
-ATGGG TCAGA AGGAT TCCTA TGTG-30 and
50-GCCAG ATTTT CTCCA TGTCG TC-30 for b-Actin, GenBank accession number: X00351. Comp-lementary DNA synthesized by reverse transcription was added to the master mix. Then, the PCR reagent mix were transferred to thermocycler and PCR profile were performed at 50 8C for 2 min, 95 8C for 10 min, and followed by 40 cycles of amplification at 95 8C for 15 s, 60 8C for 1 min, using the GeneAmp 5700 sequence detection system.
Relative expression of PDGF-A or PDGFR-a transcripts was determined by the following calcu-lation, as described in the Applied Biosystems users bulletin, ‘Relative Quantitation of Gene Expression’:
Relative expressionZ2KDDCt
where DDCtPDGF-AZ(CtPDGF-AKCtb-Actin)thyroid carcinoma cell lineK(CtPDGF-AKCtb-Actin)nodular hyperplasia
or DDCt
PDGFR-aZ(CtPDGFR-aKCtb-Actin)thyroid carcinoma
cell lineK(CtPDGFR-aKCtb-Actin)nodular hyperplasia.
2.5. Western blot analysis
Whole cell protein extracts were prepared by using cold lysis buffer consisting of 1! PBS, 1% NP-40, 0.5% sodium deoxycholate, 0.1% SDS, 10 mg/mL PMSF, and 0.2 units/mL aprotinin (Santa Cruz Bio-technology, Santa Cruz BioBio-technology, Inc., CA).
Samples were incubated on ice for 30 min and supernatants were recovered by centrifuging at 10,000!g at 4 8C for 10 min. Protein concentrations were determined by DCeprotein assay method
(BIO-RAD, Bio-Rad Laboratories, Inc., CA). Proteins were separated on 10% SDS-PAGE and transferred to PVDF membrane (Amersham Biosciences). Blocking reagent was 3% gelatin in TBS (pH 7.4). The washing buffer consisted of TBS (pH 7.4) with 0.1% Tween-20. Mouse monoclonal IgG2banti-PDGF-A (E-10) (Santa
Cruz Biotechnology) and rabbit polyclonal IgG anti-PDGFR-a (C-20) (Santa Cruz Biotechnology) were used as primary antibodies. Goat anti-mouse IgG conjugated by alkaline phosphatase (Santa Cruz Biotechnology) and goat anti-rabbit IgG conjugated by horseradish peroxidase (Santa Cruz Biotechnology) were used as respective secondary antibodies. Signals were respectively visualized by using BCIP/NBT substrate (SIGMA ) and TMB membrane peroxidase substrate (KPL, Kirkegaard and Perry Laboratories, Inc., MD).
2.6. Immunoprecipitation and Western blot for PDGF-a receptor
For immunoprecipitation of PDGF-a receptor, 2! 107cells were washed in 1! PBS and lysed in ice-cold RIPA buffer consisting of 1! PBS, 1% NP-40, 0.5% sodium deoxycholate, 0.1% SDS, 10 mg/mL PMSF, and 0.2 U/mL aprotinin (Santa Cruz Biotech-nology). Samples were incubated on ice for 30 min and supernatants were recovered by centrifuging at 10,000!g at 4 8C for 10 min. After centrifugation, 1 mL cell lysates was incubated with 2 mg rabbit polyclonal IgG anti-PDGFR-a (C-20) for 2 h at 4 8C. Then precipitation was performed by using 20 mL protein A/G plus-agarose (0.5 mL agarose/2.0 mL; Santa Cruz Biotechnology) at 4 8C overnight. The precipitates were washed 3 times with PBS, extracted by adding reducing SDS sample buffer and incubated for 5 min at 95 8C.
Samples were analyzed on 10% SDS-PAGE and transferred to PVDF membranes (Amersham Bios-ciences). Blocking reagent was 3% gelatin in TBS (pH 7.4). Washing buffer consisted of TBS (pH 7.4) with 0.1% Tween-20. Goat polyclonal IgG specific for Tyr-754-phosphorylated PDGFR-a [p-PDGFR-a (Tyr754); Santa Cruz Biotechnology] and goat
polyclonal IgG specific for Tyr-720-phosphorylated PDGFR-a [p-PDGFR-a (Tyr720); Santa Cruz Bio-technology] were used as primary antibodies. Anti-goat IgG-HRP (Santa Cruz Biotechnology) was used as secondary antibody. Signals were visualized by using TMB membrane peroxidase substrate (KPL). 2.7. Cell proliferation assays
CGTH W-1 or CGTH W-3 cells (2.5!104 per 35-mm dish) were seeded in RPMI-1640 medium with 1% fetal calf serum. Tyrphostin AG1295 (AG1295; selective inhibitor for the PDGF-receptor tyrosine kinase activity) was used at final concen-trations of 0, 0.25, 0.5, 1, and 2.5 mM (Calbiochem, Merck Biosciences, Germany), and Tyrphostin A1 (AG9; negative control) was used at a final concen-tration of 2.5 mM (Calbiochem). Triplicate dishes were used for each concentration point. Tyrphostin AG1295 and Tyrphostin A1 in RPMI-1640 media containing 1% fetal calf serum were added to the cells every other day. The cell number per dish was determined at day 7 after plating, when cells were trypsinized, suspended, and counted using a Coulter counter (Coulter Electronics, Herpendon, UK).
3. Results
3.1. Expression of PDGF-A and PDGF-a receptors in thyroid carcinoma cells
By comparing the gene expression pattern of CGTH W-1 and CGTH W-3 cell lines with benign tissues of thyroid nodular hyperplasia, we identified 41 over-expressed genes and 38 suppressed genes in CGTH W-1 cell line with more than 2-fold expression difference. We also identified 35 over-expressed genes and 22 suppressed genes in CGTH W-3 (Table 1). The differences of gene expression between nodular hyperplasia and thyroid carcinoma cell lines involve large numbers of genes, and the overall profiles of gene expression were represented graphi-cally in Fig. 1. The correlation between gene expressions in CGTH W-1 cell and those in nodular hyperplasia (R2Z0.48) is very similar to that between gene expressions in CGTH W-3 cell and those in nodular hyperplasia (R2Z0.46). The greater
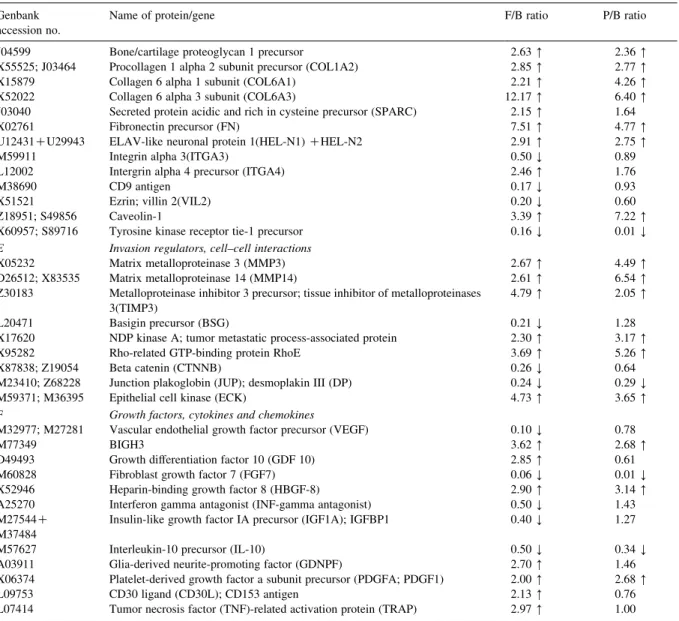
Table 1
A list of differentially expressed genes identified by using a cDNA array technique in the ratio of follicular thyroid carcinoma cell line to benign tissue (F/B ratio), together with the ratio of papillary thyroid carcinoma cell line to benign tissue (P/B ratio)
Genbank accession no.
Name of protein/gene F/B ratio P/B ratio A Cell cycle and growth regulators
X05360 Cell division control protein 2 homolog 0.36 Y 0.68 X66365 Cell division protein kinase 6 (CDK6); PLSTIRE 0.45 Y 1.08 M34065 CDC25C; M-phase inducer phosphatase 3 2.64 [ 1.93 L25676 Cell division protein kinase 9 (CDK9); PITALRE 0.15 Y 0.43 Y L33264 Cdc2-related protein kinase PISSLRE 0.26 Y 0.54 X59798; M64349 G1/S-specific cyclin D1(CCND1); bcl-1 oncogene 0.43 Y 1.54 M92287 G1/S-specific cyclin D3(CCND3) 0.50 Y 1.17 U11791; U12685 Cyclin H(CCNH); MO 15-associated protein 0.16 Y 0.68
U09579; L25610 WAF1 0.23 Y 0.84
S72008 CDC10 protein homolog 2.55 [ 3.40 [
U00001 CDC27HS protein 0.30 Y 1.22
U63131 CDC37 homolog 0.45 Y 0.96
X60188 Extracellular signal-regulated kinase 1(ERK1) 0.38 Y 1.08 L35253; L35263 Mitogen-activated protein kinase p38 (MAP kinase p38) 3.80 [ 3.22 [
X85134 RBQ-3 2.89 [ 3.01 [
AF001954 P33ING1 0.39 Y 0.61
L29511 Growth factor receptor-bound protein 2 (GRB2) isoform 0.41 Y 1.45
X03484 c-raf proto-oncogene 0.47 Y 0.92
M29039 Jun-B 2.65 [ 3.73 [
D89667 c-myc binding protein MM-1 0.27 Y 1.09 M26326 Type I cytoskeletal 18 keratin (K18) 0.18 Y 0.22 Y M34225 Type II cytoskeletal 18 keratin (KRT8) 0.10 Y 2.10 [ B Apoptosis, oncogenes and tumor suppressors
L08246 Induced myeloid leukemia cell differentiation protein MCL-1 0.32 Y 1.35 S83171; Z35491 BCL-2 binding athanogene-1(BAG-1) 0.23 Y 0.55 L41690 Tumor necrosis factor receptor 1-associated death domain protein (TRADD) 0.20 Y 0.80 AF016268 Cytotoxic TRAIL receptor 2 (TRICK2A) 2.84 [ 2.40 [
M35543; M57298 CDC42 homolog 0.42 Y 0.74
D17517 Tyrosine-protein kinase receptor tyro3 precursor; rse; sky; dtk 2.27 [ 2.98 [ C Cell fate and development regulators
J04088 DNA topoisomerase II alpha (TOP2A) 10.55 [ 64.12 [ M60974 DNA-damage-inducible transcript 1 (DDIT1) 2.46 [ 11.39 [ M87339 Activator 1 37-kDa subunit; replication factor C 37-kDa subunit (RFC37) 2.53 [ 76.12 [ L07541 Replication factor C 38-kDa subunit (RFC38) 2.76 [ 5.23 [ M87338 Replication factor C 40-kDa subunit (RFC40) 2.63 [ 32.60 [ K00065; X02317 Cytosolic superoxide dismutase 1(SOD1) 0.36 Y 1.41
U94354 Lunatic fringe 3.13 [ 15.71 [
X91940 Wnt-8B 0.46 Y 1.06
U46461 Segment polarity protein; disheveled homolog 1 (DSH homolog 1) 0.46 Y 1.78
L38518 Sonic hedgehog (SHH) 2.77 [ 8.61 [
M76125 Tyrosine-protein kinase receptor UFO precursor; axl oncogene 2.71 [ 68.15 [
X65923 fau 0.49 Y 1.23
M29366; M34309 ERBB-3 receptor protein-tyrosine kinase precursor 2.40 [ 4.60 [ M34641 Fibroblast growth factor receptor 1 precursor (FGFR1) 2.75 [ 10.20 [ M21574 Platelet-derived growth factor receptor alpha subunit (PDGFRA) 13.00 [ 137.48 [ U12140 Brain-derived neurotrophic factor (BDNF)/NT-3 growth factors receptor
precursor
0.25 Y 0.95 M32315; M55994 Tumor necrosis factor binding protein 2 (TBP2) 2.76 [ 1.34 D Cell adhesion, motility and invasion
the similarity between the gene expression patterns the more linear the dot plot graph. From Fig. 1, it showed that a number of candidate genes were obviously altered in thyroid carcinoma cell lines. Among these candidate genes, PDGF-A exhibited 2-and 2.7-fold over-expression in CGTH W-1 2-and CGTH W-3, respectively, and PDGFR-a exhibited 13- and 137.5-fold over-expressed in CGTH W-1 and CGTH W-3, respectively (Table 1). We did not
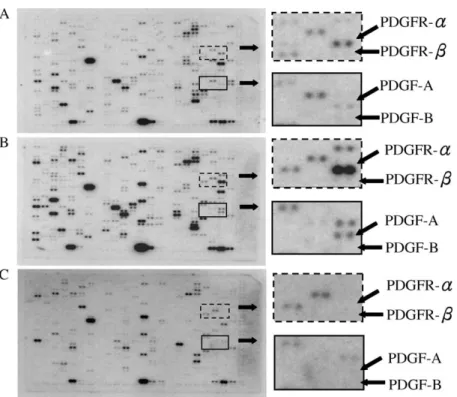
observe any expression of PDGF-A and PDGFR-a in nodular hyperplasia. On the other hand, PDGF-B did not show any obvious expression among in nodular hyperplasia, CGTH W-1, and CGTH W-3. PDGF-b receptor shows no obvious expression in both nodular hyperplasia tissues and CGTH W-1 cell line, and only slight expression in CGTH W-3 cell line (Fig. 2).
To confirm the differential expression of genes identified on the cDNA expression arrays, the total
Table 1 (continued) Genbank
accession no.
Name of protein/gene F/B ratio P/B ratio J04599 Bone/cartilage proteoglycan 1 precursor 2.63 [ 2.36 [ X55525; J03464 Procollagen 1 alpha 2 subunit precursor (COL1A2) 2.85 [ 2.77 [ X15879 Collagen 6 alpha 1 subunit (COL6A1) 2.21 [ 4.26 [ X52022 Collagen 6 alpha 3 subunit (COL6A3) 12.17 [ 6.40 [ J03040 Secreted protein acidic and rich in cysteine precursor (SPARC) 2.15 [ 1.64 X02761 Fibronectin precursor (FN) 7.51 [ 4.77 [ U12431CU29943 ELAV-like neuronal protein 1(HEL-N1) CHEL-N2 2.91 [ 2.75 [ M59911 Integrin alpha 3(ITGA3) 0.50 Y 0.89 L12002 Intergrin alpha 4 precursor (ITGA4) 2.46 [ 1.76
M38690 CD9 antigen 0.17 Y 0.93
X51521 Ezrin; villin 2(VIL2) 0.20 Y 0.60
Z18951; S49856 Caveolin-1 3.39 [ 7.22 [
X60957; S89716 Tyrosine kinase receptor tie-1 precursor 0.16 Y 0.01 Y E Invasion regulators, cell–cell interactions
X05232 Matrix metalloproteinase 3 (MMP3) 2.67 [ 4.49 [ D26512; X83535 Matrix metalloproteinase 14 (MMP14) 2.61 [ 6.54 [ Z30183 Metalloproteinase inhibitor 3 precursor; tissue inhibitor of metalloproteinases
3(TIMP3)
4.79 [ 2.05 [ L20471 Basigin precursor (BSG) 0.21 Y 1.28 X17620 NDP kinase A; tumor metastatic process-associated protein 2.30 [ 3.17 [ X95282 Rho-related GTP-binding protein RhoE 3.69 [ 5.26 [ X87838; Z19054 Beta catenin (CTNNB) 0.26 Y 0.64 M23410; Z68228 Junction plakoglobin (JUP); desmoplakin III (DP) 0.24 Y 0.29 Y M59371; M36395 Epithelial cell kinase (ECK) 4.73 [ 3.65 [ F Growth factors, cytokines and chemokines
M32977; M27281 Vascular endothelial growth factor precursor (VEGF) 0.10 Y 0.78
M77349 BIGH3 3.62 [ 2.68 [
D49493 Growth differentiation factor 10 (GDF 10) 2.85 [ 0.61 M60828 Fibroblast growth factor 7 (FGF7) 0.06 Y 0.01 Y X52946 Heparin-binding growth factor 8 (HBGF-8) 2.90 [ 3.14 [ A25270 Interferon gamma antagonist (INF-gamma antagonist) 0.50 Y 1.43 M27544C
M37484
Insulin-like growth factor IA precursor (IGF1A); IGFBP1 0.40 Y 1.27 M57627 Interleukin-10 precursor (IL-10) 0.50 Y 0.34 Y A03911 Glia-derived neurite-promoting factor (GDNPF) 2.70 [ 1.46 X06374 Platelet-derived growth factor a subunit precursor (PDGFA; PDGF1) 2.00 [ 2.68 [ L09753 CD30 ligand (CD30L); CD153 antigen 2.13 [ 0.76 L07414 Tumor necrosis factor (TNF)-related activation protein (TRAP) 2.97 [ 1.00
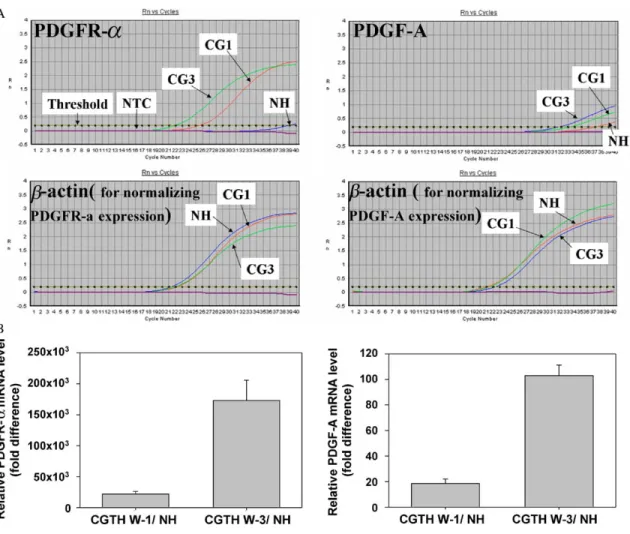
RNAs derived from thyroid carcinoma cell line and nodular hyperplasia was subjected to reverse tran-scription and quantitative PCR for PDGF-A and PDGFR-a. Fig. 3 illustrated that these gene expressions amplified by gene-specific quantitative PCR displayed the same tendency as observed by the cDNA expression arrays. No signal was detected by quantitative PCR analysis when the cDNA synthesis
step was performed without adding reverse transcrip-tase or the quantitative PCR step was performed without adding template (no template control; NTC), indicating that genomic DNA contamination and cross-contamination is negligible in our analysis condition.
The protein level of PDGF-A and PDGFR-a were detected by antibodies against an epitope of PDGF-A
Fig. 1. The list of intensity obtained from gene expressions of human cDNA expression arrays under the thyroid carcinoma cell lines and nodular hyperplasia. Scatter plots of the calibrated intensity (log scale) compared to give a correlation coefficient between CGTH W-1 cell and nodular hyperplasia (upper plot) or between CGTH W-3 cell and nodular hyperplasia (lower plot) were shown.
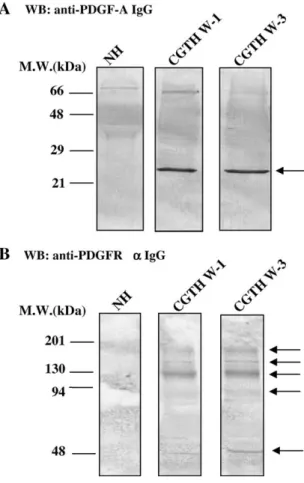
(135–211 amino acids) and PDGFR-a (carboxyl terminus), respectively. The result shows that no obvious band was detected in nodular hyperplasia, while a band of approximately 26 kDa was detected in
CGTH W-1 and CGTH W-3 (Fig. 4(A)). The
polyclonal antibody against the PDGFR-a shows that marked bands around at 180, 156, 130, 90 and 52 kDa in the Western blotting analysis of total protein lysate of CGTH W-1 and CGTH W-3 (Fig. 4(B)). These results indicated that both the transcriptional and translational levels of PDGF-A and PDGFR-a were over-expressed in CGTH W-1 and CGTH W-3 cell lines.
3.2. Phosphorylated activation of the PDGFR-a in thyroid carcinoma cells
After the expression of PDGF-A and PDGFR-a in CGTH W-1 and CGTH W-3 cell lines were
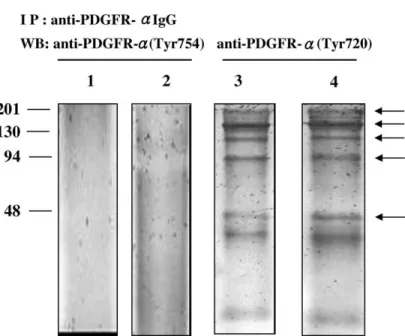
confirmed, we survey the PDGFR-a activity in terms of phosphorylation. Phosphorylation was detected by a Western blotting with phosphor-specific antibodies. The results showed that several bands (170, 156, 120, 90 and 52 KDa) were observed in CGTH W-1 and CGTH W-3 cell lines by using antibodies against p-PDGFR-a (Tyr720) (Fig. 5, lanes 3 and 4). While no obvious band was observed in same cell lines by using antibodies against p-PDGFR-a (Tyr754) (Fig. 5, lanes 1 and 2).
3.3. The proliferation of thyroid carcinoma cells is dependent on PDGFR activation
Tyrphostin AG1295 is an inhibitor that decreases the activity of protein tyrosine kinase. It selectively inhibits PDGFR kinase (IC50Z0.5 mM) and
PDGF-dependent DNA synthesis (IC50Z2.5 mM),
while it has no influence on EGF-receptor
Fig. 2. Expression pattern of genes in follicular thyroid carcinoma cell line, papillary thyroid carcinoma cell line, and nodular hyperplasia. Differential hybridization of three identical human cDNA expression arrays was performed as described in Materials and Methods. The top, middle, and bottom panels represent the expression array membrane hybridized with cDNA from (A) follicular thyroid carcinoma cell line, (B) papillary thyroid carcinoma cell line, and (C) nodular hyperplasia, respectively. Solid arrows indicate the gene expression level of PDGF-A, PDGF-B, PDGFR-a, and PDGFR-b.
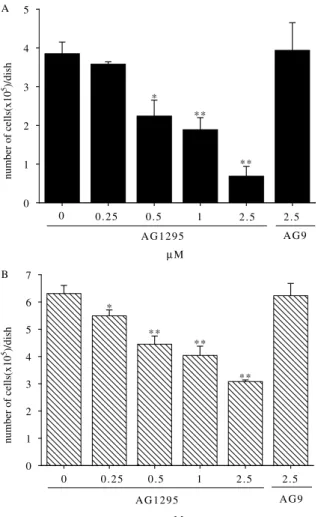
autophosphorylation and only a slight influence on EGF- or insulin-stimulated DNA synthesis [17,18]. When different concentrations of tyrphostin AG1295 were added to the cell lines, the result showed a dose-dependent inhibition for cell proliferation. After the 7-day, the average rate of inhibiting cell proliferation obtained from triplicate experiments with adding different concentrations of tyrphostin AG1295 (0, 0.25, 0.5, 1, and 2.5 mM) were 0, 7, 42, 51, and 82% in CGTH W-1 cells (Fig. 6(A)) and 0, 13, 29, 36, and 51% in CGTH W-3 cells (Fig. 6(B)). In a negative
control study, tyrphostin A1 did not affect cell proliferation of CGTH W-1 and CGTH W-3 cells.
4. Discussion
It has been reported that the signal transduction route induced by PDGF and cognate receptor can cause transformation and malignant tumors in exper-imental systems [19–22]. Then, a question aroused regarding whether PDGF and PDGFR play a role in
Fig. 3. Quantitative PCR reconfirm the tendency towards up-regulation expression of PDGF-A and PDGFR-a in CGTH W-1 and CGTH W-3 cell lines. (A) An example of a represents PDGF-A, PDGFR-a and b-actin amplification plot of the quantitative PCR. The Y-axis shows the fluorescence calculated by subtracting the background fluorescence (Rn) whereas the X-axis shows the number of PCR cycles. A higher number of PCR cycles are required to pass the fixed threshold in tissue sample from nodular hyperplasia (NH). However, samples from CGTH W-1 (CG1) or CGTH W-3 (CG3) cell lines decrease the number of cycles required to pass the threshold, which indicates that more PDGF-A and PDGFR-a gene is present in these samples. (B) The average of three independent experiments is shown, error bars indicate standard deviation. These values were normalized according to the expression data of the housekeeping gene, b-actin.
developing spontaneous carcinogenesis in humans. A number of tumor types have been found to be related to their expression of PDGF or PDGFRs[23–26]. If the expression of PDGF and its cognate receptor are found in tumor cells, then it is possible that autocrine stimulation may exist to develop carcinoma cells. By using a cDNA microarray technique, we identified the aberrant expression of PDGF-A and PDGFR-a presented in both follicular thyroid carcinoma (CGTH W-1) and papillary thyroid carcinoma (CGTH W-3) cell lines. In previous studies, it has been found that structural aberrations of the PDGF
and cognate receptor genes would lead to over-expression or over-expression of an abnormal protein [27–29]. Moreover, amplification of PDGFR-a gene could cause receptor over-expression in a few cases of glioblastoma[30–32]. These findings may imply that some variation of gene regulations lead to over-express the PDGF and PDGFR in CGTH W-1 and CGTH W-3 cells.
Generally speaking, normal thyroid cells do not exhibit PDGF and PDGFRs, which are mainly exhibited in mesenchyaml and glial origin tissues. Therefore, it is unusual to observe the expression of these genes in thyroid cells. Some studies have shown that PDGFR-b can be found in human anaplastic thyroid carcinoma cell line C643 (a kind of undiffer-entiated thyroid cell line)[14], and that both a- and b-type PDGFRs are expressed in human anaplastic thyroid carcinoma cell line HTh74 [15]. Two possibilities can be inferred from such findings. One is that the expression of PDGFR provides the cells a new route that stimulates the growth of the cells, and in this way PDGF-receptors take part in the carcinogenesis of thyroid cells. Alternatively, PDGFRs may be the remnants of immature progenitor cells.
CGTH W-1 and CGTH W-3 cell lines were obtained from well-differentiated thyroid tumor tissues. In these two cell lines, the expressions of PDGF-A and PDGFR-a proteins were observed. A segment of approximately 26 kDa was observed for PDGF-A by a Western blotting analysis, while segments around at 180, 156, 130, 90, and 52 kDa were observed for PDGFR-a. Similarly sized protein segments had been observed in glioma; these segments have been inferred to be derived from full-length receptor [33]. Huang and Huang [34] con-firmed that after PDGFR-b binding with PDGF-B, the receptor would quickly decompose. And it was also found that PDGF and receptor binding induces internalization of the complex into endosomes [35]. The PDGF-receptor complex then dissociates and recycles to the cell membrane, or alternatively the ligand-receptor complex is degraded after fusion of the endosomes with lysosomes. In addition to degradation in lysosomes, PDGF receptors also undergo cytoplasmic degradation in proteasomes after ubiquitination [36,37]. In the present study, we have similar observations in CGTH W-1 and CGTH
Fig. 4. Detection of PDGF-A and PDGFR-a by Western blotting. (A) The approximate 26 kDa PDGF-A protein was detected with mouse monoclonal IgG2b anti-PDGF-A in nodular hyperplasia
(NH), follicular thyroid carcinoma cell line (CGTH W-1), and papillary thyroid carcinoma cell line (CGTH W-3). (B) The approximate 180, 156, 130, 90 and 52 kDa protein were detected with rabbit polyclonal IgG anti-PDGFR-a in nodular hyperplasia (NH), follicular thyroid carcinoma cell line (CGTH W-1), and papillary thyroid carcinoma cell line (CGTH W-3).
W-3 cell lines regarding simultaneous expression of ligands and receptors and degradation of PDGFR-a. These observation illustrates that PDGF-A and PDGFR-a should form complex, induce internaliz-ation and undergo cytoplasmic degradinternaliz-ation. These findings indicate that the initiation event of PDGF autocrine activation and PDGFR degradation existed in CGTH W-1 and CGTH W-3 cell lines. Further-more, we would like to understand whether the aberrant expressions could appear in malignant thyroid tissues. Therefore, the immunohistochemis-trical analysis of the PDGF and PDGFR in a larger group of clinical thyroid carcinoma tissues as compared to benign tissues are currently under investigation in our laboratory.
When PDGF binding, it would induce the dimerization and autophosphorylation of PDGFR. The autophosphorylation has two important func-tions. On one hand, phosphorylation of a conserved tyrosine residue inside the kinase domains leads to an increase in the catalytic efficiencies. On the other hand, phosphorylation of tyrosine residues located outside the kinase domain creates docking sites for signal transduction molecules. We survey the PDGFR-a activation in terms of phosphorylation
and found that phosphorylation of PDGFR-a in CGTH W-1 and CGTH W-3 cell lines were detected on Tyr-720, but not on Tyr-754. The result was similar to the finding that Tyr-754 in the a-receptor was phosphorylated to a higher degree in the heterodimer compared with the homodimer [38]. Furthermore, it have been found that Tyr-720 in the PDGFR-a is required for binding of Grb2 and SHP-2 but not for activation of Ras or cell proliferation[39]. SHP-2 is a tyrosine phosphatase with two SH2 domains [40] and plays a diverse modulator. In previous studies, SHP-2 is a potential negative modulator of PDGF-related signal transduction by dephosphorylating autophosphorylated PDGFRs and substrates for the PDGF receptors [41]. However, SHP-2 may also be involved in positive signaling to act as an adaptor that binds Grb2/Sos and thus to contribute to Ras activation [42], and consequently to dephosphorylate the COOH-terminal tyrosine residue of Src and thus to contribute to Src activation [43,44]. Our data reveal that homodimeric PDGFR-a would cause autophosphorylation of tyrosine 720 in CGTH W-1 and CGTH W-3 cells. However, the possible role of tyrosine kinase phosphorylation at
tyrosine 720 concerning PDGFR-a signal
Fig. 5. Detection of phosphorylated PDGFR-a with immunoprecipitaion followed by western blotting. The rabbit polyclonal IgG anti-PDGFR-a wanti-PDGFR-as used to immunoprecipitanti-PDGFR-ate PDGFR-anti-PDGFR-a protein expressed in CGTH W-1 (lanti-PDGFR-anes 1 anti-PDGFR-and 3) anti-PDGFR-and CGTH W-3 (lanti-PDGFR-anes 2 anti-PDGFR-and 4). Immunoprecipitates were subjected to SDS-PAGE and immunoblotted with p-PDGFR-a (Tyr754) (lanes 1 and 2) and p-PDGFR-a (Tyr720) (lanes 3 and 4).
transduction must be studied further and other phosphorylation sites should be further discovered.
The growth of certain human glioma cells were blocked by PDGF antagonists[45,46]. In cell prolifer-ation assay, we demonstrated that the activity of PDGFR-a tyrosine kinase was necessary for CGTH W-1 and CGTH W-3 cell proliferations. By adding tyrphostin AG1295, which specifically inhibits
the activity of PDGFR kinase, we observed a dose-related inhibition of cell proliferation. These findings indicate that CGTH W-1 and CGTH W-3 cell lines have a PDGFR-related stimulation pathway, and which stimulates cell proliferation and consequently develops carcinogenesis. Using tyrphostin AG1295 as a proof for autocrine PDGF/PDGFR activity have preliminarily been observed. Furthermore, RNA interference, a process of homology-dependent degra-dation of cognate mRNA by short-interfering RNA (siRNA), now is being performed in our experiments by using new-designed siRNA (PDGFR-a siRNA) against PDGFR-a mRNA. The experiments by using RNAi to perturb PDGFR-a expression would recon-firm that thyroid carcinoma cell lines were growth dependent on the change of PDGFR-a expression, estimate the efficiency of PDGFR-a siRNA in suppression of the aberrant proliferation and may provide a new tool for repressing cell proliferation of thyroid tumor.
In conclusion, both the PDGF-A and PDGFR-a were over-expressed in the follicular thyroid carci-noma (CGTH W-1) and papillary thyroid carcicarci-noma (CGTH W-3) cell lines. The over-expression of PDGF-A and PDGFR-a genes might be an indication of carcinogenesis. In thyroid cells, those aberrant expressions could develop an abnormal signal trans-duction route by the PDGF-related autocrine acti-vation and consequently enhanced cell proliferation.
Acknowledgements
We thank the National Center for High-perform-ance Computing (7, R&D Rd VI, Hsinchu Science Park, Hsinchu 300, Taiwan) for analytic assistance with programs of the Genetics Computer Group sequence analysis package (GCG).
References
[1] C.J. Sherr, C.W. Rettenmier, R. Sacca, M.F. Roussel, A.T. Look, E.R. Stanley, The c-fms proto-oncogene product is related to the receptor for the mononuclear phagocyte growth factor, CSF-1, Cell 41 (1985) 665–676.
Fig. 6. Effects of Tyrphostin A1 (AG9) and Tyrphostin AG1295 on proliferation of CGTH W-1 and CGTH W-3 cell lines. Approxi-mately 2.5!104cells were seeded in RPMI-1640 medium with 1% fetal calf serum in triplicate dishes and grown in the presence of selective inhibitor for PDGF-receptor (0, 0.25, 0.5, 1, and 2.5 mM Tyrphostin AG1295) and negative control (2.5 mM Tyrphostin A1). (A) CGTH W-1. (B) CGTH W-3. Data are expressed as meanCSD The t-test was used to determine whether two experimental values were significantly different. *P!0.05 and **P!0.001 compared to the results of 0 mM Tyrphostin AG1295 treatment.
[2] \P. Besmer, J.E. Murphy, P.C. George, F.H. Qui, P.J. Bergold, L. Lederman, et al., A new acute transforming feline retrovirus and relationship of its oncogene v-kit with the protein kinase gene family, Nature 320 (1986) 415–421.
[3] Y. Yarden, W.J. Kuang, T. Yang-Feng, L. Coussens, S. Munemitsu, T.J. Dull, et al., Human proto-oncogene c-kit: a new cell surface receptor tyrosine kinase for an unidentified ligand, Eur. Mol. Biol. Org. J. 6 (1987) 3341–3351. [4] H. Bostrom, K. Willetts, M. Pekny, P. Leveen, P. Lindahl,
H. Hedstrand, et al., PDGF-A signaling is a critical event in lung alveolar myofibroblast development and alveogenesis, Cell 85 (1996) 863–873.
[5] A. Sasahara, J.N. Kott, M. Sasahara, E.W. Raines, R. Ross, L.E. Westrum, Platelet-derived growth factor B-chain-like immunoreactivity in the developing and adult rat brain, Dev. Brain Res. 68 (1992) 41–53.
[6] E.J. Battegay, J. Rupp, L. Iruela-arispe, E.H. Sage, M. Pech, PDGF-BB modulates endothelial proliferation and angiogen-esis in vitro via PDGF b-receptors, J. Cell Biol. 125 (1994) 917–928.
[7] C.H. Heldin, B. Westermark, in: C. Raf (Ed.), Role of Platelet-Derived Growth Factor In Vivosecond ed., Plenum, New York, 1996, pp. 249–273.
[8] A. Guha, A. Glowacka, R. Carroll, K. Dashner, P.M. Black, C.D. Stiles, Expression of platelet-derived growth factor and platelet derived growth factor receptor mRNA in a glioblas-toma from a patient with Li-Fraumeni syndrome, J. Neurol. Neurosurg. Psychiatry 58 (1995) 711–714.
[9] K.R. Harmon, C.J. Witkop, J.G. White, R.A. King, M. Prterson, D. Moore, et al., Pathogenesis of pulmonary fibrosis: platelet-derived growth factor precedes structural alterations in the Hermansky Pudlak syndrome, J. Lab. Clin. Med. 123 (1994) 617–627.
[10] H.E. Abboud, Role of platelet-derived growth factor in renal injury, Annu. Rev. Physiol. 57 (1995) 297–309.
[11] P. Heldin, H. Pertoft, H. Nordlinder, C.H. Heldin, T.C. Laurent, Differential expression of platelet-derived growth factor a- and b-receptors on fat-storing cells and endothelial cells of rat liver, Exp. Cell Res. 193 (1991) 364–369.
[12] C.H. Heldin, B. Westermark, A. Wastesson, Specific receptors for platelet-derived growth factor on cells derived from connective tissue and glia, Proc. Natl Acad. Sci. USA 78 (1981) 3664–3668.
[13] B. Westermark, L. Claesson-Welsh, C.H. Heldin, Structural and functional aspects of the receptors for platelet-derived growth factor, Prog. Growth Factor Res. 1 (1989) 253–266.
[14] N.E. Heldin, B. Gustavsson, L. Claesson-Welsh, A. Hammacher, J. Mark, C.H. Heldin, B. Westermark, Aberrant expression of receptors for platelet-derived growth factor in an anaplastic thyroid carcinoma cell line, Proc. Natl Acad. Sci. USA 85 (1988) 9302–9306.
[15] N.E. Heldin, D. Cvejic, S. Smeds, B. Westermark, Coexpres-sion of functionally active receptors for thyrotropin and platelet-derived growth factor in human thyroid carcinoma cells, Endocrinology 129 (1991) 2187–2193.
[16] J.D. Lin, T.C. Chao, H.F. Weng, H.S. Huang, Y.S. Ho, Establishment of xenografts and cell lines from well-differentiated human thyroid carcinoma, J. Surg. Oncol. 63 (1996) 112–118.
[17] A. Levitzki, A. Gazit, Tyrosine kinase inhibition: an approach to drug development, Science 267 (1995) 1782–1788. [18] L.M. Strawn, G. McMahon, H. App, R. Schreck,
W.R. Kuchler, M.P. Longhi, et al., Flk-1 as a target for tumor growth inhibition, Cancer Res. 56 (1996) 3540–3545. [19] M. Pech, A. Gazit, P. Arnstein, S.A. Aaronson, Generation of
fibrosarcomas in vivo by a retrovirus that expresses the normal B chain of platelet-derived growth factor and mimics the alternative splice pattern of the v-sis oncogene, Proc. Natl Acad. Sci. USA 86 (1989) 2693–2697.
[20] L. Uhrbom, G. Hesselager, M. Nister, B. Westermark, Induction of brain tumors in mice using a recombinant platelet-derived growth factor B-chain retrovirus, Cancer Res. 58 (1998) 5275–5279.
[21] L.A. Nilson, D. Dimaio, Platelet-derived growth factor receptor can mediate tumorigenic transformation by the bovine papillomavirus E5 protein, Mol. Cell. Biol. 13 (1993) 4137–4145.
[22] D.J. Goldstein, W. Li, L.M. Wang, M.A. Heidaran, S. Aaronson, R. Shinn, et al., The bovine papillomavirus type 1 E5 transforming protein specifically binds and activates the beta-type receptor for the platelet-derived growth factor but not other related tyrosine kinase-containing receptors to induce cellular transformation, J. Virol. 68 (1994) 4432–4441. [23] K. Fudge, D.G. Bostwick, M.E. Stearns, Platelet-derived growth factor A and B chain and the alpha and beta receptor in prostatic intraepithelial neoplasia, Prostate 29 (1996) 282– 286.
[24] M. Ebert, M. Yokoyama, H. Friess, M.S. Kobrin, M.W. Bochler, M. Korc, Induction of platelet-derived growth factor A and B chains and over-expression of their receptors in human pancreatic cancer, Int. J. Cancer 62 (1995) 529–535. [25] C. Sundberg, M. Branting, B. Gerdin, K. Rubin, Tumor cell
and connective tissue cell interactions in human colorectal adenocarcinoma: transfer of platelet-derived growth factor-AB/BB to stromal cells, Am. J. Pathol. 151 (1997) 479–492. [26] K. Anan, T. Morisaki, M. Katano, A. Ikubo, H. Kitsuki,
A. Uchiyama, et al., Vascular endothelial growth factor and platelet-derived growth factor are potential angiogenic and metastatic factors in human breast cancer, Surgery 119 (1996) 333–339.
[27] M.P. Simon, F. Pedeutour, N. Sirvent, J. Grosgeorge, F. Minoletti, J.M. Coindre, et al., Deregulation of the platelet-derived growth factor B-chain gene via fusion with collagen gene COL1A1 in dermatofibrosarcoma protuberans and giant-cell fibroblastoma, Nat. Genet. 15 (1997) 95–98. [28] M. Carroll, M.H. Tomasson, G.F. Barker, T.R. Golub,
D.G. Gilliland, The TEL/platelet-derived growth factor b receptor (PDGFbR) fusion in chronic myelomonocytic leukemia is a transforming protein that self-associates and activates PDGFbR kinase-dependent signaling pathways, Proc. Natl Acad. Sci. USA 93 (1996) 14845–14850.
[29] T.R. Golub, G.F. Barker, M. Lovett, D.G. Gilliland, Fusion of PDGF receptor b to a novel ets-like gene, tel, in chronic myelomonocytic leukemia with t(5;12) chromosomal translo-cation, Cell 77 (1994) 307–316.
[30] T.P. Fleming, A. Saxena, W.C. Clark, J.T. Robertson, E.H. Oldfield, S.A. Aaronson, I.U. Ali, Amplification and/or overexpression of platelet-derived growth factor receptors and epidermal growth factor receptor in human glial tumors, Cancer Res. 52 (1992) 4550–4553.
[31] M. Hermanson, K. Funa, J. Koopmann, D. Maintz, A. Waha, B. Westermark, et al., Association of loss of heterozygosity on chromosome 17p with high platelet-derived growth factor a receptor expression in human malignant gliomas, Cancer Res. 56 (1996) 164–171.
[32] T. Kumabe, Y. Sohma, T. Kayama, T. Yoshimoto, T. Yamamoto, Amplification of a-platelet-derived growth factor receptor gene lacking an exon coding for a portion of the extracellular region in a primary brain tumor of glial origin, Oncogene 7 (1992) 627–633.
[33] M. Nister, L. Claesson-Welsh, A. Eriksson, C.H. Heldin, B. Westermark, Differential expression of platelet-derived growth factor receptors in human malignant glioma cell lines, J. Biol. Chem. 266 (1991) 16755–16763.
[34] S.S. Huang, J.S. Huang, Rapid turnover of the platelet-derived growth factor receptor in sis-transformed cells and reversal by suramin: implications for the mechanism of autocrine transformation, J. Biol. Chem. 263 (1988) 12608–12618.
[35] A. Sorkin, B. Westermark, C.H. Heldin, L. Claessonwelsh, Effect of receptor kinase inactivation on the rate of internalization and degradation of PDGF and the PDGF beta-receptor, J. Cell Biol. 112 (1991) 469–478.
[36] S. Mori, C.H. Heldin, L. Claesson-Welsh, Ligand-induced polyubiquitination of the platelet-derived growth factor beta-receptor, J. Biol. Chem. 267 (1992) 6429–6434.
[37] S. Mori, K. Tanaka, S. Omura, Y. Saito, Degradation process of ligand-stimulated platelet-derived growth factor beta-receptor involves ubiquitin-proteasome proteolytic pathway, J. Biol. Chem. 270 (1995) 29447–29452.
[38] E. Rupp, A. Siegbahn, L. Ronnstrand, C. Wernstedt, L. Claesson-Welsh, C.H. Heldin, A unique autophosphoryla-tion site in the platelet-derived growth factor alpha receptor from a heterodimeric receptor complex, Eur. J. Biochem. 225 (1994) 29–41.
[39] C.E. Bazenet, J.A. Gelderloos, A. Kazlauskas, Phosphoryl-ation of tyrosine 720 in the platelet-derived growth factor alpha receptor is required for binding of Grb2 and SHP-2 but not for activation of ras or cell proliferation, Mol. Cell. Biol. 16 (1996) 6926–6936.
[40] S. Pluskey, T.J. Wandless, C.T. Walsh, S.E. Shoelson, Potent stimulation of SH-PTP2 phosphatase activity by simultaneous occupancy of both SH2 domains, J. Biol. Chem. 270 (1995) 2897–2900.
[41] R.A. Klinghoffer, A. Kazlauskas, Identification of a putative Syp substrate, the PDGF b-receptor, J. Biol. Chem. 270 (1995) 22208–22217.
[42] W. Li, R. Nishimura, A. Kashishian, A.G. Batzer, W.J.H. Kim, J.A. Cooper, J. Schlessinger, A new function for a phospho-tyrosine phosphatase: linking GRB2-Sos to a receptor phospho-tyrosine kinase, Mol. Cell. Biol. 14 (1994) 509–517.
[43] L. Ronnstrand, A.K. Arvidsson, A. Kallin, C. Rorsman, U. Hellman, U. Engstrom, et al., SHP-2 binds to Tyr703 and Tyr1009 in the PDGF b-receptor and mediates PDGF-induced activation of the Ras/MAP kinase pathway and chemotaxis, Oncogene 18 (1999) 3696–3702.
[44] D. Stokoe, F. Mccormick, Activation of c-Raf-1 by Ras and Src through different mechanisms: activation in vivo and in vitro, Eur. Mol. Biol. Org. J. 16 (1997) 2384–2396. [45] F.S. Vassbotn, A. Ostman, N. Langeland, H. Holmsen,
B. Westermark, C.H. Heldin, M. Nister, Activated platelet-derived growth factor autocrine pathway drives the trans-formed phenotype of a human glioblastoma cell line, J. Cell Physiol. 158 (1994) 381–389.
[46] S.M. Shamah, C.D. Stiles, A. Guha, Dominant-negative mutants of platelet-derived growth factor revert the trans-formed phenotype of human astrocytoma cells, Mol. Cell. Biol. 13 (1993) 7203–7212.