甲狀腺刺激素受體和細胞間素在甲狀腺上眼瞼攣
縮所扮演的角色
Purpose: To investigate thyrotropin receptor (TSHr), the candidate autoantigen shared by Graves’ disease and Graves’ ophthalmopathy, and cytokine profiles in the pathogenesis of patients with thyroid lid retraction.
Methods & materials: Data from all Müllerectomy patients, with clinical characteristics of thyroid lid retraction, were collected retrospectively. The diseased and controlled Müller‘s muscles were collected from patients after IRB approval and given of informed consent by patients. All muscle specimens were embedded in paraffin solution for subsequent pathological examination including routine H&E and immunohistochemistry (IHC). Some parts of the Müller‘s muscle were homogenized for test of local cytokine expression. Peripheral blood from GO patients was also collected for investigating the effects of exogenous cytokines.
Results: A total of 31 patients, 29 female and 2 male, with thyroid lid retraction were enrolled into this study. We found a correlation between theseverity of upper lid retraction (MRD1) and age (r=0.407, p=0.0203), initial thyroid state (p=0.045), and the period of
hyperthyroidism (r=0501, p=0.004). On pathological analyses, variation of fat and fibrosis, as well as a decrease in normal muscle volume, were noted in the thyroid Müller‘s muscle. Neither cells stained positive of TSHr in both groups using IHC. The determination of local cytokine expression within the Müller‘s muscle could not be performed using the current Western blot method owing to the very low quantity of total protein. However, there is significant difference of serum IL-4 levels between GO and control groups (p<0.005).
Conclusion: In clinical association analyses, there was a positive correlation between the severity of upper lid retraction and age, along with the initial thyroid state and the period of hyperthyroidism. Although TSHr could not be detected using IHC, the existence of TSHr involving thyroid Müller‘s muscle, using PCR or Q-PCR amplification, remains to be studied in near future. From data of peripheral cytokine in GO patients, the serum Th2 cytokine level may correlate with clinical activity but not with the degree of severity of GO.
Keywords: Graves’ ophthalmopathy, thyroid eye disease, Muller’s muscle, upper lid retraction, thyrotropin receptor antibody, cytokines
二、緣由與目的
Approximately 25-50 % of patients with Graves’ disease (GD) may have ocular complications in terms of Graves’ ophthalmopathy (GO) or thyroid eye disease (TED). 1,2 The pathogenesis of GO is less understood, but most investigators believe it is an autoimmune disease in nature because of the close temporal association between them. 3,4,5 Most investigations of GO focus on the research of extra ocular muscles and proptosis, which are resulted from accumulation of products secreted by activated infiltrating orbital fibroblasts after propagated stimulation of inflammatory mediators or cytokines. 6,7,8,9 Nearly 90% of GD patients manifest upper lid retraction, which is the most common pathognomonic characteristic in patients with GO; however, there is a lack of research for the pathogenesis of upper lid retraction.10 Not only several conjectures have been postulated to provide possible mechanisms for upper lid retraction 11,12,13, but pathology of the Müller‘s muscle that is a smooth muscle contributive to upper lid excursion, there exist many controversies. 14,15,16 The purpose of our study is to investigate the role of thyrotropin receptor (TSHr), the potential autoantigen shared between GD and GO, as well as of contribution of systemic cytokines to the severity of thyroid lid retraction.
Methods and Materials
Collection of Clinical Data and the Müller’s Muscle
Data from all Müllerectomy patients, with clinical characteristics of thyroid lid retraction, were collected retrospectively, including age, sex, pre-operative severity of upper lid retraction, degree of exophthalmos, initial thyroid state, duration of hyperthyroidism and GO, status of thyrotropin binding inhibitory immunoglobulin (TBII), smoking, drugs and family history of GO. The degree of upper lid retraction was measured
and recorded as upper marginal reflex distance (MRD1, mm). The degree of proptosis was
evaluated by a Hertel exophthalmometer (mm). The diseased Müller‘s muscle was collected from patients with thyroid lid retraction, in a stable thyroid and ophthalmic state for at least 3 to 6 months, before operation. On the other hand, normal Müller‘s muscle was obtained from patients without thyroid disease, in the control group, during scheduled correction for ptosis.
Immunohistochemistry (IHC)
The excised Müller‘s muscle of both groups was fixed and embedded in formalin solution and paraffin, respectively. Sections of 3µm thickness were cut and stained with hematoxyline and eosin (H&E), and several IHC including LCA, CD3, CD20, and TSHr. Most of the pre-treatment was performed by the heat induced epitode retrieval (HIER) method in a microwave for 10 minutes while immersed in 10mmol/L citrate buffer (pH 6.5). Tonsil or lymph nodes were used as a positive control for LCA, CD3, CD20, whereas the thyroid gland of patients with goiter was employed as a positive control for TSHr. As a negative control, each sample was stained with a secondary antibody, with a primary antibody omitted.
Cytokine Immunoassay
Approximately one half sized Müller‘s muscles (5 samples) were collected and stored at -20 ºC for homogenization and cytokine test using Western blot method. The levels of cytokine in serum of patients with thyroid lid retraction were measured with an enzyme immunoassay kit (Bender MedSystems, Austria). In the control group, the blood samples of patients were collected from routine healthy examination. All serum samples after centrifugation were stored at -20ºC until test. A 50 µl of each sample was applied to a 96-well flat-bottom plate. Standards and samples were incubated in at room temperature for 2 hours. Finally, the plate was read by an ELISA Labsystem Multiscan RC at 450 nm.
Serum and muscle collected in both the diseased and control groups were obtained and approved by the Institutional Review Board of the National Taiwan University Hospital. Informed consent was acquired from patients after given an explanation of the
study’s purpose.
Statistics
The correlation was evaluated using the Pearson correlation coefficient. The differences of MRD1 between clinical categorical data (sex, thyroid state, TBII, drug,
smoking, and family history) were analyzed by Wilcoxon Rank-Sum test. All statistical analyses were performed with SAS and significant level was p< 0.05.
三、結果與討論 Results
Results of Clinical Data and Their Correlation to the Severity of Upper Lid Retraction
A total of 31 patients, 29 female and 2 male, with thyroid lid retraction, aged from 23 to 64 years old with a mean age of 39.13 ± 9.40 years, were enrolled into this study. In contrast, a total of 7 patients (6 female, 1 male), ages from 45 to 73 years, underwent a correction of ptosis were collected in the control group. All clinical characters of patients are summarized in Table 1. We found a correlation between the severity of upper lid retraction (MRD1) and age (r=0.407, p=0.0203), initial thyroid state (p=0.045), and the
period of hyperthyroidism (r=0501, p=0.004); however, there is no significant association between the severity of upper lid retraction and other clinical variables. The analyses of clinical data in patients with upper lid retractionare listed in Table 2.
The pathology of the Müller’s Muscle
Under H&E stain, undulated bundles of smooth muscle fibers were coated with a thin connective tissue, as well as a diminutive amount of fatty tissue infiltrated into normal Müller‘s muscle. There was no existence of fibrotic composition. While a variable degree of fat and fibrosis, as well as a decrease in normal muscle volume, were noted in the thyroid Müller‘s muscle. (Fig 1A & 1B)
Semi-quantification of Inflammatory Cells and Immunohistochemistry
higher than that in thyroid Müller‘s muscle (p=0.023). The numbers of T cells (CD3), in normal Müller‘s muscle, were also higher than those in the thyroid group; however, this did not achieve a significant level, statistically (p=0.161). Neither cells stained positive of CD20 or TSHr in both groups.
Results of cytokines in the diseased Müller’s Muscle
We collected 5 samples of one half sized Müller’s Muscle for cytokines test, whereas the quantity of total protein within the Müller’s Muscle was too low to detect by spectrophotometry at 578 nm. The determination of local cytokine expression could not be performed using the current Western blot method.
Results of Serum cytokines
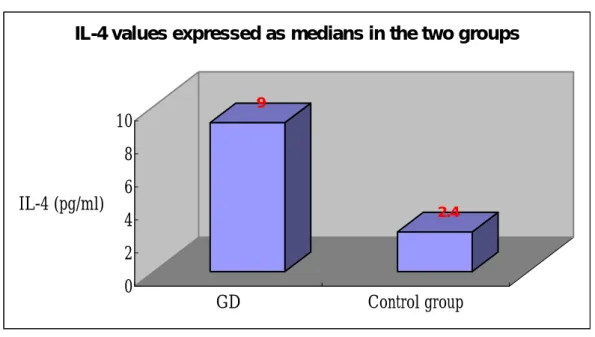
A total of 56 serum samples from patients with thyroid lid retraction in an active or chronic stage, in contrast of 15 healthy volunteers, were collected for cytokine test. In Th2 profile, the serum IL-4 concentrations ranged from 1.1 to 36.8 pg/ml (mean: 6.0, median: 9.0, SD: ± 8.3 pg/ml). In the control group, IL-4 levels ranged from 0.0 to 13.1 pg/ml (mean: 2.2, median: 2.4, SD: ± 3.6 pg/ml). There is significant difference between the median values of serum IL-4 between these two groups (p<0.005). The result was illustrated in Fig.2. On associative analyses, there is no significant correlation between IL-4 level and thyroid function profiles such as freeT4, TSH, and TBII. We also correlated the IL-4 level to the severity of upper lid retraction or GO, however, there no association exits. Surprisingly in serial follow-up, there is a tendency of increased IL-4 value in GO patients whose clinical manifestations have progressed. The data of serum Th1 profile in GO patients with upper lid retraction including INF-γ, TNF-α will be tested in near future.
Discussion
In clinical association analyses, there was a positive correlation between the severity of upper lid retraction and age, along with the initial thyroid state and the period of hyperthyroidism. Kendler et al ever proposed that elder patients with GO were prone to
have a more severe course of the disease such as strabismus or compressive optic neuropathy, which is similar to our findings. The status of TBII or TRAb of GO patients seems no influence in the severity of upper lid retraction, which is more accepted in correlation with clinical activity not with the severity of clinical manifestations.
Using IHC, there was no evidence of cells stained with TSHr. Our data suggest that there were two possibilities for this finding: (1) a low abundance of TSHr contained in the thyroid Müller‘s muscle, which can not be detected with IHC, (2) TSHr is not the major autoantigen in the thyroid Müller‘s muscle. The existence of TSHr involving thyroid Müller‘s muscle, using PCR or Q-PCR amplification, remains to be studied in near future. There is no correlation between IL-4 level and severity of upper lid retraction or GO; however, we noted a trend between the increased IL-4 value and progression of clinical manifestation. It seems that serum Th2 cytokine level may correlate with clinical activity but not with the degree of severity of GO. To prove this association, we necessitate enrolling more GO patients with a serial time course to determine the role of new serum markers contributing in an earlier detection of disease progression.
五、參考文獻
1. Teng CS, Yeo PP. Ophthalmic Graves’ disease. The natural history and detailed thyroid function studies. Br Med J. 1977; 1: 273-275.
2. Bahn RS, Heufelfelder AE. Pathogenesis of Graves’ ophthalmopathy. N Engl J Med 1993; 329: 1468-1475.
3. Kazim M, Goldberg RA, Smith TJ. Insights into the pathogenesis of thyroid associated orbitopathy. Ar Ophthalmol 2002; 120: 380-386.
4. Ludgate M, Baker G. Unlocking the immunological mechanisms of orbital inflammation in thyroid eye disease. Clin Exp Immunol 2002; 127: 193-198.
5. Bahn RS. Pathophysiology of Graves’ Ophthalmopathy: The cycle of disease. J Clin Endocrinol Metab 2003; 88: 1939-1946
6. Hiromatsu, Y, Kaku H, Miyake I, Murayama S, Soejima E. Role of cytokines in the pathogenesis of thyroid-associated ophthalmopahty. Thyroid 2002; 12: 217-221. 7. Ajjan RA, Weetman AP. New understanding of the role of cytokines in the
pathogenesis of Graves’ ophthalmopathy. J Endocrinol Invest 2004; 27: 237-245. 8. Kahaly G, Stover C, Otto E, Beyer J, Schuler M. Glycosaminoglycans in
thyroid-associated ophthalmopathy. Autoimmunity 1992; 13:81-88.
9. Kahaly G, Hansen C, Stover C, Kuhlemann K, Beyer J, Otto E. Glycosaminoglycans and endocrine orbitopathy. Exp Clin Endocrinol 1994; 102: 151-161.
10. Bartley GB, Fatourechi V, Kadrmas EF, Jacobsen SJ, Ilstrup DM, Garrity JA, Gorman CA. Clinical features of Graves’ ophthalmopathy in an incidence cohort. Am J Ophthalmol 1996; 121: 284-290.
11. Putterman AM, Urist M. Surgical treatment of upper eyelid retraction. Arch ophthalmol 1972; 87: 401-405.
12. Jampolsky A. What can electromyography do for the ophthalmologist? Invest Ophthalmol 1970; 9: 570-599.
ophthalmopathy. Ophthalmology 1989; 96: 424-30.
14. Rootman J, Patel S, Berry K, Nugent R. Pathological and clinical study of the Müller’s muscle in Graves’ ophthalmology. Can J Ophthalmol 1987; 22: 32-36.
15. Gaag RVD, Schmidt D, Koornneef L. Retrobulbar histology and immunohistochemistry in endocrine ophthalmopathy. Dev Ophthalmol 1993; 25: 1-10.
16. Cockerham KP, Hidayat AA, Brown HG, Cockerham GC, Graner SR. Clinicopathologic Evaluation of the Mueller Muscle in Thyroid-Associated Orbitopathy. Ophthal Plast Reconstr Surg 2002; 18(1): 11-17.
Figure 1 A.
B.
Undulated bundles of smooth muscle fibers were coated with a thin connective tissue, as well as a diminutive amount of fatty tissue infiltrated into normal Müller‘s muscle. There was no existence of fibrotic composition. (Fig 1A) While a variable degree of fat and fibrosis, as well as a decrease in normal muscle volume, were noted in the thyroid Müller‘s muscle. (Fig 1B)
Figure 2. 9 2.4 0 2 4 6 8 10 IL-4 (pg/ml) GD Control group
IL-4 values expressed as medians in the two groups
There is significant difference between the median values of serum IL-4 between GD group (median 9.0) and control group (median 2.4) (p<0.005).
Table 1. Clinical characters of patients with thyroid lid retraction N N Age 31 39.13 ± 9.40 (23.00 ~ 64.00) Sex 31 Female 29 (93.6%) Male 2 (6.5%) MRD1 (mm) 31 7.58 ± 0.81 (6.00 ~ 9.00) Proptosis (mm) 31 19.68 ± 2.56 (15.00 ~ 26.00)
Original thyroid state 31
Normal 3 (9.7%)
Hyper 28 (90.3%)
TBII* 19
Normal 6 (31.6%)
Abnormal 13 (68.4%)
Duration of hyperthyroidism (month) 31 68.58 ± 76.98 (12.00 ~ 336.00)
Duration of GO (month) 31 43.32 ± 33.20 (3.00 ~ 120.00) Drug history ATD† 31 27 (87.1%) Steroid 31 16 (51.6%) Thyroidectomy 31 5 (16.1%) Sandostatin 31 1 (3.2%) Smoking 31 4 (12.9%) Family history of GO 31 3 (9.7%)
*TBII: Thyrotropin binding inhibitory immunoglobulin
13
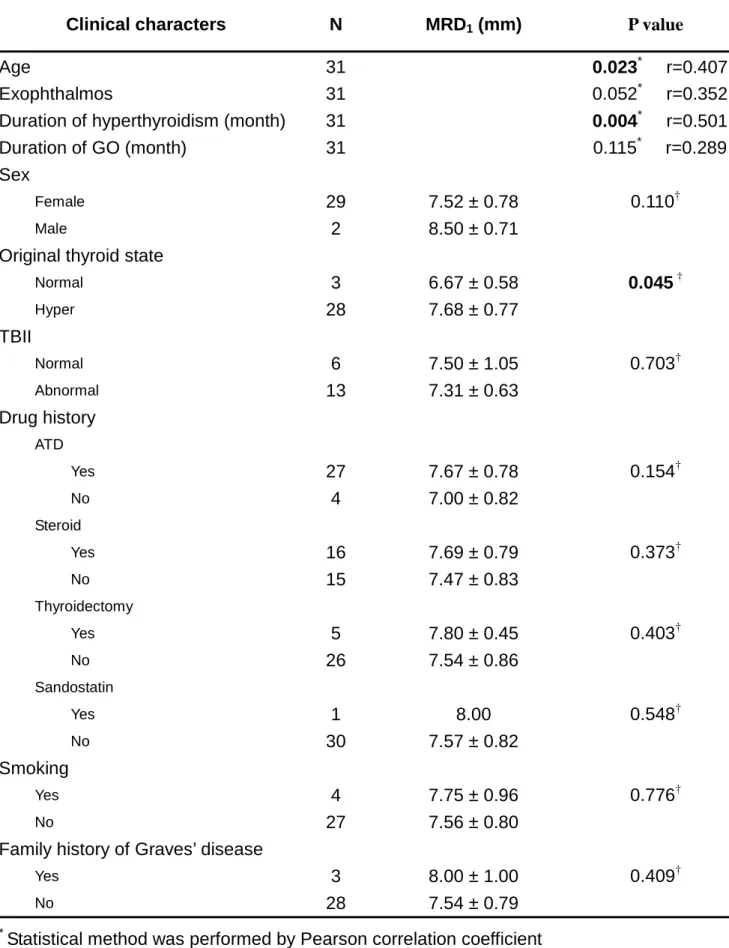
Table 2. Correlation between clinical findings and the severities of upper lid retraction
Clinical characters N MRD1 (mm) P value
Age 31 0.023* r=0.407
Exophthalmos 31 0.052* r=0.352
Duration of hyperthyroidism (month) 31 0.004* r=0.501
Duration of GO (month) 31 0.115* r=0.289
Sex
Female 29 7.52 ± 0.78 0.110†
Male 2 8.50 ± 0.71
Original thyroid state
Normal 3 6.67 ± 0.58 0.045 † Hyper 28 7.68 ± 0.77 TBII Normal 6 7.50 ± 1.05 0.703† Abnormal 13 7.31 ± 0.63 Drug history ATD Yes 27 7.67 ± 0.78 0.154† No 4 7.00 ± 0.82 Steroid Yes 16 7.69 ± 0.79 0.373† No 15 7.47 ± 0.83 Thyroidectomy Yes 5 7.80 ± 0.45 0.403† No 26 7.54 ± 0.86 Sandostatin Yes 1 8.00 0.548† No 30 7.57 ± 0.82 Smoking Yes 4 7.75 ± 0.96 0.776† No 27 7.56 ± 0.80
Family history of Graves’ disease
Yes 3 8.00 ± 1.00 0.409†
No 28 7.54 ± 0.79
*
Statistical method was performed by Pearson correlation coefficient
†