( ~ Pergamon
0040-4020(94)00943-0
Tetrahedron Voh 51, No. I, pp. 193-202, 1995 Copyright © 1994 Elsevier Science Ltd Printed in Great Britain. All rights reserved 0040-4020/95 $9.50+0.00
S y n t h e s i s a n d D i e l s - A l d e r R e a c t i o n s o f F u r o [ 2 , 3 - c ] p y r r o l e s a n d B e n z o f u r o [ 2 e 3 - c ] p y r r o l e s
Chin.Kang Sha* and Ren-Sheng Lee Department of Chemistry, National Tsing Hua University
Hsinchu, Taiwan 300, R. O. C. Yu W a n g
Department of Chemistry, National Taiwan University Taipei, Taiwan 100, R. O. C.
A b s t r a c t : Furo[2,3-c]pyrroles l a - d and benzofuro[2,3-c]pyrroles 6a-e were synthesized. Diels-Alder reactions of l b and 6b gave 1:2 cycloadduct 13 and 1:1
cycloadduct 20, respectively. Parent compound 17 of benzofuro[2,3-c]pyrrole ring system was trapped as N-tert-butoxycarbonyl derivative 18. Oxidative extrusion of the N-bridge in Diels-Alder adduct 20 gave dibenzofuran 22.
Furo[2,3-c]pyrroles 1 and benzofuro[2,3-c]pyrroles 6 are highly labile heterocyclic ring systems according to theoretical calculation. 1 Their synthesis and reaction are unknown. Among the related ring systems 2-5, 7, and 8, only 32 and 73 were synthesized. However, the method used in these syntheses are unsuitable for the preparation of furo[2,3-c]pyrrole 1 and benzofuro[2,3-c]pyrrole 6 ring systems. We developed three methods for the synthesis of !so-condensed heteroaromatic pyrroles. 4 In our preliminary communication, we have reported the synthesis of benzo[2,3-c]pyrrole 6. 4b Herein we report the preparation of furo[2,3-c]pyrrole 1 and benzofuro[2,3-c]pyrrole 6 in detail, as well as Diels-Alder reactions of these heterocycles.
R I ! ! R R 1 2 3 4 5 R ! R 6 7 8 193
194 C.-K. SHA et aL
K n o e v e n a g e l c o n d e n s a t i o n of 3 - m e t h y l - 2 - f u r o c a r b o x a l d e h y d e (9) with diethyl m a l o n a t e gave alkylidenemalonate 10. 4 Bromination of 10 with N-bromosuccinimide in t h e p r e s e n c e of dibenzoyl peroxide afforded b r o m i d e 11. T r e a t m e n t of 11 with b e n z y l a m i n e , isopropylamine, tert-butylamine a n d 3 - h y d r o x y p r o p y l a m i n e in ethanol yielded furo[2,3-c]pyrroles l a , l b , l c and l d , respectively. The yields are only moderate (16- 46%) because these compounds are highly sensitive to acid, and p a r t i a l l y polymerized upon silica gel chromatography. Subsequently, Diels-Alder reaction of l b with two equiv. dimethyl acetylenedicarboxylate (DMAD) at room t e m p e r a t u r e in benzene gave a 1:2
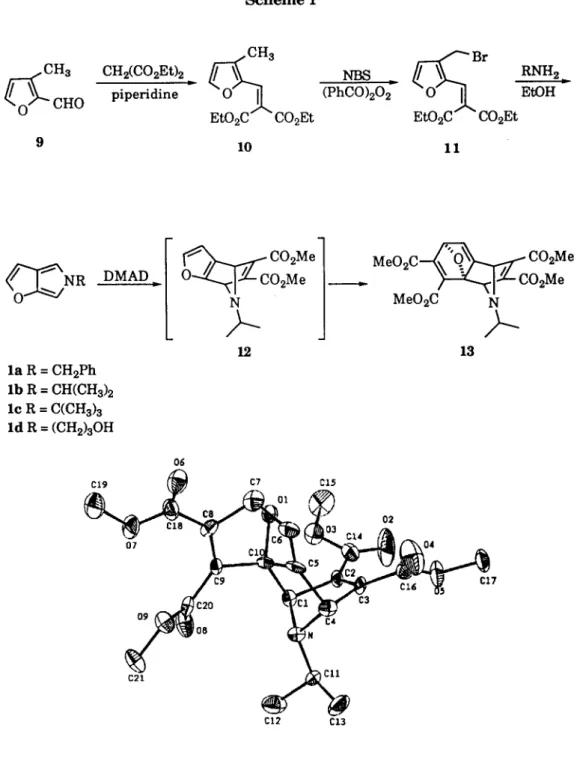
cycloadduct 13. The expected 1:1 cycloadduct 12 was not detected. Diels-Alder reaction of l b with 0.9 equiv. DMAD also gave only 1:2 cycloadduct 13 and recovered l b . Apparently, the reactive furan moiety of 1:1 cycloadduct 12 underwent a second Diels-Alder reaction with DMAD v e r y rapidly, Scheme 1. A s t r u c t u r e of 13 from X - r a y c r y s t a l l o g r a p h i c analysis is shown in Figure 1. 5
We a p p l i e d the s a m e m e t h o d for s y n t h e s i s of b e n z o f u r o [ 2 , 3 - c ] p y r r o l e s 6. K n o e v e n a g e l c o n d e n s a t i o n of 2 - m e t h y l - 3 - b e n z o f u r o c a r b o x a l d e h y d e ( 1 4 ) w i t h d i e t h y l m a l o n a t e gave 15. Bromination of 15 with N - b r o m o s u c c i n i m i d e afforded bromide 16. T r e a t m e n t of bromide 16 with methylamine, isopropylamine, benzylamine, phenylamine and p-toluidine afforded benzofuro[2,3-c]pyrroles 6a-e respectively. In addition, t r e a t m e n t of 16 with a m m o n i a in ethanol gave p a r e n t compound 17, which was not isolable, but reacted i m m e d i a t e l y with di-tert-butyl dicarboxylate and 4-dimethylaminopyridine to give stable derivative 18. 6 Diels-Alder reactions of compounds 6 and 18 with DMAD afforded c y c l o a d d u c t s 20 and 19 smoothly, m oChloroperbenzoic acid oxidation of 20 with spontaneous extrusion of the R-N=O group gave 3,4-dimethoxycarbonyldibenzofuran (22)
via intermediate 21. 7
In s u m m a r y , we have succeeded in synthesis of two new heteroeyclic ring systems, furo[2,3-c]pyrrole 1 and benzofuro[2,3-c]pyrrole 6. Diels-Alder reactions of l b and 6b with DMAD gave 1:2 cycloadduct 13 and 1:1 cycloadduct 20 respectively. Oxidative extrusion of the nitrogen bridge in Diels-Alder adduct 20 afforded dibenzofuran 22. P a r e n t system 6 was also p r e p a r e d a n d t r a p p e d with di-tert-butyl dicarbonate to give 18, which also underwent Diels-Alder reaction with DMAD.
E x p e r i m e n t a l Section
General. 1H NMR spectra were recorded on a Varian EM-390, a J E O L HX-100 or a B r u k e r AM-400 s p e c t r o m e t e r . 13C NMR spectra were recorded on a B r u k e r AM-400 spectrometer. Mass spectra refer to the electron impact m a s s spectra and were recorded on a J E O L TMS-D-100 m a s s spectrometer. High-resolution m a s s spectra were recorded on
Furo[2,3-c]pyrroles and benzofuro[2,3-c]pyrroles
195
Scheme 1
CH3
C H O 9CH2(CO2Et)2
piperidine
C H 3 "~
CO2Et
10
NBS
~
RNH2.
(PhCO)20 2 : t O ~ c ~ C
EtOH
O2Et
11
o ~ N R
DMAD
la R = CH2Ph
lb R = CH(CH3)2
lc R = C(CH3) 3
ld R = (CH2)3OH
O••C
CO2Me
2Me
N
12MeO2C ~
C02Me
. ~'-~I-~--- C 0 2 M e M e 0 2 C N)-_
13 O6 C7 C15 C21 C3 7 C21 C12 C13196
C.-K. SHA
et al.Scheme2
14CH2(CO2Et)2
piperidine
15NtkS
(PhCO)202
n r~
CO2EI NH2 *
16NH3/EtOH
~
NR
6a R = CH 3
6b R = CH(CH3) 2
6c R = CH2Ph
6dR = Ph
6e R = p-CH3Ph
DMAD~
~
N~
17
Y
N
~--'~-0~.-'~
/~---.. C 0 2 M e 2 0I MCPBA
21(t'BuCO2)20.
DMAP,
N- CO2tBu
CH2C12
18NJxCO2tBu
19
- R - N--- 0~
COsMe C02Me 22Furo[2,3-c]pyrroles and bcnzofuro[2,3-c]pyrroles 197
a J E O L H X - I I 0 m a s s spectrometer. IR spectra were recorded on a Perkin-Elmer 781 spectrometer, and U V spectra on a Perkin-Elmer L a m b d a 5 U V - V I S spectrometer. Single crystal X-ray analysis was performed on a Enraf-Nonius C A D - 4 diffractometer. Melting points determined with a Bfichi 530 melting-point apparatus and are uncorrected. Flash- column chromatography w a s performed as follows: silica gel, M e r c k No. 7736 Kieselgel 60H, w a s placed in a sintered-glass column packed dry. Solvent was flushed through the silica gel under a water-aspirator vacuum. The c o m p o u n d w a s then deposited with a minimal a m o u n t of solvent and eluted with solvent under a water aspirator vacuum. Diethyl ether and tetrahydrofuran (THF) were distilled from potassium/sodium metal
under a nitrogen atmosphere with benzophenone ketyl as the indicator. All reactions were conducted under a nitrogen atmosphere.
D i e t h y l [ ( 3 - B r o m o m e t h y l - 2 . f u r y l ) m e t h y l e n e ] p r o p a n e d i o a t e (U). To a solution of 10
(290 mg, 1.2 mmol) in carbon tetrachloride (25 ml) was added N-bromosuccinimide (204 rag, 1.2 mmol) and dibenzoyl peroxide (10 rag). The reaction mixture was stirred and heated at reflux for i h. After the mixture was cooled in an ice bath, the solid was removed by filtration and washed with carbon tetrachloride. Concentration and silica gel flash column c h r o m a t o g r a p h y (hexane-ethyl acetate, 10:1) gave 11 (209 mg, 55%) as a yellow oil: IR (neat) 2980, 1730, 1635 cm-1; 1H NMR (400 MHz, CDC13) 8 7.44 (d, 1 H, J = 1.7 Hz), 7.38 (s,1 H), 6.49 (d, 1 H, J = 1.7 Hz), 4.56 (s, 2 H), 4.34 (q, 2 H, J = 7.2 Hz), 4.23 (q, 2 H, J = 7.2 Hz), 1.30 (t, 3 H, J = 7.2 Hz), 1.26 (t, 3 H, J = 7.2 Hz); MS m/z (relative intensity) 322 (M++2, 44%), 330 (M+, 48%), 251 (100%).
N - B e n z y l f u r o [ 2 , 3 - c ] p y r r o l e (la). To a solution ofbenzylamine (114 mg, 1.1 retool) in 95% ethanol (2 ml) was added dropwise a solution of 11 (160 mg, 0.48 mmol) in 95% ethanol (7ml). The reaction mixture was stirred at room t e m p e r a t u r e for 31 h. Concentration and silica gel flash column chromatography (hexane-ethyl acetate, 20:1) gave l a (19mg, 20%) as a yellow oil: IR (neat) 3120, 3030, 2930, 1605 cm-1; 1H NMR (400 MHz, CDC13) 8 7.34-7.24 (m, 4 H), 7.12 (s, 1 H), 7.11 (d, 1 H, J = 4.1 Hz), 6.50 (d, 1 H, J = 4.1 Hz), 6.49 (s, 1 H), 6.40 (s, 1 H), 5.13 (s,2 H); MS m/z (relative intensity) 197 (M+,77), 91 (100); HRMS calcd C13HllNO 197.0834, found 197.0841.
N - I s o p r o p y l f u r o [ 2 , 3 - c ] p y r r o l e (lb). To a solution of isopropylamine (38 nag, 0.64 mmol) in 95% ethanol (2 ml) was added dropwise a solution of 11 (96 mg, 0.29 retool) in 95% ethanol (10 ml). The reaction mixture was stirred at room t e m p e r a t u r e for 32 h. Concentration and silica gel flash column c h r o m a t o g r a p h y (hexane-ethyl acetate, 20:1) gave l b (10.8 mg, 25%) as a yellow oil: IR (Neat) 3115, 2920, 1380 cm-1; 1H NMR (90 MHz, CDC13) 3 7.18 (bd, 1 H), 6.90 (bd, 1 H), 6.41 (d, 1 H, J = 3.0 Hz), 6.32 (d, 1 H, J = 3.0 Hz), 4.24 (m, 1 H), 1.45 (d, 6 H, J = 6.0 Hz); MS m/z (relative intensity) 149 (M+,100), 107 (45).
N-tert-Butylfuro[2,3-c]pyrrole (lc). To a solution of tert-butylamine (36 rag, 0.49 mmol) in 95% ethanol (2 ml) was added dropwise a solution of 11 (73 rag, 0.22 mmol) in
198 C.-K. SI-IA et al.
95% ethanol (10 ml). The reaction mixture was stirred and heated at reflex for 24 h. Concentration and silica gel flash column chromatography (hexane-ethyl acetate, 20:1) gave l c (7.1 rag, 20%) as a yellow oil: IR (neat) 3120, 2940, 1640 cm-1; 1H NMR (400 MHz, CDC13) ~ 7.25 (d, 1 H, J = 2.1 Hz), 6.66 (d, 1 H, J = 1.6 Hz), 6.62 (d, 1 H, J = 1.6 Hz), 6.39 (d, 1 H, J = 2.1 Hz), 1.65 (s, 9 H); MS m / z (relative intensity) 163 (M +, 77), 107 (100).
N - ( 3 - H y d r o x y p r o p y l ) f u r o [ 2 , 3 - c ] p y r r o l e (ld). To a solution of 3-hydroxypropylsmlne (145 rag, 1.49 retool) in 95% ethanol (2 re_l) was added dropwise a solution of 11 (128 rag, 0.39 retool) in 95% ethanol (10 ml). The reaction mixture was stirred at room temperature for 18 h. Concentration and silica gel flash column charomatography (hexane-ethyl acetate, 5:1) gave l d (6 rag, 10%) as a yellow oil: IR (neat) 3415, 3010, 1376 cm-1; 1H NMR (90 MHz, CDCI3) 8 7.26 (d, 1 H, J = 1.5 Hz), 6.48 (d, 1 H, J = 1.5 Hz), 6.42 (d, 1 H, J = 2.4 Hz), 6.36 (d, 1 H, J = 2.4 Hz), 4.11 (t, 2 H, J = 6.0 Hz), 4.61 (t, 2 H, J = 6.0 Hz), 2.09 (quintet, 2 H, J = 6.0 Hz), 1.59 Cos, 1 H); MS m / z (relative intensity) 165 (M +, 100), 121 (54).
C y c l o a d d u c t 13 o f N - I s o p r o p y l f u r o [ 2 , 3 - c ] p y r r o l e ( l b ) a n d D i m e t h y l A c e t y l e n e d i c a r b o x y l a t e . A solution of l b (6 rag, 0.04 mmol) and dimethyl acetylenedicarboxylate (11.4 rag, 0.08 mmol) in benzene (3 ml) were stirred at room temperature for 5 h. Concefitration and silica gel flash column chromatography (hexane- ethyl acetate, 5:1) gave 13 (15.3 mg, 88%) as a white solid: mp 107-108°C; IR (neat) 3130, 1732, 1718, 1635 cm-1; 1H NMR (400 MHz, CDC13) 8 6.47 (s, 1 H), 5.67 (d, 1 H, J = 1.4 Hz), 5.01 (s, 1 H), 4.98 (s, 1 H), 3.81 (s, 3 H), 3.80 (s, 3 H), 3.79 (s, 3 H), 3.77 (s, 3 H), 2.76 (m, 1 H), 0.97 (d, 3 H, J = 6.2 Hz), 0.94 (d, 3 H, J = 6.2 Hz); 13C NMR (100.6 MHz, CDCI 3) ~ 163.70 (s), 163.48 (s), 163.29 (s), 162.40 (s), 157.31 (s), 151.09 (s), 149.58 (s), 143.10 (s), 140.52 (s), 126.21 (s), 98.59 (s), 87.20 (d), 66.33 (d), 65.91 (d), 52.50 (q), 52.42 (q), 52.19 (q), 52.11 (q), 45.40 (q), 22.01 (q), 21.90 (q); MS m / z (relative intensity) 433 (M ÷, 27), 401 (100), 358 (27); HRMS calcd CmH23NO9 433.1376, found 433.1373.
D i e t h y l [ ( 3 - M e t h y l - 2 - b e n z o f u r y l ) m e t h y l e n e ] p r o p a n e d i o a t e (15). To a solution of 14 (lg, 6.25 retool) in dry benzene (30 ml) was added diethyl malonate (3 g, 18.75 retool), piperidine (0.15 ml) and acetic acid (0.1 ml). The reaction mixture was refluxed for 26 h with a Dean-Stark water separator attached. After the mixture were washed with H20 (20 ml x 2), 5% hydrochloride acid (10 ml x 2), saturated sodium carbonate (15 ml) and then dried (MgS04). Concentration and silica gel flash column chromatography (hexane-ethyl acetate, 10:1) gave 15 (1.76g, 94%) as a yellow solid: mp 89-90°C; IR (KBr) 2965, 1745, 1690, 1620 cm'l; 1H NMR (90 MHz, CDC13) 5 7.61 (s, 1 H), 7.40-7.12 (m, 4 H), 4.45 (q, 2 H, J = 6.8 Hz), 4.28 (q, 2 H, J = 6.8 Hz), 2.41 (s, 3 H), 1.41 (t, 3 H, J = 6.8 Hz), 1.30 (t, 3 H, J = 6.8 Hz); MS m / z (relative intensity) 302 (M+, 93), 256 (100), 184 (33); HRMS calcd C17Hls05 302.1149, found 302.1154.
Diethyl [(3-Bromomethyl-2-benzofuryl)methylene]propanedloate (16). To a solution of 15 (1.65 g, 5.4 retool) in carbon tetrachloride (50 ml) was added N-bromosuccinimide
Furo[2,3-c]pyrroles and benzofuro[2,3-c]pyrroles 199
(970 rag, 5.4 mmol) and dibenzoyl peroxide (80 mg). The reaction mixture was stirred and heated at reflux for 1 h. After the mixture was cooled in an ice bath, the solid was removed by filtration and washed with carbon tetrachloride. Concentration and silica gel flash column chromatography (hexane-ethyl acetate, 10:1) gave 16 (1.85 g, 90%) as a yellow solid: mp 115-116°C; IR (KBr) 2985, 1732, 1718, 1636 cm'l; 1H NMR (400 MHz, CDCla) 8 7.67 (dd, 1 H, J = 7.2, 0.8 Hz), 7.41-7.27 (m, 4 H), 4.69 (s, 2 H), 4.46 (q, 2 H, J = 7.3 Hz), 4.31 (q, 2 H, J = 7.3 Hz), 1.41 (t, 3 H, J = 7.3 Hz), 1.33 (t, 3 H, J = 7.3 Hz); 13C NMR (100.6 MHz, CDCI3) 5 165.70 (s), 163.71 (s), 155.29 (s), 147.21 (s), 127.78 (d), 126.80 (s), 125.91 (s), 123.70 (d), 123.63 (d), 123.00 (s), 120.68 (d), 111.59 (d), 62.01 (t), 61.80 (t), 20.01 (t), 14.09 (q), 14.02 (q); MS m/z
(relative intensity) 382 (M++2, 26), 380 (M ÷, 26), 301 (100), 255 (35); HRMS calcd
C17H17BrO5
380.0259, found 380.0224.
N-Methylbenzofuro[2,3-c]pyrrole (6a). To a solution of 35% methylsmine (29.7 mg, 0.96 mmol) in 95% ethanol (2 ml) was added drepwise a solution of 16 (166 rag, 0.44 retool) in 95% and ethanol-tetrahydrofuran (1:1, 6 ml). The reaction mixture was stirred at room emperature for 16 h. Concentration and silica gel flash column chromatography (hexane- ethyl acetate, 20:1) gave 6a (41 mg, 55%) as a white solid: mp 102-103°C; IR (KBr) 3118, 2935, 1635, 1580, 1385 cm-1; 1 H NMR (400 MHz, CDC13) 5 7.63 (dd, 1 H, J = 7.3, 1.0 Hz), 7.40 (d, 1 H, J = 7.3Hz), 7.22 (m, 2 H), 6.72 (d, 1 H, J = 1.3Hz), 6.49 (d, 1 H, J = 1.3 Hz), 3.77 (s, 3 H); 13C NMR (100.6 MHz, CDCIs) 5 161.10 (s), 150.29 (s), 121.41 (d), 122.70 (s), 122.01 (d), 120.33 (d), 114.74 (s), 111.39 (d), 108.50 (d), 97.58 (d), 37.40 (q); MS m/z (relative intensity) 171 (M +, 100); HRMS calcd CllHgNO 171.0684, found 171.0674.
N-Isopropylbenzofuro[2,3-c]pyrrole (6b). To a solution ofisopropylamine (38 rag, 0.64 retool) in 95% ethanol (2 ml) was added dropwise a solution of 16 (110 mg, 0.29 mmol) in 95% ethanol-tetrahydrofuran (2:1, 15 ml). The reaction mixture was stirred at room t e m p e r a t u r e for 18 h. Concentration and silica gel flash column c h r o m a t o g r a p h y (hexane-ethyl acetate, 20:1) gave 6b (34 rag, 80%) as a white solid: mp 54-55°C; IR (KBr) 2975, 1625, 1570, 1405, 1370 cm'l; 1H NMR (90 MHz, CDC13) 5 7.53 (m, 1 H), 7.39-7.04 (m, 3 H), 6.81 (d, 1 H, J = 2.7Hz), 6.51 (d, 1 H, J = 2.7 Hz), 4.25 (m, 1 H), 1.56 (d, 6 H, J = 6.0 Hz); MS m/z (relative intensity) 199 (M ÷, 100); HRMS calcd C13HmNO 199.0998, found 199.0997.
N - B e n z y l b e n z o f u r o [ 2 , 3 - c ] p y r r o l e (6c). To a solution of benzylamine (102 mg, 0.95 mmol) in 95% ethanol (2 ml) was added dropwise a solution of 16 (165 mg, 0.43 retool) in 95% e t h a n o l - t e t r a h y d r o f u r a n (1:1, 6 ml). The reaction mixture was stirred at room t e m p e r a t u r e for 22 h. Concentration and silica gel flash column c h r o m a t o g r a p h y (hexane-ethyl acetate, 20:1) gave 6c (84.5 mg, 79%) as a white solid: mp 88-89°C; IR (KBr) 3025, 1630, 1580, 1395, 1198cm'1; aH NMR (400 MHz, CDC13) 5 7.64 (d, 1 H, J = 7.4Hz), 7.41 (d, 1 H, J = 7.4 Hz), 7.36-7.15 (m, 7 H), 6.85 (d, 1 H, J = 1.4 Hz), 6.57 (d, 1 H, J = 1.4 Hz), 5.16 (s, 2 H); 13C NMR (100.6 MHz, CDC13) 5 161.40 (s), 150.50 (s), 138.01 (s), 128.90 (d, 2c), 127.91 (d), 127.12 (d, 2c), 124.50 (d), 122.73 (s), 122.20 (d), 120.59 (d), 115.09 (s), 111.61 (d), 108.19 (d),
200 C.-K. SHA et al.
97.40 (d), 54.81 (t); MS m / z (relative intensity) 247(M ÷, 100); HRMS calcd C17H13NO 247.0998, found 247.0982.
N - P h e n y l b e n z o f u r o [ 2 , 3 - c ] p y r r o l e (6d). To a solution of aniline (88.7 mg, 0.95 retool) in 95% ethanol (2 ml) was added dropwise a solution of 16 (165 rag, 0.43 retool) in 95% ethanol-tetrahydrofuran (1:1, 6 ml). The reaction mixture was stirred and heated at 65°C for 20 h. Concentration and silica gel flash column c h r o m a t o g r a p h y (hexane-ethyl acetate, 20:1) gave 6d (39.6 rag, 17%). IR (KBr) 3031, 1640, 1382, 1220, 745 cm-1; 1H NMR (400 MHz, CDC13) 5 7.67(d, 1 H, J = 7.5Hz), 7.45 (d, 4 H, J = 4.2 Hz), 7,39 (d, 1 H, J = 7.5 Hz), 7.27 (m, 2 H), 7.20 (m, 1 H), 7.20 (d, 1 H, J = 1.4Hz), 6.95 (d, 1 H, J = 1.4 Hz); MS m / z (relative intensity) 233 (M +, 100); HRMS calcd C16H11NO 233.0851, found 233.0840.
N-p-Tolylbenzofuro[2,3.c]pyrrole (6e). To a solution of p-toluidine (98 rag, 0.91 mmol) in 95% ethanol (2 ml) was added dropwise a solution of 16 (134 mg, 0.35 retool) in 95% ethanol (6 ml). The reaction mixture was stirred at room t e m p e r a t u r e for 34 h. Concentration and silica gel flash column chromatography (hexane-ethyl acetate, 20:1) gave 6e (17.2 mg, 20%) as a yellow solid: mp 137-138°C; IR (KBr) 3008, 1645, 1380 cm-1; 1H NMR (400 MHz, CDC13) 5 7.67 (d, 1 H, J = 6.8 Hz), 7.39 (d, 1 H, J = 7.8 Hz), 7.34 (d, 2 H, J = 6.8 Hz), 7.25 (m, 3 H), 7.20 (d, 1 H , J = 7.8 Hz), 7.16 (d, 1 H , J = 1.9Hz), 6.91 (d, 1 H , J = 1.9 Hz), 2.38 (s, 3 H); MS rn/z (relative intensity) 247 (M ÷, 100), 232 (31); HRMS calcd for C17H13NO 247.1003, found 247.0997.
N-tert-Butoxycarbonylbenzofuro[2,3.c]pyrrole (18). To a solution of 16 (78 rag, 0.21 mmol) in 95% ethanol (10 ml) was added dropwise 25% ammonia water (0.15 ml). The reaction mixture was stirred at room t e m p e r a t u r e for 52 h. After concentration, a solution of 4-dimethylAmlnopyridine (50 rag, 0.41 retool), di-tert-butyl dicarbonate (134 mg, 0.61 retool) in dry dichloromethane (10 ml) was added. The reaction mixture was stirred at room t e m p e r a t u r e for 2 h. Concentration and silica gel flash column chromatography (hexane-ethyl acetate, 5:1) gave 18 (12.6 rag, 25%) as a yellow oil: IR (neat) 2980, 1740, 1415, 1360, 1250, 1160 cm-1; 1H NMR (90 MHz, CDCls) 5 7.66 (dd, 1 H, J = 6.6, 1.5 Hz), 7.40-7.18 (m, 4 H), 7.04 (d, 1 H, J = 1.5 Hz), 1.65 (s, 9 H); MS m / z (relative intensity) 257 (M ÷, 56), 201 (100), 157 (63); HRMS calcd for C15H15NO3 257.1053, found 257.1051.
C y c l o a d d u c t 19 of N-tert.butoxycarbonylbenzofuro[2~c]pyrrole (18) a n d Dimethyl A c e t y l e n e d i c a r b o x y l a t e . A solution of 18 (14 rag, 0.05 mmol) and dimethyl acetylenedicarboxylate (15.5 rag, 0.11 retool) in benzene (5 ml) were refluxed for 9 h. Concentration and silica gel flash column chromatography (hexane-ethyl acetate, 7:1) gave 19 (17.8mg, 82%) as a yellow oil: IR (neat) 2945, 1770-1710, 1530, 1385, 1255 cm-1; 1H NMR (90 MHz, CDCls) 5 7.75 (s, 2 H), 7.30 (s, 2 H), 3.78 (s, 6 H), 3.69 (m, 2 H), 1.57 (s, 9 H); MS ra/z (relative intensity) 399 (M ÷, 24), 283 (50), 227 (32), 183 (100); HRMS calcd for
Furo[2,3-c]pyrroles and benzofuro[2,3-c]pyrroles 201
C y c l o a d d u c t 20 of N - i s o p r o p y l b e n z o f u r o [ 2 , 3 - c ] p y r r o l e (6b) a n d D i m e t h y l A c e t y l e n e d i c a r b o x y l a t e . A solution of 6b (18 rag, 0.09 retool) and dimethyl
acetylenedicarboxylate (15.4 mg, 0.11 mmol) in benzene (5 ml) were stirred at room t e m p e r a t u r e for 60 h. Concentration and silica gel flash column chromatography (hexane-ethyl acetate, 5:1) gave 20 (22.9 rag, 75%) as a yellow oil: IR (neat) 1950, 1740, 1715, 1580, 1572, 1432 cm'l; 1H NMR (90 MHz, CDC13) 5 7.50-7.30 (m, 2 H), 7.28-7.08 (m, 2 H), 5.55 (d, 1 H, J = 0.2 Hz), 5.09 (d, 1 H, J = 0.2 Hz), 3.79 (s, 3 H), 3.72 (s, 3 H), 2.88 (m, 1 H), 1.10 (d, 6 H, J = 6.0 Hz); MS m / z (relative intensity) 341 (M ÷, 90), 225 (100), 199 (70).
D i m e t h y l D i b e n z o f u r a n . 2 $ . d l c a r b o x y l a t e (22). To a solution of 20 (22 mg, 0.06 retool) in dichloromethane (5 ml) was added dropwise a solution of m-chloroperbenzoic acid (15.3 mg, 0.07 mmol) in dichloromethane (3 ml). The reaction mixture was stirred at room t e m p e r a t u r e for 3 h. The reaction mixture was then washed with saturated sodium thiosulfate solution, w a t e r and brine. Concentration and silica gel flash column chromatography (hexane-ethyl acetate, 2:1) gave 22 (9.5 mg, 56%) as a yellow oil: IR (neat) 3100, 2900, 1720, 1640 cm-1; 1H NMR (400 MHz, CDCI3) ~ 8.36 (s, 1 H), 7.98 (d, 1 H, J = 7.6 Hz), 7.88 (s, 1 H), 7.61 (d, 1 H, J = 8.3Hz), 7.54 (dd, 1 H, J = 8.3, 7.2 Hz), 7.40 (dd, 1 H, J = 7.6, 7.2 Hz), 3.9 (s, 6 H); MS m / z (relative intensity) 284 (M ÷, 100), 253 (26), 236 (27); HRMS calcd
for C16H1205 284.0685, found 284.0684.
A c k n o w l e d g e m e n t : We thank the National Science Council of the Rupublic of China
for the financial support (NSC-83-0208-M007-028).
References and Notes:
1. Milum, M.; Trinajustic, N. Croa. Chem. Acta. 1977, 107.
2. Soth, S.; Farnier, M.; Paulmier, C. Can. J. Chem. 1978, 56, 1429.
3. Saruwatari, M.; Hatano, S.; Isomura, K.; Taniguchi, H.; Fukusokan Kagaku Toronkai
Koen Yoshishu 12th 1979, 211. C. A. 1980, 93, 95115y; Krutosikova, A.; Kovac, J.;
Dandarova, M.; Bobalova, M.; Collect. Czech. Chem. Commun. 1982, 47 (12), 3288. 4. (a) Sha, C.-K.; Tsou, C.-P. J. Org. Chem. 1990, 55, 2446. (b) Sha, C.-K.; Tsou, C.-P, Li,
Y.-C.; Lee, R.-S.; Tsai, F.-Y.; Yeh, R.-H. J. Chem. Soc., Chem. Commun. 1988, 1081. (c) Sha, C.-K.; Tsou, C.-P. J. Chem. Soc., Chem. Commun. 1986, 310.
5. Crystal data of 13: C21H23NOg: M=433.41, monoclinic, space group P21/c, a=9.819(7), b=8.906(4), c=23.503(7)/k, a=90 °, 9=91.72 °, Z=4. 2712 Unique reflections were measured of which 892 were considered observed [I > 1.5 (~ (I)]. The structure was solved by direct
202 C.-K. SHA et al.
method to an R value of 0.066. All calculations were performed with the N R C C - S D P package. The supplementary materials have been deposited at the Cambridge Crystallographic Data Center.
6. Grieco, P. A.; Flynn, D. L.; Zelle, R. E. J. Org. Chem. 1983, 48, 2424. 7. Gribble, G. W.; Allen, R. W. Tetrahedron Lett. 1976, 17, 3673.