Pergamon Tetrahedron Letters, Vol. 37, No. 33, pp. 5913-5916, 1996 Copyright © 1996 Elsevier Science Ltd Printed in Great Britain. All rights reserved P I I : S0040-4039(96)01275-0 0040-4039/96 $15.00 + 0.00
S y n t h e s i s a n d C h a r a c t e r i z a t i o n o f O l i g o ( 2 , 7 - b i p h e n y l e n y l e n e - ( E ) - v i n y l e n e ) s
Chi-Yuen Kwong, b Man-kit Leung,*,a, b Shi-Chun Lin, a Tze-Lock Chan, b Hak-Fun Chow b
a Department of Chemistry, National Taiwan University, Taipei, Taiwan, R. O. C. b Department of Chemistry, The Chinese University of Hong Kong, Shatin, Hong Kong
Abstract: The syntheses, spectral behavior, and electrochemical properties of novel oligo(2,7- biphenylenylene-(E)-vinylene)s (OBPV's) 1 to 3 have been reported. On the basis of the spectral analysis of OBPV's, a band gap of 2.5 ev for poly(2,7-bipbenylenylene-(E)-vinylene) was estimated. Copyright © 1996 Elsevier Science Ltd
Because of their unusual electrical and optical properties, conjugative polymers containing aromatic (4n+2)x-electron units as the fundamental building blocks have been extensively studied during the past few decades. However, conjugated polymers based on (4n)x-electron systems are surprisingly rare. 1 To exploit the potential of applying the anti-aromatic (4n)x-electron units to conjugated polymer systems, we have chosen oligo(2,7-biphenylenylene-(E)-vinylene)s (OBPV's) 1 to 3 as the models for poly(2,7-biphenylenylene-(E)- vinylene) to explore. 2
The synthetic sequences leading to OBPV's 1, 2 and 3 are summarized in Schemes 1 and 2 respectively. Dimethyl(biphenylene-2,7-dicarboxylate) 4 was prepared by a modified Marvel's procedure. 3 LAH reduction of 4, followed by PCC oxidation led to biphenylene-2,7-dialdehyde. However, Wittig olefination of the dialdehyde only afforded OBPV 1 as a mixture of cis-trans isomers. To obtain OBPV 1 with all trans double bonds, we adopted the recently reported modified Ramberg-Backlund reaction as the key synthetic tool. 4, 5 Dehydroxy-chlorination of alcohol 5 led to bischloride 6 which was further converted to sulfone 7, followed by Ramberg-B~tcklund olefination to provide OBPV 1 as a yellowish solid. The (E)- configurations of both newly formed double bonds were confirmed by 1H NMR ( J = 16.3 Hz). 6
M e O 2 C . ~ ~ . CO2M e i - i v X C H 2 . . ~ ~ CH2 x
4 5 X=OH
6 X = C l
7 X = 3,5-((CH3)3C)2CtH3CH2SO 2- v
Reagents: (i) LiAIH4, ft., 95%; (ii) SOC12, rt., 85%; (iii) Cs2CO3,
3,5-di-tert-butylbenzylthiol,
50"C, 73%; (iv) mCPBA, rt., 91%; (v) KOH/AI203, CBr2F2, 0°C, 80%.S c h e m e 1 5913
5914
On the other hand, selective hydrolysis of 4, followed by BH3 reduction gave unsymmetrical methyl 7-hydroxymethylbiphenylene-2-carboxylate 8 (Scheme 2). By using a similar synthetic strategy described for OBPV 1, alcohol 8 was transformed to sulfone 9 which was further subjected to the modified Ramberg- Backlund olefination, during which the carboxylic ester was partially hydrolysed, to provide a mixture of carboxylic acid and methyl ester. The mixture was then converted to chloride 10 via a LAH reduction and SOC12 chlorination sequence. The (E)-configuration of the double bond of 10 was again confirmed by 1H NMR (J = 16.4 Hz). The successful synthesis of 10 allows us to prepare OBPV 2 and OBPV 3. In addition, compounds 8 and 10 are important precursors for the iterative approach which may allow the conjugation length to grow step by step. 5
MeO2 C ' ~ ~ X vi, viii,._ M e O 2 C ~ 4 CO2Me- v I~ 8 X=CI~2-~(cHq-I3)3C)2C6H3CH2SO 2 - C
ix, X ~
10 + ~ xi-xiii ~
HSI ' 1 2 SH
Reagents : (i) KOH, EtOH, 40"C; (ii) BH3-THF, 0°C, 82%; (iii) SOC12, rt., 85%; (iv)Cs2CO3,
3,5-di-tert-butylbenzylthiol,
50*C, 79%; (v) mCPBA, rt., 92%; (vi) KOH/A1203, CBr2F2, 0°C, (vii) LAH, 0°C, followed by SOC12, rt., 75 % from the suifone 9; (viii) Na2S, EtOH, rt., 55%; (ix) Oxone ®, MeOH, CH2CI2, 82%; (x) KOI-I/AI203, CBr2F2, 0*C, 83%; (xi) KOH, EtOH, THF, rt., 51%; (xii) Oxone ®, MeOH, CH2CI2, rt., 51%; (xiii) KOH/A1203, CBr2F2, 0*C, 93%..S c h e m e 2
To synthesize OBPV 2, two equivalents of 10 were coupled together to one equivalent of Na2S to give sulfide 11. By employing the aforementioned oxidation and Ramberg-Backlung olefination protocol, OBPV 2 was obtained in good yield. 6 It is worth mentioning that Oxone ® was used, instead of MCPBA as the oxidizing agent in order to prevent from epoxidation of the existent double bonds. 7
OBPV 3 was assembled in a [1+1+1] coupling fashion (Scheme 2). Thus, two equivalents of 10 were coupled to one equivalent of dithiol 12 8 to give the corresponding bissulfide which was further transformed in a similar way to OBPV 3 as an orange solid. 6
5915
For OBPV 2, the olefinic protons of the newly formed double bond are chemically equivalent 05 = 6.84 ppm); while for OBPV 3, the IH NMR resonances of the newly formed olefinic protons appeared coincidentally as a singlet at 6.83 ppm. As a consequence, the stereochemical configurations of these newly formed double bonds are difficult to confirm. Nevertheless, both OBPV 2 and OBPV 3 were isolated as a single component from the reaction mixture. Since the modified Ramberg-Backlund olefination usually results in trans olefins, 4 we tentatively assign (E)-configurations to the newly formed double bonds in OBPV 2 and OBPV 3.
OBPV's 1, 2 and 3 exhibit a major UV absorption band with the ~max at 414, 455, and 466 nm, respectively. These absorption maxima show a linear correlation between En=hc/'Amax and l/n, 9 where n is the number of the biphenylenylene units in the molecule (Figure 1). It is noteworthy that the ~-max for poly(2,7-biphenylenylene-(E)-vinylene) (PBPV, n = Qo) can be estimated by extrapolating of the results from OBPV's. Thus, a peak band-gap of 2.5 ev (~ 500 nm) for PBPV was obtained on the basis of our spectral analysis. This band gap is far smaller than that of poly(para-phenylene-(E)-vinylene) (3.1 eV). 10 This result implies that PBPV will be a new class of organic conducting materials.
En (ev) 3.00 - 2.90 - 2 . 8 0 - 2.70 - 2.60 - 2.50 - 2.40 • I • ! • ! • ! , I • 0.0 0.2 0.4 0.6 0.8 1.0 1.2
Reciprocal chain length 1/n Figure 1: Reciprocal chain length versus
absorption maxima for OBPV's 1, 2, and 3.
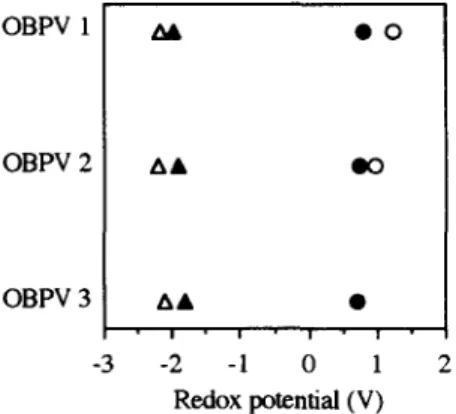
OBPV 1 OBPV 2 OBPV 3 / k ~ O O A A O O A A • ! ! ! ! -3 -2 - 1 0 1 2 Redox potential (V)
Figure 2: Redox potential diagram for OBPV's 1, 2, and 3 (A: 1st red. potential; A: 2nd red. potential; O: 1st oxid. potential; o: 2nd oxid. potential).
The electrochemical studies of OBPV's I to 3 by cyclic voltammetry suggest that all three oligomers can either be oxidized or reduced reversibly in solution. More interestingly, the potential difference between subsequent oxidations (AEoxid.=E4-E 3 in Table 1) 11 decreases dramatically from OPBV 1 to OPBV 2, and for OPBV 3, the first and the second oxidation potentials merge to a single oxidation potential (Figure 2). This can be explained by the delocalization of the positive charges over the conjugative n systems which reduces the Coulombic repulsion between the charges. This observation is complementary to the electrochemical reduction behavior of oligo(p-phenylenevinylene)s (OPPV's) of which the potential difference between the first two reduction waves vanishes at a certain conjugation length. 12
On the other hand, the reduction potential differences (AEred.-E2-E 1 in Table 1) It of OBPV's 1 to 3 remain almost unchanged with increasing the conjugation-length. We tentatively interpret these effects as due to the localization of the negative charges of which the electrostatic repulsion could not be relaxed simply by increasing the chain length.
5916
The detailed discussion about the preparation, theoretical studies, electrochemical behavior and fluorescence properties of OBPV's will be reported in due course. Potential applications of these materials for electroluminescent devices are under investigation.
A c k n o w l e d g e m e n t s
We thank the National Science Council of the Republic of China (NSC-84-2113-M-002-036 and -013) and the Department of Chemistry, The Chinese University of Hong Kong for financial support.
References and Notes
1. Auchter-Krummel, P.; MUllen, K. Angew. Chem. Int. Ed. Engl., 1991, 30, 1003.
2. For general reviews of biphenylene chemistry, see (a) Barton, J. W. "Non-benzenoid Aromatics, " Vol. 1, Academic Press, New York. 1969, 32. (b) Lloyd, D. 'Non-benzenoid Conjugated Carbocyclic Compounds," Elsevier, Amsterdam, 1984, 216. (c) VOgtle, F. "Fascinating Molecules in Organic Chemistry." Wiley, New York. 1992, 111.
3. (a) Hanson, M. P. ; Marvel, C. S. J. Polymer Science: Polymer Lett. Ed. 1978,16, 653. (b) Lin S.C.; Marvel, C. S. J. Polymer Science: Polymer Chem. Ed. 1979, 17, 2337.
4. Chan, T. L.; Fong, S.; Li, Y.; Man T.-O.; Poon, C.-D. J. Chem. Soc., Chem. Commun., 1994, 1771. 5. Chan, T. L.; Chow, H. F.; Fong, S.; Leung M.-k.; Tu, J. J. Chem. Soc., Chem. Commun., 1994,
1919.
6. All new compounds were characterized on the basis of 1H NMR, 13C NMR, mass spectral data and elemental analyses, for satisfactory results were obtained. Physical and selected spectral data for: OBPV 1: yellow crystals: mp 227-229"C; 6H 1.36 (s, 36H), 6.66 (d, 2H, J = 7.4 Hz), 6.87 (d, 2H, J = 7.5 Hz), 6.94 (d, 2H, J = 16.3 Hz), 7.01 (s, 2H), 7.03 (d, 2H, J = 16.3 Hz), 7.33 (s, 6H); 8C 31.5, 34.9, 114.4, 117.5, 120.9, 122.1, 128.3, 128.4, 129.0, 136.6, 138.2, 149.9, 150.9, 151.1; OBPV 2: orange crystals, mp > 245°C; 6H 1.36 (s, 36H), 6.66 (d, 2H, J = 7.2 Hz), 6.67 (d, 2H, J = 7.2 Hz), 6.84 (d, 2H, J = 7.2 Hz), 6.84 (s, 2H), 6.88 (d, 2H, J = 7.2 Hz), 6.93 (d, 2H, J = 16.4 Hz), 6.96 (s, 2H), 7.04 (s, 2H), 7.05 (d, 2H, J = 16.4 Hz), 7.34 (bs, 6H); 6C 31.48, 34.88, 114.19, 114.41, 117.53, 117.62, 120.84, 122.14, 128.09, 128.35, 128.42, 128.55, 129.05, 136.56, 137.87, 138.28, 149.82, 150.16, 150.82, 150.96, 151.11; OBPV 3: red crystals, mp > 245°C; 0H 1.36 (s, 36H), 6.66 (d, 4H, J = 7.2 Hz), 6.68 (d, 2H, J = 7.2 Hz), 6.83 (d, 4H, J = 7.2 Hz), 6.83 (s, 4H), 6.88 (d, 2H, J = 7.2 Hz), 6.93 (d, 2H, J = 16.2 Hz), 6.95 (s, 2H), 6.83 (s, 2H), 7.04 (d, 2H, J = 16.2 Hz), 7.04 (s, 2H), 7.33 (s, 4H), 7.34 (s, 2H); 6C (only 26 carbon signals were observed for OBPV 3) 31.48, 34.89, 114.26, 114.31, 114.43, 117.52, 117.61, 120.84, 122.14, 128.05, 128.16, 128.34, 128.44, 128.59, 128.64, 129.08, 136.57, 137.84, 137.96, 138.31, 149.81, 150.08, 150.19, 150.81, 150.90, 151.13. 7. (a) Trost, B. M.; Curran, D. P. Tetrahedron Lett., 1981, 22, 1287. (b) Trost, B. M.; Braslau, R. J. Org.
Chem., 1988, 53, 532.
8. By reaction of 5 with thiourea, followed by NaOH hydrolysis, 2,7-(bismercaptomethyl)biphenylene was obtained in moderated yield.
9. Tolbert, L. M. Acc. Chem. Res., 1992, 25, 561 and references cited therein.
10. Extrapolated from the data reported in the following papers: (1) Schenk, R.; Gregorius, H.; MUllen, K. Adv. Mater. 1991, 3, 492. (c) Schenk, R.; Gregorius, H.; Meerholz, K.; Heinze, J.; Mttllen, K. J. Am. Chem. Soc,, 1991, 113, 2634.
11. Table 1. Oxidation and reduction potentials (V) of 1, 2, and 3 vs Ag/AgC! electrode, scan rate = 100 mV/s, calibration vs ferrocene, in CH2C12 containing TBABF4 (0.1M) as the supporting electrolyte.
Reduction Oxidation
E1 E2 AEred.~E2-E1 E3 E4 dEoxid.=E4-E3
OBPV 1 -2.16 -1.96 0.20 0.82 1.23 0.41
OBPV 2 -2.20 - 1.90 0.30 0.73 0.96 0.20
OBPV 3 -2.10 - 1.80 0.30 0.72
12. Schenk, R.; Gregorius, H.; Mtillen, K.; Heinze, J. Adv. Mater. 1994, 6, 671.