Association of Cyclooxygenase 2 Single Nucleotide Polymorphisms and
Hepatocellular Carcinoma in Taiwan
Wen-Shin Chang1, Mei-Due Yang1, Chia-Wen Tsai1,2, Li-Hao Cheng2, Long-Bin Jeng1, Woei-Chung Lo4, Chih-Hsueh Lin5, Chih-Yang Huang2,6,# and Da-Tian
Bau1,2,3,#
1Terry Fox Cancer Research Laboratory and 5Department of Family Medicine, China
Medical University Hospital, Taichung, Taiwan;
Graduate Institutes of 2Basic Medical Science, 3Clinical Medical Science, China Medical University, Taichung, Taiwan, R.O.C.
4Division of Hematology and Oncology, Department of Internal Medicine, China
Medical University Hospital, Taichung, Taiwan, R.O.C.
6Department of Health and Nutrition Biotechnology, Asia University, Taichung 413,
Taiwan
Running head: COX-2 GENOTYPES IN HEPATOCELLULAR CARCINOMA
Research Lab, China Medical University Hospital, 2 Yuh-Der Road, Taichung, 404 Taiwan, Tel: +886 422053366 Ext 3312, Fax: +886 422053366 Ext 1511, E-mail:
Abstract
Hepatocellular carcinoma (HCC) is a worldwide neoplasm, for which early diagnosis is difficult and the prognosis is usually poor. Overexpression of cyclooxygenase 2 (COX-2) has been suggested to be associated with hepatocarcinogenesis. Although several COX-2 inhibitors have been used in hepatoma therapy, it remains largely unknown about the genetic background between COX-2 and HCC. In this study, the association of genotypic polymorphisms in COX-2 with HCC was investigated. 298patients with HCC and 298 healthy controls recruited from the China Medical Hospital in Taiwan were genotyped by PCR-RFLP method. We have investigated six polymorphic variants of COX-2, including A-1195G, G-765C, T+8473C, intron 1, intron 5, and intron 6, and analyzed the association of specific genotype with susceptibility to HCC. The results showed that, for each genotype of COX-2 A-1195G, G-765C, T+8473C, intron 1, intron 5, and intron 6, no different distribution between the HCC and control groups was found. There was neither obvious joint effect of COX-2 G-765C/intron 6 haplotype nor its genotypes with smoking or alcohol drinking on HCC risk. Environmental factors, other than smoking and alcohol drinking, may affect the post-natal expression of COX-2 in the etiology of HCC, which is an outcome of complex genetic and environmental interactions. Moreover, our immunohistochemistrical result indicated that the COX-2
protein significantly over-expression in well-differentiated HCC, but not significantly increased in poor-differentiated HCC. We suggest that COX-2 may be a determinant of the differentiation grade of HCC.
Introduction
Hepatocellular carcinoma (HCC) is the leading cause of malignant cancer death in the world (7) and Taiwan (13). Limited treatment and poor prognosis of this disease emphasize the importance in developing an effective chemoprevention. However, the exact molecular mechanism of hepatocarcinogenesis is still unclear (7).
Cyclooxygenases (COX) are key enzymes for the conversion of arachidonic acid to prostaglandin and other eicosanoids (14). Whereas COX-1 is constitutively expression, COX-2 is a highly inducible protein. Increased COX-2 expression has been associated with tumorigenesis in various types of human cancer, including in early stages of hepatocarcinogenesis (2, 19, 28, 29). In several animal and clinical studies, COX-2 specific inhibitors have both preventive and therapeutic effects as anticancer drugs for breast, bladder, lung and pancreas cancers (12, 20, 25, 27). However, the association of COX-2 genotypes with HCC has never been investigated. In addition, the mRNA and protein levels of COX-2 may vary among individuals, and this variability may be partially genetically determined under different molecular mechanisms, which may depends on single nucleotide polymorphisms (SNPs) of COX-2 (11, 26).
To clarify the hypothesis that the SNP variants of COX-2 are associated with the risk of HCC, we analyzed the genetic polymorphisms of six COX-2 SNPs, including
A-1195G (rs689466), G-765C (rs20417), T+8473C (rs5275), intron 1 (rs2745557), intron 5 (rs16825748), and intron 6 (rs2066826), in a large Taiwanese HCC population (control/case=298/298).
Materials and Methods Study Population and Sample Collection
Two hundred and ninety-eight patients diagnosed with HCC were recruited at the Departments of General Surgeon at the China Medical University Hospital, Taiwan, in 2004-2010. Each patient and non-cancerous healthy person (matched by gender, age and individual habits, such as smoking and alcohol drinking, from a random sampling from the Health Examination Cohort of China Medical University Hospital) completed a self-administered questionnaire and provided their peripheral blood samples.
Genotyping Assays
Genomic DNA was prepared from peripheral blood samples using a QIAamp Blood Mini Kit (Blossom, Taipei, Taiwan) and further processed according to previous studies (5, 6, 8-10, 16, 17, 33). The polymerase chain reaction (PCR) cycling conditions were: one cycle at 94oC for 5 min; 35 cycles of 94oC for 30 sec, 55oC for
30 sec, and 72oC for 30 sec, and a final extension at 72oC for 10 min. Pairs of PCR primer sequences and restriction enzyme for each DNA product are all listed in Table I.
Immunohistochemical Staining for COX-2
For liver specimens, tissue sections (5 μm) mounted on silanized slides (DAKO Japan, Kyoto, Japan) were deparaffinized with xylene and dehydrated in a graded series of ethanol. After rehydration in absolute ethanol for 15 s, the slides were heated by microwave in 10 mmol/liter citrate buffer (pH 6.0; Zymed Lab Inc., San Francisco, CA) for 8 min. After washing in ice-cold phosphate-buffered saline (PBS), sections were pre-blocked for 10 min in an autoblocker (Leica Biosystems Newcastle Ltd., Newcastle Upon Tyne, UK). Then, they were incubated overnight with mouse monoclonal anti-human COX-2 antibody (1:100; Transduction Lab Inc., Franklin Lakes, NJ). After three washes in PBS, the sections were incubated with horseradish peroxidase (HRP) conjugate anti-mouse IgG (Santa Cruz, CA, USA) antibody at room temperature for one hour. Finally, 3, 3’-diaminobenzidine (Sigma, Missouri, USA) was added. Counter-staining was done with hematoxylin (Sigma, Missouri, USA). The image capture was used an Olympus BX 50 fluorescence microscope (Olympus, Optical, Tokyo, Japan) and a Delta Vision disconsolation microscopic
system operated by SPOT software (Diagnostic Instruments Inc., USA).
Western Blotting Analysis
The liver specimens were homogenized in RIPA lysis buffer (Upstate Inc., Lake Placid, NY, USA), the homogenates were centrifuged at 10 000 g for 30 min at 4°C, and the supernatants were used for western blotting. Samples were denatured by heating at 95°C for 10 min, then separated on a 10% SDS-PAGE gel, and transferred to a nitrocellulose membrane (Bio-Rad). The membrane was blocked with 5% non-fat milk and incubated overnight at 4°C with mouse monoclonal anti-human COX-2 antibody (1:1000; Transduction Lab Inc., Franklin Lakes, NJ), then with the corresponding horseradish peroxidase-conjugated goat anti-mouse IgG secondary antibody (Chemicon, Temecula, CA) for 1 h at room temperature. After reaction with ECL solution (Amersham, Arlington Heights, IL, USA), a bound antibody was visualized using a chemiluminescence imaging system (Syngene, Cambridge, UK). Finally, the blots were incubated at 56°C for 18 min in stripping buffer (0.0626 M Tris-HCl, pH 6.7, 2% SDS, 0.1 M mercaptoethanol) and re-probed with a monoclonal mouse anti-β-actin antibody (Sigma) as the loading control. The optical density of each specific band was measured using a computer-assisted imaging analysis system (Gene Tools Match software; Syngene).
Statistical Analyses
Only those with both genotypic and clinical data (control/case=298/298) were selected for final analysis. To ensure that the controls used were representative of the general population and to exclude the possibility of genotyping error, the deviation of the genotype frequencies of COX-2 SNPs in the controls from those expected under the Hardy-Weinberg equilibrium was assessed using the goodness-of-fit test. Pearson’s chi-square test or Fisher’s exact test (when the expected number in any cell was less than five) was used to compare the distribution of the genotypes between cases and controls. Data were recognized as significant when the statistical P-value was less than 0.05.
Results
The frequency distributions of selected characteristics of 298 HCC patients and 298 controls are shown in Table II. These characteristics of patients and controls are all well matched. None of the differences between both groups were statistically significant (P>0.05) (Table II).
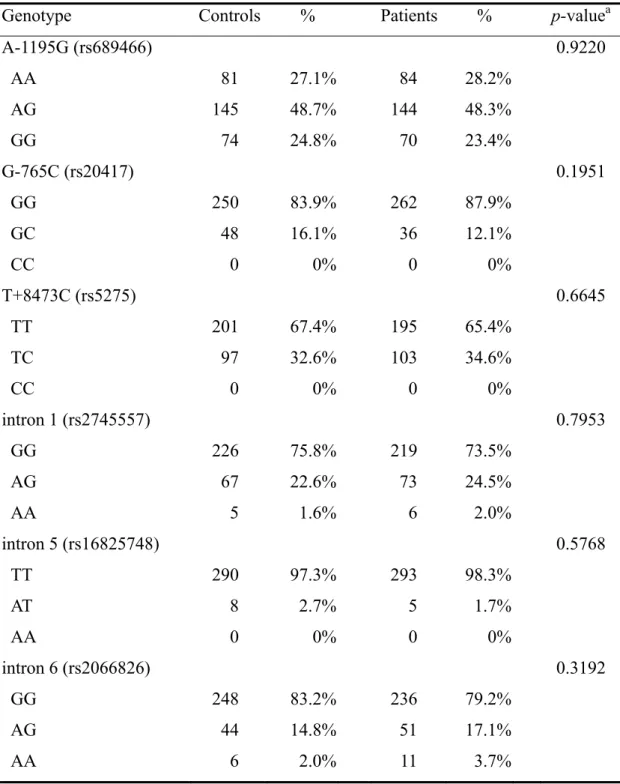
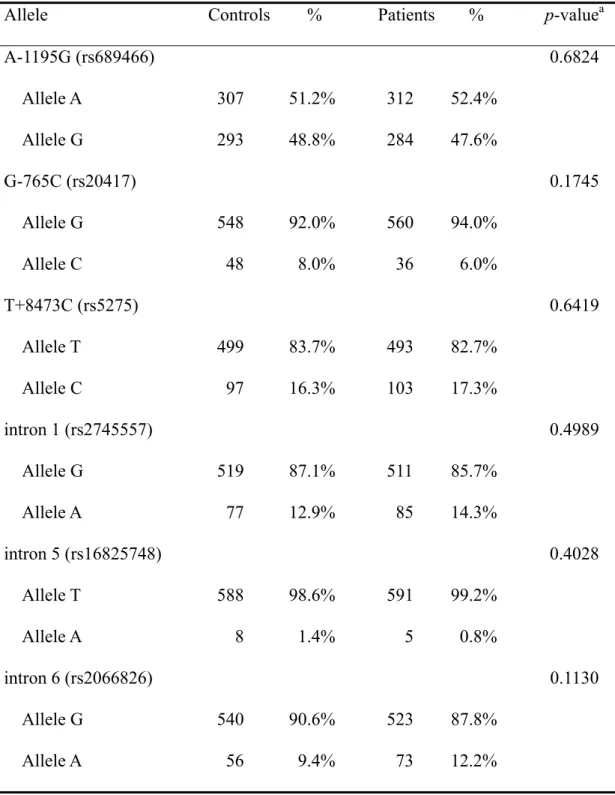
The frequencies of the genotypes for the COX-2 SNPs in controls and HCC patients are shown in Table III. The genotype distributions of the genetic polymorphisms of COX-2 of the six polymorphisms investigated were not significant between the two groups (P>0.05) (Table III). The frequencies of the alleles for COX-2 SNPs in controls and HCC patients are shown in Table IV. Neither of the allele of the COX-2 of the SNPs were found to be associated with HCC (P>0.05).
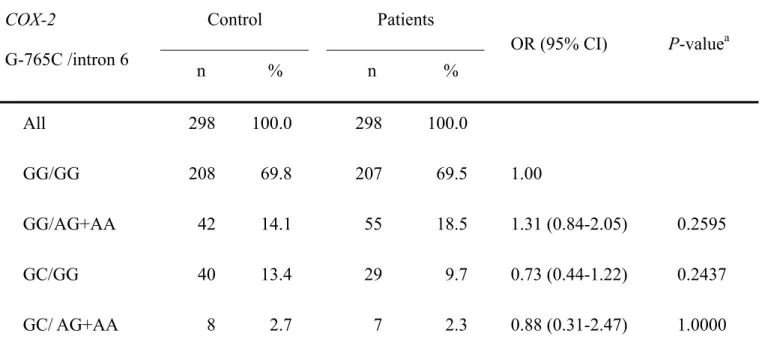
To further investigate the association of genotype COX-2 and HCC, the two-SNP COX-2 interactions among SNPs were investigated by genotype analysis. There were no significant differences in frequencies of any combined genotypes between the two groups for each combined genotype (data not shown). We can take the two SNPs with lowest p-values for example, G-765C and intron 6, the odds ratios (ORs) of the GG/AG+AA, GC/GG, GC/ and AG+AA combined genotypes compared with common GG/GG reference genotype were 1.13 (95% confidence interval, CI=0.84-2.05; P=0.2595), 0.73 (95% CI=0.44-1.22; P=0.2437), and 0.88 (95% CI=0.31-2.47;
P=1.0000), respectively (Table V). We have also investigated the joint effects of COX-2 genotypes and environmental factors, including smoking and alcohol drinking while no significant interaction was found (data not shown).
Although, the genetic polymorphisms of COX-2 of the six polymorphisms were not significant between HCC and control group, but in immunohistochemistrical assay and Western blot (Fig. 1) we found that the COX-2 protein significantly over-expression in well-differentiated HCC, and slight but not significantly increased in poor-differentiated HCC then in non-tumor portion.
Discussion
The abnormal expression of COX-2 has been reported to play an important role in hepatocarcinogenesis (22, 28). In order to reveal the role of COX-2 and to find potential tumor-markers for HCC, we chose six SNPs of the COX-2 gene and investigated their association with the HCC susceptibility in a Taiwan population. We found that for any single SNP, the variant genotypes of COX-2 were not significantly associated with the susceptibility for HCC (Tables III and IV). This may not be explained by the limited sample size (for this is a relatively large in HCC population studies), but more likely that COX-2 may play a minor role in the etiology of HCC, which is an outcome of complex genetic and environmental interactions. Since we
could not find direct association of COX-2 genotype with hepatocellular carcinoma risk, the transcriptional and translational and post-translational levels could be involved in hepatocarcinogenesis. Therefore, the factors involved in COX-2 expression need more concerned, for it is known that transcriptional modulation of
COX-2 is cell-specific (3). In literature, the expression level of COX-2 is mainly
regulated by C/EBPs (15) and reactive oxygen species (ROS) in hepatocytes (1, 21). Therefore, the involvements of C/EBPs and ROS in the regulation of COX-2 expression in hepatocytes may also be a direction of future investigation of our study.
The supporting evidence comes from the study documented that increased COX-2 expression has been associated with inhibition of apoptosis (4, 24), increased angiogenesis (30) and enhanced metastatic ability (18). Moreover, the overexpression of COX-2 is associated with tumorigenesis in a number of human cancers, including HCC (19, 29). Regarding oncogenesis, COX-2 contributes to tumor formation or growth, although the in vivo mechanism by which COX-2 affect tumor growth has not been determined. In addiction, both tumor and stromally derived COX-2 could influence tumor angiogenesis and/or immune function (32). Also, study indicated that COX-2 over-expression is important in mediating drug resistance to apoptosis in HCC (2). Pharmacological suppression of COX-2 induced the apoptosis of hepatoma cells (2, 31), and over-expression of COX-2 mRNA (18) and protein (28) were closed
related to a poor survival rate. According to the histological grade, we were found that COX-2 expression was well correlated with the differentiation grade of HCC. COX-2 was up-regulated in well-differentiated HCC, whereas it was down-regulated in the poorly-differentiated HCC. Such a close relationship between COX-2 expression and the differentiation grade of HCC has been reported previously (19, 28). These suggest that the modulation of COX-2 expression may be a determinant of cellular differentiation in HCC. Such a biological role of COX-2 can be supported by a recent observation that, when epithelial cells are transfected with the COX-2 gene, the adhesion to the extracellular matrix increases and apoptosis is inhibited (23).
To sum up, this is the first study which focuses on the SNPs of COX-2 and their effects on HCC risk. Further investigations of multiple SNPs of other cancer related genes, gene-gene and gene-environment interactions, and phenotypic assays of the HCC-associated SNPs are urgently needed in the future. Moreover, COX-2 might play a role in the advanced as well as the early stages of hepatocarcinogenesis.
Acknowledgements
We thank Tzu-Ting Weng and the Tissue Bank at the China Medical University for their technical assistance. This study was supported by research grants from the Terry Fox Cancer Research Foundation, and China Medical University and Hospital
Figure legend
Fig. 1 The expression levels of COX-2 at different differentiation grade HCC (×400). A, non-tumor portion. B, tumor portion in well-differentiated (WD) HCC. C, tumor portion in poor-differentiated (PD) HCC. D, Western blot analysis of COX-2 expression. E, Quantification of the Western blot data from above group. β-actin was used as the loading control. Data are averaged from six tissues per group with 15 μg total sample protein for each lane. #P<0.05 compared to the non-tumor portion tissues.
References
1. Avila, M.A., Berasain, C., Sangro, B. and Prieto, J. New therapies for hepatocellular carcinoma. Oncogene 25:3866-3884, 2006.
2. Bae, S.H., Jung, E.S., Park, Y.M., Kim, B.S., Kim, B.K., Kim, D.G. and Ryu, W.S. Expression of cyclooxygenase-2 (COX-2) in hepatocellular carcinoma and growth inhibition of hepatoma cell lines by a COX-2 inhibitor, NS-398. Clin. Cancer Res. 7:1410-1418, 2001.
3. Casado, M., Callejas, N.A., Rodrigo, J., Zhao, X., Dey, S.K., Bosca, L. and Martin-Sanz, P. Contribution of cyclooxygenase 2 to liver regeneration after partial hepatectomy. Faseb. J. 15:2016-2018, 2001.
4. Casado, M., Molla, B., Roy, R., Fernandez-Martinez, A., Cucarella, C., Mayoral, R., Bosca, L. and Martin-Sanz, P. Protection against Fas-induced liver apoptosis in transgenic mice expressing cyclooxygenase 2 in hepatocytes. Hepatology 45:631-638, 2007.
C.S., Lin, C.C. and Bau, D.T. Significant association of an XRCC4 single nucleotide polymorphism with bladder cancer susceptibility in Taiwan. Anticancer Res. 29:1777-1782, 2009.
6. Chang, C.H., Chiu, C.F., Liang, S.Y., Wu, H.C., Chang, C.L., Tsai, C.W., Wang, H.C., Lee, H.Z. and Bau, D.T. Significant association of Ku80 single nucleotide polymorphisms with bladder cancer susceptibility in Taiwan. Anticancer Res. 29:1275-1279, 2009.
7. Chen, C.J., Yu, M.W. and Liaw, Y.F. Epidemiological characteristics and risk factors of hepatocellular carcinoma. J. Gastroenterol. Hepatol. 12:S294-308, 1997.
8. Chiu, C.F., Tsai, M.H., Tseng, H.C., Wang, C.L., Wang, C.H., Wu, C.N., Lin, C.C. and Bau, D.T. A novel single nucleotide polymorphism in XRCC4 gene is associated with oral cancer susceptibility in Taiwanese patients. Oral Oncol. 44:898-902, 2008.
D.T. A novel single nucleotide polymorphism in XRCC4 gene is associated with gastric cancer susceptibility in Taiwan. Ann. Surg. Oncol. 15:514-518, 2008.
10. Chiu, C.F., Wang, H.C., Wang, C.H., Wang, C.L., Lin, C.C., Shen, C.Y., Chiang, S.Y. and Bau, D.T. A new single nucleotide polymorphism in XRCC4 gene is associated with breast cancer susceptibility in Taiwanese patients. Anticancer Res. 28:267-270, 2008.
11. Cok, S.J. and Morrison, A.R. The 3'-untranslated region of murine cyclooxygenase-2 contains multiple regulatory elements that alter message stability and translational efficiency. J. Biol. Chem. 276:23179-23185, 2001.
12. Davies, G., Salter, J., Hills, M., Martin, L.A., Sacks, N. and Dowsett, M. Correlation between cyclooxygenase-2 expression and angiogenesis in human breast cancer. Clin. Cancer Res. 9:2651-2656, 2003.
13. Department of Health, Taiwan. Cancer registration system annual report. Taiwan, Department of Health, 2008.
14. DeWitt, D.L. Prostaglandin endoperoxide synthase: regulation of enzyme expression. Biochim. Biophys. Acta. 1083:121-134, 1991.
15. Gorgoni, B., Caivano, M., Arizmendi, C. and Poli, V. The transcription factor C/EBPbeta is essential for inducible expression of the cox-2 gene in macrophages but not in fibroblasts. J. Biol. Chem. 276:40769-40777, 2001.
16. Hsu, C.F., Tseng, H.C., Chiu, C.F., Liang, S.Y., Tsai, C.W., Tsai, M.H. and Bau, D.T. Association between DNA double strand break gene Ku80 polymorphisms and oral cancer susceptibility. Oral Oncol. 45:789-793, 2009.
17. Hsu, N.Y., Wang, H.C., Wang, C.H., Chiu, C.F., Tseng, H.C., Liang, S.Y., Tsai, C.W., Lin, C.C. and Bau, D.T. Lung cancer susceptibility and genetic polymorphisms of Exo1 gene in Taiwan. Anticancer Res. 29:725-730, 2009.
18. Jiang, M.C., Liao, C.F. and Lee, P.H. Aspirin inhibits matrix metalloproteinase-2 activity, increases E-cadherin production, and inhibits in vitro invasion of tumor cells. Biochem. Biophys. Res. Commun. 282:671-677, 2001.
19. Koga, H., Sakisaka, S., Ohishi, M., Kawaguchi, T., Taniguchi, E., Sasatomi, K., Harada, M., Kusaba, T., Tanaka, M., Kimura, R., Nakashima, Y., Nakashima, O., Kojiro, M., Kurohiji, T. and Sata, M. Expression of cyclooxygenase-2 in human hepatocellular carcinoma: relevance to tumor dedifferentiation. Hepatology 29:688-696, 1999.
20. Levitt, R.J. and Pollak, M. Insulin-like growth factor-I antagonizes the antiproliferative effects of cyclooxygenase-2 inhibitors on BxPC-3 pancreatic cancer cells. Cancer Res. 62:7372-7376, 2002.
21. Lim, W., Kwon, S.H., Cho, H., Kim, S., Lee, S., Ryu, W.S. and Cho, H. HBx targeting to mitochondria and ROS generation are necessary but insufficient for HBV-induced cyclooxygenase-2 expression. J. Mol. Med. 88:359-369, 2010.
22. Martin-Sanz, P., Mayoral, R., Casado, M. and Bosca, L. COX-2 in liver, from regeneration to hepatocarcinogenesis: what we have learned from animal models? World J. Gastroenterol. 16:1430-1435, 2010.
23. Mayoral, R., Fernandez-Martinez, A., Bosca, L. and Martin-Sanz, P. Prostaglandin E2 promotes migration and adhesion in hepatocellular carcinoma cells. Carcinogenesis 26:753-761, 2005.
24. Mayoral, R., Molla, B., Flores, J.M., Bosca, L., Casado, M. and Martin-Sanz, P. Constitutive expression of cyclo-oxygenase 2 transgene in hepatocytes protects against liver injury. Biochem. J. 416:337-346, 2008.
25. Mizutani, Y., Kamoi, K., Ukimura, O., Kawauchi, A. and Miki, T. Synergistic cytotoxicity and apoptosis of JTE-522, a selective cyclooxygenase-2 inhibitor, and 5-fluorouracil against bladder cancer. J. Urol. 168:2650-2654, 2002.
26. Papafili, A., Hill, M.R., Brull, D.J., McAnulty, R.J., Marshall, R.P., Humphries, S.E. and Laurent, G.J. Common promoter variant in cyclooxygenase-2 represses gene expression: evidence of role in acute-phase inflammatory response. Arterioscler. Thromb. Vasc. Biol. 22:1631-1636, 2002.
27. Sanchez-Alcazar, J.A., Bradbury, D.A., Pang, L. and Knox, A.J. Cyclooxygenase (COX) inhibitors induce apoptosis in non-small cell lung
cancer through cyclooxygenase independent pathways. Lung Cancer 40:33-44, 2003.
28. Shiota, G., Okubo, M., Noumi, T., Noguchi, N., Oyama, K., Takano, Y., Yashima, K., Kishimoto, Y. and Kawasaki, H. Cyclooxygenase-2 expression in hepatocellular carcinoma. Hepatogastroenterology 46:407-412, 1999.
29. Sung, Y.K., Hwang, S.Y., Kim, J.O., Bae, H.I., Kim, J.C. and Kim, M.K. The correlation between cyclooxygenase-2 expression and hepatocellular carcinogenesis. Mol. Cells 17:35-38, 2004.
30. Tsujii, M., Kawano, S., Tsuji, S., Sawaoka, H., Hori, M. and DuBois, R.N. Cyclooxygenase regulates angiogenesis induced by colon cancer cells. Cell 93:705-716, 1998.
31. Vecchini, A., Ceccarelli, V., Susta, F., Caligiana, P., Orvietani, P., Binaglia, L., Nocentini, G., Riccardi, C., Calviello, G., Palozza, P., Maggiano, N. and Di Nardo, P. Dietary alpha-linolenic acid reduces COX-2 expression and induces apoptosis of hepatoma cells. J. Lipid Res. 45:308-316, 2004.
32. Williams, C.S., Mann, M. and DuBois, R.N. The role of cyclooxygenases in inflammation, cancer, and development. Oncogene 18:7908-7916, 1999.
33. Yang, M.D., Hsu, Y.M., Kuo, Y.S., Chen, H.S., Chang, C.L., Wu, C.N., Chang, C.H., Liao, Y.M., Wang, H.C., Wang, M.F. and Bau, D.T. Significant association of Ku80 single nucleotide polymorphisms with colorectal cancer susceptibility in Central Taiwan. Anticancer Res. 29:2239-2242, 2009.
Table I. The primer sequences, PCR and restriction fragment length polymorphism (RFLP) conditions for COX-2 gene polymorphisms.
Polymorphism
(location)
Primers sequences (5’ to 3’) Restriction
enzyme SNP sequence DNA fragment size (bp) A-1195G (rs689466) F: CCCTGAGCACTACCCATGAT R: GCCCTTCATAGGAGATACTGG Hha I A G 273 220 + 53 G-765C (rs20417) F: TATTATGAGGAGAATTTACCTTTCGC R: GCTAAGTTGCTTTCAACAGAAGAAT PvuⅡ C G 100 74 + 26 T+8473C (rs5275) F: GTTTGAAATTTTAAAGTACTTTTGAT R: TTTCAAATTATTGTTTCATTGC Bcl I T C 147 124 + 23 intron 1 (rs2745557) F: GAGGTGAGAGTGTCTCAGAT R: CTCTCGGTTAGCGACCAATT Taq I G A 439 353 + 76 intron 5 (rs16825748) F: GCGGCATAATCATGGTACAA R: CAGCACTTCACGCATCAGTT BsrG I T A 417 314 + 103 intron 6 (rs2066826) F: ACTCTGGCTAGACAGCGTAA R: GCCAGATTGTGGCATACATC Aci I A G 327 233 + 94
Talbe II. Characteristics of 298 HCC patients and 298 controls Controls (n = 298) Patients (n = 298) Characteristic n % Mean (SD) n % Mean (SD) p-valuea Age (years) 54.1 (4.6) 52.3 (4.5) 0.68 Gender 1.00 Male 213 71.5% 213 71.5% Female 85 28.5% 85 28.5% Habit Smoking 213 71.5% 224 75.2% 0.35 Alcohol drinking 198 66.4% 206 69.1% 0.54
Table III. Distribution of COX-2 genotypes among the HCC patient and control groups.
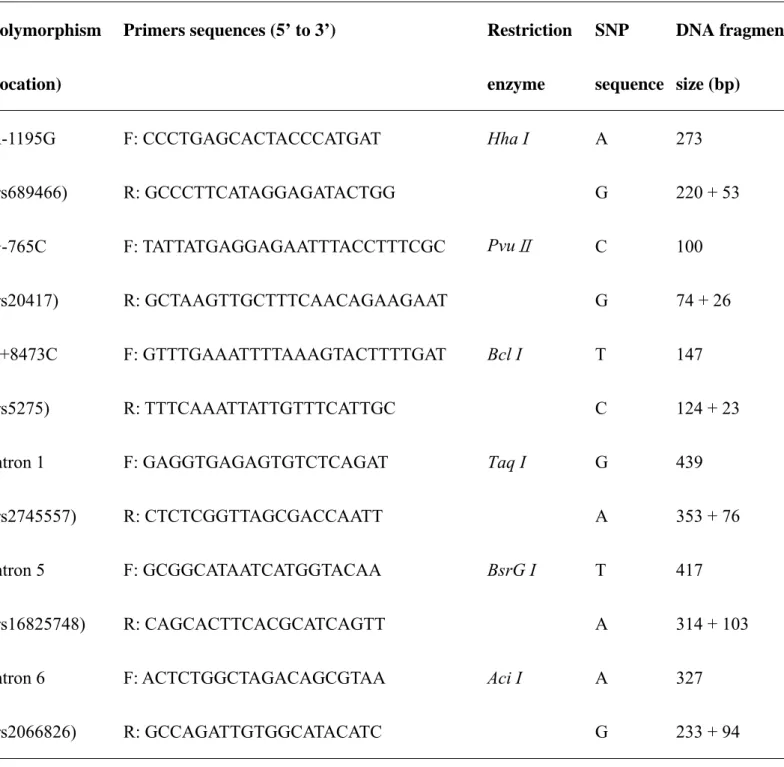
Genotype Controls % Patients % p-valuea
A-1195G (rs689466) 0.9220 AA 81 27.1% 84 28.2% AG 145 48.7% 144 48.3% GG 74 24.8% 70 23.4% G-765C (rs20417) 0.1951 GG 250 83.9% 262 87.9% GC 48 16.1% 36 12.1% CC 0 0% 0 0% T+8473C (rs5275) 0.6645 TT 201 67.4% 195 65.4% TC 97 32.6% 103 34.6% CC 0 0% 0 0% intron 1 (rs2745557) 0.7953 GG 226 75.8% 219 73.5% AG 67 22.6% 73 24.5% AA 5 1.6% 6 2.0% intron 5 (rs16825748) 0.5768 TT 290 97.3% 293 98.3% AT 8 2.7% 5 1.7% AA 0 0% 0 0% intron 6 (rs2066826) 0.3192 GG 248 83.2% 236 79.2% AG 44 14.8% 51 17.1% AA 6 2.0% 11 3.7%
Table IV. COX-2 allelic frequencies among the HCC patient and control groups.
Allele Controls % Patients % p-valuea
A-1195G (rs689466) 0.6824 Allele A 307 51.2% 312 52.4% Allele G 293 48.8% 284 47.6% G-765C (rs20417) 0.1745 Allele G 548 92.0% 560 94.0% Allele C 48 8.0% 36 6.0% T+8473C (rs5275) 0.6419 Allele T 499 83.7% 493 82.7% Allele C 97 16.3% 103 17.3% intron 1 (rs2745557) 0.4989 Allele G 519 87.1% 511 85.7% Allele A 77 12.9% 85 14.3% intron 5 (rs16825748) 0.4028 Allele T 588 98.6% 591 99.2% Allele A 8 1.4% 5 0.8% intron 6 (rs2066826) 0.1130 Allele G 540 90.6% 523 87.8% Allele A 56 9.4% 73 12.2%
Table V. Frequencies of combined COX-2 G-765C and intron 6 genotype polymorphisms among the HCC and control groups.
Control Patients COX-2 G-765C /intron 6 n % n % OR (95% CI) P-valuea All 298 100.0 298 100.0 GG/GG 208 69.8 207 69.5 1.00 GG/AG+AA 42 14.1 55 18.5 1.31 (0.84-2.05) 0.2595 GC/GG 40 13.4 29 9.7 0.73 (0.44-1.22) 0.2437 GC/ AG+AA 8 2.7 7 2.3 0.88 (0.31-2.47) 1.0000