Title:
Fracture risk and adjuvant therapies amongin young breast cancer patients: aA
population-based study
Running title: Breast Cancer and Fracture
Chun-Hung Chang,1,2 Shaw-Ji Chen,3,4 Chieh-Yu Liu5* 1China Medical University Hospital, Taichung, Taiwan, R.O.C.
2Institute of Clinical Medicine, China Medical University, Taichung, Taiwan, R.O.C.; 3Department of Psychiatry, Mackay Memorial Hospital Taitung Branch, Taitung, R.O.C. 4Institute of Medical Sciences, Tzu Chi university, Hualien, Taiwan, R.O.C.
5Biostatistical Consulting Lab, Institute of Nursing-Midwifery, National Taipei University of
Nursing and Health Sciences, Taipei, Taiwan, R.O.C.
*Corresponding author: Chieh-Yu Liu, Ph.D.
365, Min-der Rd., Beitou district, Taipei City, Taiwan, R.O.C. Biostatistical Consulting Lab, Institute of Nursing-Midwifery, National Taipei University of Nursing and Health Sciences Tel: +886-2-28227101 ext 3312/2500
Fax: +886-2-28213233
E-mail: b89401068@ntu.edu.tw ; chiehyu@ntunhs.edu.tw
Financial Disclosure: The authors have indicated they have no relevant financial
relationships to disclose for this article. 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26
Conflicts of Interest: The authors declare that there is no conflict of interest.
Contributor’s Statement:
Chun-Hung Chang conceptualized and designed the study, database processing and drafted the initial manuscript.
Shaw-Ji Chen provided expert opinions and reviewed the final submitted manuscript. Chieh-Yu Liu is in charge of this study, including applying the research database, conducting the data analysis and critically reviewed the manuscript, and approved the final submitted manuscript.
27 28 29 30 31 32 33 34 35 36 37
Abstract
Background: Breast cancer survivors have an increased risk of bone fracture. But the
risk among young patients with adjuvant therapies remain unknown. This population-based study is aimed to assess the incidence and risk of fracture among young (age of 20 to 39 years) breast cancer patients who underwent adjuvant therapies.
Methods: From January 2001 to December 2007, 5,146 newly diagnosed breast
cancer patients were enrolled from the National Health Insurance Research Database(NHIRD) in Taiwan. Subjects were observed for a maximum of 7 years to determine the incidences of newly onset fracture. Kaplan-Meier and Cox regression analyses was used to evaluate the risk of fracture in young breast cancer patients who underwent adjuvant treatments.
Results: Of the total 5,146 young (age of 20 to 39 years) breast cancer patients, the
Cox multivariate proportional hazards analysis showed that AIs, radiotherapy, and monoclonal antibody were significantly associated with a higher risk of fracture. Moreover, patients with longer AIs prescription duration (>180 days) or more radiotherapy visits (>4 visits) have higher risk of fracture (HR of AIs = 1.77; 95% CI, 0.68 to 4.57; p=0.255) (HR of radiotherapy = 2.54; 95% CI, 1.07 to 6.06; p<0.05). And young breast cancer patients with AIs therapy had the greatest risk of hip fracture (HR = 8.520, 95% CI, 1.711- 42.432, P<0.05), while patients with radiotherapy had 38 39 40 41 42 43 44 45 46 47 48 49 50 51 52 53 54 55 56
the greatest risk of vertebrae fracture (HR = 5.512, 95% CI, 1.847 to 16.451, P<0.01). Conclusion: Young breast cancer patients with AIs therapy, radiotherapy or
monoclonal antibody were associated the increased risk of fracture.
Keywords: breast cancer, fracture, aromatase inhibitors (AIs). 57
58 59 60 61
Introduction
Breast cancer is the most prevalent malignancy in women worldwide and the leading cause of death from cancer-related mortality in women worldwide [1,2]. In Taiwan, the 5-year survival rates have increased from 69.79% in 1986 to 82.85% in 2003 [3] because of early screening [4], surgery, and adjuvant therapies including such as the use of selective o estrogen receptor modulators (e.g. tamoxifen) [5], third-generation aromatase inhibitors (AIs; e.g. anastrozole, letrozole, or exemestane), or monoclonal antibody (e.g. trastuzumab) [2,6], chemotherapy [7], and radiotherapy [8].
Increased An increased risk of fracture among has been observed in breast cancer survivors has been noted [9-11]. But little research investigates However, the risk of fracture following adjuvant therapies, which are increasingly are used for breast cancer increasingly treatment, has not been investigated thoroughly . Two stu dies Aromatase inhibitors (AIs) have shown associated AIs the association with an increase in the increased risk of fracture in postmenopausal breast cancer patients [3,12]; however, . But they these two studies were did not designed to address the risk of fracture risk among in young patients. On the other han d Conversely , 62 63 64 65 66 67 68 69 70 71 72 73 74 75 76 77 78 79 80
tamoxifen, a selective estrogen receptor modulator, is was reported to preserve the bone mineral density of the lumbar spine in postmenopausal women [13], but ; however, evidence regarding on fractures has been mixed has been conflicting [14-17]. Moreover, the risk of fracture among in young breast cancer survivors receiving monoclonal antibody antibodies , chemotherapy, and radiotherapy has not been evaluated.
Besides, young breast cancer women Young women with breast cancer are considered a special group of breast cancer patient because they have poor prognosis, because and the survival rates are comparatively lower for women aged <40 years of age are comparatively lower than those for older women [18,19]. Approximately 7% of women with breast cancer are diagnosed before the age of 40 years [20], but the incidence of young breast cancer increase in recent years [2]. Moreover, multivariate analysis has one study reported shown younger age to be an independent predictor of adverse outcome outcomes of adjuvant therapies [20].
This study was aimed to investigate We investigated the risk of fracture of resulting from adjuvant therapies among in young breast cancer patients at age aged of 20-39 years by using retrieving claims data from the population-based retrospective database which was retrieved from of the 81 82 83 84 85 86 87 88 89 90 91 92 93 94 95 96 97 98 99
National Health Insurance Research Database (NHIRD) in Taiwan. Methods
Database
We used available claims data from Taiwan’s National Health Insurance (NHI) program, which was launched by the Taiwan government in 1995 and providesprovided comprehensive health care for 98.29% of its citizensresidents in 2006 [21]. The NHI research database (NHIRD) contains comprehensive information including outpatient, inpatient, prescription drugs, and traditional Chinese medicine services. The diagnostic and procedure codes are based on the International Classification of Diseases, ninthNinth revision, Clinical Modification (ICD-9-CM) and Procedure Coding System (ICD-9-PCS).
Ethics Sstatement
The Institutional Review Board of the China Medical University Hospital approved this study (CMUH103-REC3-077). The National Health Research Institutes encrypt patients’the personal information of patients for privacy protection. The NHI Bureau of NHI and Institutional Review Board of China Medical University Hospital guarantee the data to protect the confidentiality of patientsthe personal and health information of patients.
Study Populationpopulation
We identified adult female of at least 20 years of agepatients aged 20 to 39 years 100 101 102 103 104 105 106 107 108 109 110 111 112 113 114 115 116 117 118 119
with a primaryan initial diagnosis of breast cancer (ICD-9-CM code=174.XX) for the first time between January 1, 2002, and December 31, 2007 from the NHIRD. Study population was divided in two categories: age 20-39 years and 40 and up. Young was defined as patients at the age of 20 to 39 years. This breast cancer cohort was followed until the date of fracture (ICD-9-CM codes: 800-829), death, withdrawal from the National Health Insurance program, or the end of 2007. We further investigated the risk of fracture at three sites: including the hip (ICD-9-CM 820), vertebrae (ICD-9-CM 806.20-806.9), and forearm (ICD-9-CM 813) [10] (Fig.ure 1). Covariate Aassessment
We took into accountconsidered adjuvant therapies including selective oestrogen receptor modulator (tamoxifen), AIs (anastrozole, letrozole, and exemestane), monoclonal antibody (trastuzumab), chemotherapy, and radiotherapy based onaccording to diagnostic and procedure codes. From Based on the medications (including tamoxifen, anastrozole, letrozole, exemestane, and trastuzumab) prescribed to the study cohortpatients, the ‘prescription duration’ (sum of prescription’s days of supply) for each patient was calculated for dosage effect evaluation. Patients who received chemotherapy PCS codes: V581 and 992.5), or radiotherapy (ICD-9-PCS codes: 922 and V580) were also identified. BesidesFurthermore, we defined distant metastases as metastases to the lungs, liver, brain, bones, and other organs 120 121 122 123 124 125 126 127 128 129 130 131 132 133 134 135 136 137 138
(ICD-9-CM codes 197.x, 198.0, 198.1, and 198.3-198.7). The Charlson comorbidity index (CCI) was also calculated to represent degree of comorbidity [22].
Statistical Analysisanalysis
The incidence densities and hazard ratio (HR) of fractures amongin young breast cancer patients with and without specific adjuvant treatment were calculated. UniviriaateUnivariate and multivariate Cox proportional- hazard regressionsregression were used to examineevaluate the effects of adjuvant therapies on the risk of fracture,; as shown the results were expressed usingby HRs with 95% confidence intervals (CIs). The multivariablemultivariate model was used to control for age, and the comorbidities. We estimated the cohort-specific cumulative incidences by 1- (Kaplan- Meier survival rate) for unadjusted curves and tested the differences by using log-rank tests. Regarding database processing, MyY Structured Query Language (MySQL) was used for extraction, linkage, and processing of the data. All statistical analyses were performed using IBM SPSS statistical software versionVersion 20 (IBM Corp., New York, NY, USA). TheA two-tailed p -value < 0.05 was considered statistically significant.
Results
Clinical characteristics of the study population
Based on the selection criteria (Fig. 1), thisThe samplestudy comprised 5,146 young breast cancer patients at the age ofaged 20 to 39 years without fracture 139 140 141 142 143 144 145 146 147 148 149 150 151 152 153 154 155 156 157 158
fromdiagnosed between January 1, 2002 toand December 31, 2007. The mean age was 35.32 years (standard deviation, 3.83). The mean follow-up periods in our study werewas 2.94 ± 1.69 years. The most common adjuvant treatments among breast cancer patients werewas tamoxifen ([63.5%]), chemotherapy ([52.1%]), and radiotherapy ([24.2%]). The most common underlying diseases were hypertension (0.4%), autoimmune diseases (0.4%), and diabetes (0.3%) (Table 1).
Incidences of fracture
The incidence of subsequent fractures of breast cancer survivors was 54.34 per 10,000 person-years. (Fig. 2). The older patients (age 40 years and up) had higher cumulative incidence of fracture than young patients (age 20-39 years) (59.48 vs. 24.43 per 10,000 person-years), with a HR of 2.4265(95% CI, 1.9199 to 3.0667, P<0.001) (Figure 2). It is worth noting that young patients with AIs therapy exhibited significantly higher risk of fracture than those without AIs therapy (cumulative incidence 6.4% vs. 0.7%, p<0.001), and with radiotherapy compared to those without radiotherapy (cumulative incidence 4.0% vs. 0.6%, p<0.001) (Figure 3).Moreover, young patients who received AIs exhibited a significantly higher risk of fracture than that of those who did not (cumulative incidence 6.4% vs 0.7%, p < 0.001). Similarly, patients who received radiotherapy exhibited a higher risk of fracture than that of those who did not (cumulative incidence 4.0% vs 0.6%, p < 0.001) (Fig. 3).
159 160 161 162 163 164 165 166 167 168 169 170 171 172 173 174 175 176 177
Risk factors associated with fracture in breast cancer patients receiving adjuvant therapies
InAs shown in Table 2, we appliedperformed univariate analysis to predict the riskdevelopment of fracture in the young breast cancer patientscohort,; and the results demonstrated that patients who received tamoxifen, AIs, trastuzumab, chemotherapy, and radiotherapy hadwere at a higher risk. After controlling the age and comorbidities, were controlled for, the Cox multivariate proportional hazards analysis showed that AIs (adjusted HR [aHR] = 7.221, 95% CI, = 3.743 -13.929, Pp < 0.001), trastuzumab (aHR = 5.032, 95% CI, = 2.091-12.112, Pp < 0.001), and radiotherapy (aHR = 4.403, 95% CI, = 2.271-8.536, Pp < 0.001) were significantly associated with a higher risk of fracture.
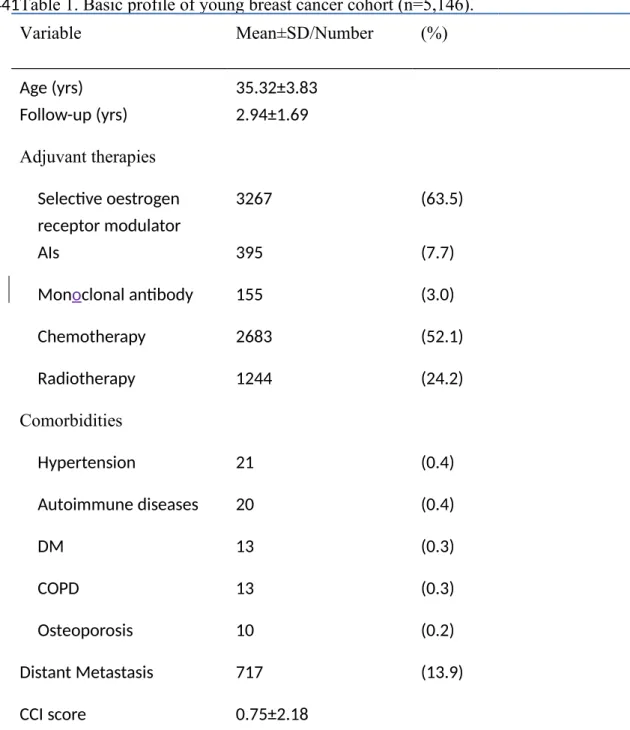
Table 3 showed that 17 (6+11) of 395 (204+191) patients with AIs developed fracture, and 22 (9+13) of 1244 (825+419) patients with radiotherapy developed fracture. Moreover, young patients with longer AIs prescription duration (>180 days) or more radiotherapy visits (>4 visits) have higher risk of fracture (HR of AIs = 1.77; 95% CI, 0.68 to 4.57; p=0.255) (HR of radiotherapy = 2.54; 95% CI, 1.07 to 6.06; p<0.05). As shown in Table 3, we focused on the incidence of fracture in patients receiving AIs and radiotherapy. The risk of fracture was high in patients receiving 178 179 180 181 182 183 184 185 186 187 188 189 190 191 192 193 194 195 196
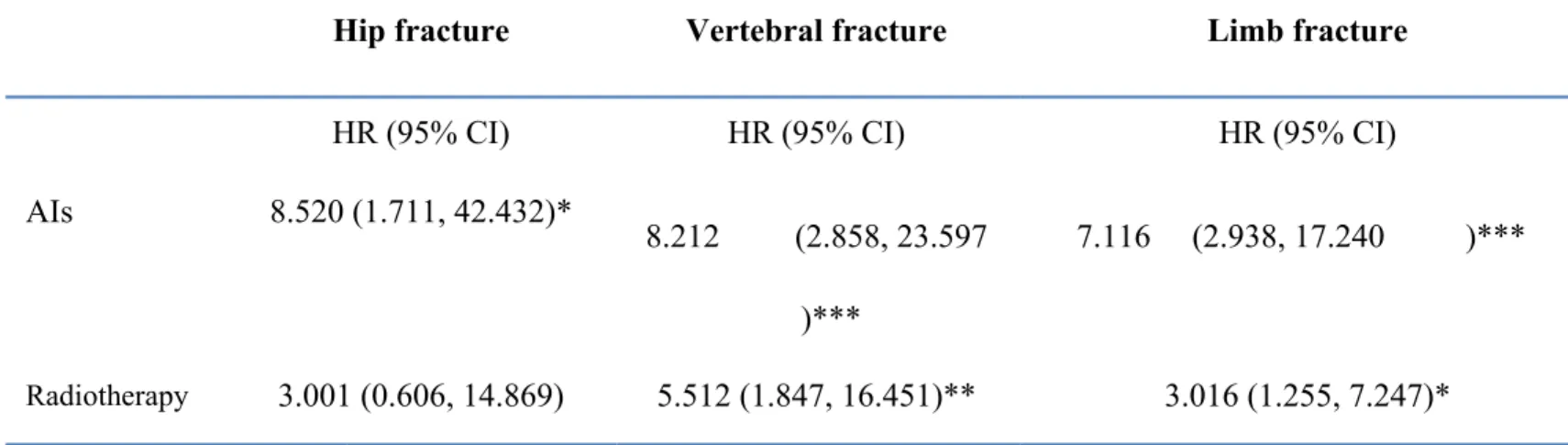
AIs. Compared with the patients who received AIs for fewer than 180 days, those who received AIs for more than 180 days had a high HR of 1.77 (95% CI = 0.68- 4.57), which was not significant. However, the HR of fracture was 2.54 (95% CI = 1.07-6.06 in patients who received more than four radiotherapy visits. Moreover, we further investigated the risk of fracture at three sites and listed the observations in Table 4. Under the site-specific analysis, young breast cancer patients who received AIs had the highest risk of hip fracture (HR = 8.520, 95% CI = 1.711-42.432, p < 0.04), whereas patients who received radiotherapy had the highest risk of vertebral fracture (HR = 5.512, 95% CI = 1.847-16.451, p < 0.01) (Table 4). Furthermore, we divided cases who developed fracture into three groups by fracture sites. In the site-specific analysis, limb is the most common site of fracture. But young breast cancer patients with AIs therapy had the greatest risk of hip fracture (HR = 8.520, 95% CI, 1.711-42.432, P<0.05), while patients with radiotherapy had the greatest risk of vertebrae fracture (HR = 5.512, 95% CI, 1.847 to 16.451, P<0.01) (Table 4).
Discussion
ToBased on our knowledgeresearch, this is the first study to analyze the subsequent risk of fracture in young breast cancer survivors aged 20-39 yrsyears who received adjuvant therapies. The results of this nationwide, population-based cohort 197 198 199 200 201 202 203 204 205 206 207 208 209 210 211 212 213 214 215
study shows that (1) the incidence rate of fracture in young breast cancer survivors was 1.4%.; (2) tThe risk of fracture was higher whenin patients underwentwho received AIs or radiotherapy;than in those who did not. (3)A higher number of radiotherapy visits exposed patients to a higher risk of fracture. longer AIs prescription days and radiotherapy visits exhibited higher risk; (4) tThe most common fracture site is limb, but patients withPatients who received AIs therapy havehad the highest hazard ratioHR offor hip fracture. compared with those without AIs therapy; and (5) pPatients who underwentreceived radiotherapy havehad the highest hazard ratioHR offor vertebral fracture compared with those didn’t.
In our study, we found breast cancer patients had higher incidence than the incidence in general population in Taiwan (54.34 vs. 42.52 per 10,000 person-years) A study using a population-based representative sample showed that the incidence of fracture in the general Taiwanese female population was approximately 42.52 per 10,000 [10]. In addition, the incidence of subsequent fractures was higher in the elder patients than in the young patients. The cumulative incidence of fracture in our study is 24.43 per 10,000 person-years for young breast cancer group, which is lower than the incidence (42.52 per 10,000 person-years) in general population in Taiwan In this study, 561 fractures were reported among 37,231 breast patients. The relative risk ratio (RR) of fracture in breast cancer patients was (95% CI = 3.185-3.947, p < 216 217 218 219 220 221 222 223 224 225 226 227 228 229 230 231 232 233 234
0.001). Therefore, the incidence of fracture in breast cancer patients was significantly higher than that in the general population [10] or (90-228 per 10,000 person-years) in general population [23]. These findings agree with previous studies and higher incidence in elder patients may be resulted from major risk factor like age and menopause[24].Moreover, we compared the incidence of subsequent fractures between young breast cancer patients and young non breast cancer patients from the Taiwan Cancer Registry database [3]. The cumulative incidence of fracture in our study was 1.4% for the young breast cancer group, which is significantly higher than that for (0.3%) the young non breast cancer patients in Taiwan (RR = 4.762, 95% CI = 3.996-5.764, p < 0.001).
However, to our knowledge, we are Furthermore, according to our research, our study is the first to report a higher risk of fracture following adjuvant therapies including AIs, radiotherapy, and monoclonal antibodiesy. Young breast cancer patients who underwentreceived AIs therapy had a higher aHR (adjusted hazard ratio) compared to those didn’tthan that of those who did not (aHR = 7.221, 95% CI, = ( 3.743, -13.929), Pp < 0.001). The trials of AIs treatment showing benefit for breast cancer patients have shown increases in the total number of bony fractures [25-28].An increase in the number of bony fractures has been reported in clinical trials on AIs used in treating breast cancer patients [23-26]. A population-based cohort of 2,003 235 236 237 238 239 240 241 242 243 244 245 246 247 248 249 250 251 252 253
older female breast cancer patients revealsrevealed that subjectspatients usingwho received AIs hashad a higher risk of fracture (adjusted hazardaHR = 1.34, CI = ([0.92, 1.94])) [27][29]. In Aanother study ofon 211 postmenopausal breast cancer patients, the aHR washas adjusted hazard ratio (aHR) = 20.08, (95 % CI = 1.72–234.08, Pp = 0.017) in patients who received endocrine therapy compared with those who did notcomparison with non-endocrine therapy group (1.1 %, 1/89)[12]. However, these two studies are focushave focused on postmenopausal patients. We areOur study is the first to investigate the risk of fracture ofon receiving AIs in young patients. BesidesFurthermore, our results also showed that radiotherapy and monoclonal antibodyantibodies were significantly associated with a higher risk of fracture.
Furthermore, we evaluated the dose effect by using the prescription duration for AIs and the number of visits for radiotherapy because the radiation dose-related details were is not available in the NHIRD., which is one of the limitations of this study. The number ofFew patients who underwentreceived monoclonal antibodiesy and developed fractures; was too few for further analysistherefore further analysis could not be performed because of the small sample size. We found young group patients with longer AIs prescription duration (>180 days) or more than 4 visits of radiotherapy (>4 visits) had higher risk of fracture (HR: 1.77 and 2.54 respectively). Further studies are needed to investigate accurate dosage of AIs and 254 255 256 257 258 259 260 261 262 263 264 265 266 267 268 269 270 271 272
radiotherapy.Young breast cancer patients who received AIs for more than 180 days or those who received more than four radiotherapy visits had a higher risk of fracture (HR : 1.77 and 2.54, respectively, although the HR was not statistically significant for AIs). Additional studies are necessary to investigate the dose effects of AIs and radiotherapy.
In the fracture site-specific analysis, limbs fracture was most common fracture sites, and this agreesfinding is consistent with those of with a review ofon adult fractures [28][23]. However, we foundobserved that young patients withwho received AIs therapy had the highest hazard ratiorisk of hip fracture compared with those without AIs therapy; whereas young subjectspatients who underwentreceived radiotherapy had the highest riskhazard ratio of vertebral fracture compared with those didn’t. IncreasedAn increased risk of vertebral fracture has been notedreported in previous studies. Kanis et. al. reported that the incidence of vertebral fracture was nearly five times greaterhigher than normal in women from the time of the first diagnosis [(odds ratio ([OR]), = 4.7,; 95% confidence interval (95% CI),CI = 2.3– 9.9]) [30][29], while Chen et al. reportedfound that the increased risk offor vertebral fracture was statistically significant among breast cancer survivors who had a breast cancer diagnosis before the age of 55 years (HR,= 1.78,; 95% CI,= 1.28 -2.46) [9]. Another study showed that the incidence of fracture was higher in the breast cancer 273 274 275 276 277 278 279 280 281 282 283 284 285 286 287 288 289 290 291
cohort than in the comparison cohort with adjusted HRs (aHRs) of 1.24 (95% CI,= 1.04–1.48) for vertebral fractures [10]. ButHowever, the possible mechanisms of the association between breast cancer and vertebral fractures remain unknown.
To date, the pathophysiological mechanisms for fracture following AIs treatment using AIs have not yet been clearly identified. Published sStudies have revealed early breast cancer women underwent who received AIs are at a risk of bone loss, which resulted from because AIs almostAI-induced nearly completely inhibit estrogen production [31,32][30, 31]. AIs therapy may cause estrogen suppression and increases in bone turnover rates, whichthus acceleratinge bone loss. Therefore, these effects may ultimately result in osteoporosis and an increased fracture risk of fracture, especiallyparticularly at the site of hip [33-35][32-34].
The relationship between radiotherapy and fracture may be associationassociated with the chest wall radiation therapy because radiation therapy has been commonly used to minimize the risk of local cancer recurrence after lumpectomy [2,8]. The fFrequent chest wall radiation may increase the finding of vertebral fracture. Besides, bBone metastases represent are the most common manifestation of metastatic breast cancer and are associated with significanthigh morbidity [36][35]. Therefore, vertebral metastasis may also result in a higher risk of vertebral fracture.
Our study has some limitations. First, the National Health Insurance Research 292 293 294 295 296 297 298 299 300 301 302 303 304 305 306 307 308 309 310
Database NHIRD did not provide information ofon potential confounders includingsuch as cancer staging, pregnancy/ or lactation status, bone mineral density (BMD), subjects’ lifestyles habits (smoking, alcoholism, and so on), and the use of self-paid medications (vitamin D, calcium, and so on). However, young breast cancer patients are less affected by the risk factors likesuch as age or menopause compared withthan elderolder patients [24][36]. Besides, aAdjuvant therapies have the potential to impact more significantly on affect younger patients more significantly than eolder patients [20]. Therefore, our study results may show morebe heavily influenced influence ofby adjuvant therapies. Second, the fractures history may have been diagnosed before 2000, and; thisthese data could not be detected or excluded in this study. Third, patients with minor fractures who might neglect to may not seek treatment or did not know that they had experienced fracturesbe aware of their fractures. However, these data should bewere distributed equally between two groups (with/without those who received adjuvant therapies and those who did not) and caused no bias in the results.
In conclusion, this study found a subsequent risk of fracture following adjuvant therapies including AIs, radiotherapy, and monoclonal antibodiesy. AndFurthermore, these the risks may increase with the duration of AIs use and radiotherapy. Patients who underwentreceived AIs had the highest risk at theof hip sitefractures, 311 312 313 314 315 316 317 318 319 320 321 322 323 324 325 326 327 328 329
whilewhereas those withwho received radiotherapy had the highest risk of at vertebral sitefractures. Further Additional studies would beare requirednecessary to evaluatedetermine more accurate dose effects andthe possible mechanisms and the dose effects that are more accurate.
330 331 332 333
References
1. DeSantis C, Ma J, Bryan L, Jemal A (2014) Breast cancer statistics, 2013. CA Cancer J Clin 64: 52-62.
2. Benson JR, Jatoi I, Keisch M, Esteva FJ, Makris A, et al. (2009) Early breast cancer. Lancet 373: 1463-1479.
3. (2015) The Taiwan Cancer Registry. pp. http://tcr.cph.ntu.edu.tw/main.php? Page=A5B3.
4. Warner E (2011) Clinical practice. Breast-cancer screening. N Engl J Med 365: 1025-1032.
5. Early Breast Cancer Trialists' Collaborative G, Davies C, Godwin J, Gray R, Clarke M, et al. (2011) Relevance of breast cancer hormone receptors and other factors to the efficacy of adjuvant tamoxifen: patient-level meta-analysis of randomised trials. Lancet 378: 771-784.
6. Hudis CA (2007) Trastuzumab--mechanism of action and use in clinical practice. N Engl J Med 357: 39-51.
7. Berry DA, Cronin KA, Plevritis SK, Fryback DG, Clarke L, et al. (2005) Effect of screening and adjuvant therapy on mortality from breast cancer. N Engl J Med 353: 1784-1792.
8. Buchholz TA (2009) Radiation therapy for early-stage breast cancer after breast-conserving surgery. N Engl J Med 360: 63-70.
9. Chen Z, Maricic M, Bassford TL, Pettinger M, Ritenbaugh C, et al. (2005) Fracture risk among breast cancer survivors: results from the Women's Health Initiative Observational Study. Arch Intern Med 165: 552-558.
10. Tsai CH, Muo CH, Tzeng HE, Tang CH, Hsu HC, et al. (2013) Fracture in asian women with breast cancer occurs at younger age. PLoS One 8: e75109.
11. Newcomb PA, Trentham-Dietz A, Egan KM, Titus-Ernstoff L, Baron JA, et al. (2001) Fracture history and risk of breast and endometrial cancer. Am J Epidemiol 153: 1071-1078.
12. Xu L, Wang J, Xue DD, He W (2014) Aromatase inhibitors associated musculoskeletal disorders and bone fractures in postmenopausal breast cancer patients: a result from Chinese population. Med Oncol 31: 128.
13. Love RR, Mazess RB, Barden HS, Epstein S, Newcomb PA, et al. (1992) Effects of tamoxifen on bone mineral density in postmenopausal women with breast cancer. N Engl J Med 326: 852-856.
14. Kristensen B, Ejlertsen B, Mouridsen HT, Andersen KW, Lauritzen JB (1996) Femoral fractures in postmenopausal breast cancer patients treated with adjuvant tamoxifen. Breast Cancer Res Treat 39: 321-326.
334 335 336 337 338 339 340 341 342 343 344 345 346 347 348 349 350 351 352 353 354 355 356 357 358 359 360 361 362 363 364 365 366 367 368 369 370
15. Vestergaard P, Rejnmark L, Mosekilde L (2008) Effect of tamoxifen and aromatase inhibitors on the risk of fractures in women with breast cancer. Calcif Tissue Int 82: 334-340.
16. Powles TJ, Hickish T, Kanis JA, Tidy A, Ashley S (1996) Effect of tamoxifen on bone mineral density measured by dual-energy x-ray absorptiometry in healthy premenopausal and postmenopausal women. J Clin Oncol 14: 78-84.
17. Kristensen B, Ejlertsen B, Dalgaard P, Larsen L, Holmegaard SN, et al. (1994) Tamoxifen and bone metabolism in postmenopausal low-risk breast cancer patients: a randomized study. J Clin Oncol 12: 992-997.
18. Bleyer AOL, M.; Barr, R.; Ries, L. (2006) NIH Publication No 06-5767. Bethesda, MD: National
Cancer Institute; 2006. Cancer epidemiology in older adolescents and young adults 15 to 29 years
of age, including SEER incidence and survival: 1975-2000.
19. Ademuyiwa FO, Gao F, Hao L, Morgensztern D, Aft RL, et al. (2014) US breast cancer mortality trends in young women according to race. Cancer.
20. Anders CK, Johnson R, Litton J, Phillips M, Bleyer A (2009) Breast cancer before age 40 years. Semin Oncol 36: 237-249.
21. Cheng CL, Kao YH, Lin SJ, Lee CH, Lai ML (2011) Validation of the National Health Insurance Research Database with ischemic stroke cases in Taiwan. Pharmacoepidemiol Drug Saf 20: 236-242.
22. Charlson ME, Pompei P, Ales KL, MacKenzie CR (1987) A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 40: 373-383.
23. Court-Brown CM, Caesar B (2006) Epidemiology of adult fractures: A review. Injury 37: 691-697.
24. Cummings SR, Melton LJ (2002) Epidemiology and outcomes of osteoporotic fractures. Lancet 359: 1761-1767.
25. Goss PE, Ingle JN, Martino S, Robert NJ, Muss HB, et al. (2003) A randomized trial of letrozole in postmenopausal women after five years of tamoxifen therapy for early-stage breast cancer. N Engl J Med 349: 1793-1802.
26. Coombes RC, Kilburn LS, Snowdon CF, Paridaens R, Coleman RE, et al. (2007) Survival and safety of exemestane versus tamoxifen after 2-3 years' tamoxifen treatment (Intergroup Exemestane Study): a randomised controlled trial. Lancet 369: 559-570.
27. Jakesz R, Jonat W, Gnant M, Mittlboeck M, Greil R, et al. (2005) Switching of postmenopausal women with endocrine-responsive early breast cancer to anastrozole after 2 years' adjuvant tamoxifen: combined results of ABCSG trial 371 372 373 374 375 376 377 378 379 380 381 382 383 384 385 386 387 388 389 390 391 392 393 394 395 396 397 398 399 400 401 402 403 404 405 406 407 408
8 and ARNO 95 trial. Lancet 366: 455-462.
28. Howell A, Cuzick J, Baum M, Buzdar A, Dowsett M, et al. (2005) Results of the ATAC (Arimidex, Tamoxifen, Alone or in Combination) trial after completion of 5 years' adjuvant treatment for breast cancer. Lancet 365: 60-62.
29. Neuner JM, Yen TW, Sparapani RA, Laud PW, Nattinger AB (2011) Fracture risk and adjuvant hormonal therapy among a population-based cohort of older female breast cancer patients. Osteoporos Int 22: 2847-2855.
30. Kanis JA, McCloskey EV, Powles T, Paterson AH, Ashley S, et al. (1999) A high incidence of vertebral fracture in women with breast cancer. Br J Cancer 79: 1179-1181.
31. Simpson ER, Dowsett M (2002) Aromatase and its inhibitors: significance for breast cancer therapy. Recent Prog Horm Res 57: 317-338.
32. Geisler J, Lonning PE (2005) Endocrine effects of aromatase inhibitors and inactivators in vivo: review of data and method limitations. J Steroid Biochem Mol Biol 95: 75-81.
33. Coleman RE, Banks LM, Girgis SI, Kilburn LS, Vrdoljak E, et al. (2007) Skeletal effects of exemestane on bone-mineral density, bone biomarkers, and fracture incidence in postmenopausal women with early breast cancer participating in the Intergroup Exemestane Study (IES): a randomised controlled study. Lancet Oncol 8: 119-127.
34. Eastell R, Adams JE, Coleman RE, Howell A, Hannon RA, et al. (2008) Effect of anastrozole on bone mineral density: 5-year results from the anastrozole, tamoxifen, alone or in combination trial 18233230. J Clin Oncol 26: 1051-1057.
35. Eastell R, Hannon RA, Cuzick J, Dowsett M, Clack G, et al. (2006) Effect of an aromatase inhibitor on bmd and bone turnover markers: 2-year results of the Anastrozole, Tamoxifen, Alone or in Combination (ATAC) trial (18233230). J Bone Miner Res 21: 1215-1223.
36. Coleman RE (2001) Metastatic bone disease: clinical features, pathophysiology and treatment strategies. Cancer Treat Rev 27: 165-176.
409 410 411 412 413 414 415 416 417 418 419 420 421 422 423 424 425 426 427 428 429 430 431 432 433 434 435 436 437 438 439 440
Table 1. Basic profile of young breast cancer cohort (n=5,146). Variable Mean±SD/Number (%) Age (yrs) 35.32±3.83 Follow-up (yrs) 2.94±1.69 Adjuvant therapies Selective oestrogen receptor modulator 3267 (63.5) AIs 395 (7.7) Monoclonal antibody 155 (3.0) Chemotherapy 2683 (52.1) Radiotherapy 1244 (24.2) Comorbidities Hypertension 21 (0.4) Autoimmune diseases 20 (0.4) DM 13 (0.3) COPD 13 (0.3) Osteoporosis 10 (0.2) Distant Metastasis 717 (13.9) CCI score 0.75±2.18
Abbreviations: CCI=Charlson Comorbidity Index; AIs=aromatase inhibitors. 441
442 443 444
Table 2. Cox regression analysis of fracture incidence among young breast cancer survivors with adjuvant treatments.
Type of Adjuvant therapies HRUnivariate analysis95% CI of HR p-value aHR Multivariate analysis95% CI of aHR p-value Selective oestrogen receptor modulat
or 1.782 0.814 to 3.898 0.148 1.825 0.831 to 4.008 0.134 AIs 7.251 3.786 to 3.889 < 0.001 7.221 3.743 to 13.929 <0.001 Monoclonal antibody 4.899 2.041 to 11.759 < 0.001 5.032 2.091 to 12.112 <0.001 Chemotherapy 1.854 0.943 to 3.643 0.073 1.856 0.944 to 3.650 0.073 Radiotherapy 4.447 2.307 to 8.573 <0.001 4.403 2.271 to 8.536 <0.001
Abbreviations: AIs=aromatase inhibitors; HR=hazard ratio; aHR=adjusted hazard ratio.
Multivariable analysis including age, and comorbidities of hypertension, autoimmune diseases, DM, COPD, and osteoporosis. 446 447 448 449 450 451 452
Table 3. Risk of fracture among young breast cancer patients with AIs or Radiotherapy.
N Events Person-years IR HR
(95% CI) p-value
1 p-value2
AIs prescription days
≤180 204 6 722 8.31 1.00(Reference) 0.2553 >180 191 11 774 14.21 1.77 (0.68 to 4.57) Radiotherapy visits ≤4 825 9 2411 3.73 1.00(Reference) 0.0258* 0.0202* >4 419 13 1344 9.67 2.54 (1.07 to 6.06) 25 49 50 453 454 455 456 457 458 459 460 461 462 463 464 465 466 467 468
Abbreviations: AIs=aromatase inhibitors; HR, hazard ratio.
N Event Person-years IR HR
(95% CI) p-Value
AIs prescription days
≤180 204 6 722 8.31 1.00(Reference) 0.2553 >180 191 11 774 14.21 1.77 (0.68 to 4.57) Radiotherapy visits ≤4 825 9 2411 3.73 1.00(Reference) 0.0258* >4 419 13 1344 9.67 2.54 (1.07 to 6.06) IR, incidence rate, per 1000 person-years
HR, hazard ratio
* p<0.05, ** p<0.01, *** p<0.001
1p-value: estimated using Cox regression models
2p-value: exact two-tailed probability estimated using hypergeometric tests
26 51 52 469 470 471 472 473 474 475 476 477 478 479 480 481 482 483 484 485 486 487 488
Table 4. Hazard ratio and 95% confidence intervals of site specific fracture among young breast cancer group with AIs and radiotherapy.
Hip fracture Vertebral fracture Limb fracture
HR (95% CI) HR (95% CI) HR (95% CI)
AIs 8.520 (1.711, 42.432)*
8.212 (2.858, 23.597 )***
7.116 (2.938, 17.240 )***
Radiotherapy 3.001 (0.606, 14.869) 5.512 (1.847, 16.451)** 3.016 (1.255, 7.247)*
Abbreviations: AIs=aromatase inhibitors; HR, hazard ratio. *p<0.05, **p<0.01, ***p<0.001. 489 490 491 492 493 494 495 496 497
Figure 1. Selection of study patients. 498
499 500
Figure 2. Cumulative incidence of fracture in young breast cancer cohort by Kaplan-Meier analysis (n=5,146). 501
502 503
Figure 3. Cumulative incidence of fracture in young breast cancer cohort (a) with/without AIs, and (b) with/without radiotherapy by Kaplan-Meier analysis. 504 505 506 507