行政院國家科學委員會補助專題研究計畫成果報告
登革病毒血清型專一性抗體的 B 細胞抗原決定位之研究及評估其
為偵檢試劑之可行性
計畫類別:■個別型計畫 □整合型計畫
計畫編號:NSC 89-2320-B-002-208
執行期間:90 年 8 月 1 日至 91 年 7 月 31 日
計畫主持人:吳 漢 忠
共同主持人:
本成果報告包括以下應繳交之附件:
□赴國外出差或研習心得報告一份
□赴大陸地區出差或研習心得報告一份
□出席國際學術會議心得報告及發表之論文各一份
□國際合作研究計畫國外研究報告書一份
執行單位:臺大醫學院口腔生物研究所
中 華 民 國 91 年 10 月 27 日
行政院國家科學委員會專題研究計畫成果報告
計畫編號:NSC 89-2320-B-002-208
執行期限:90 年 8 月 1 日至 91 年 7 月 31 日
主持人:吳 漢 忠 臺大醫學院口腔生物研究所
共同主持人:xxxxxx 執行機構及單位名稱
計畫參與人員:鍾美櫻
一、中文摘要 病毒感染後,經常會借由活化宿主的 免疫反應產生抗體,對抗病毒蛋白之抗原 決定位。這些病毒蛋白上抗原決定位的確 認,有助於病毒感染機制的暸解,對於發 展有效的疫苗及偵檢試劑也有重大的助 益。本計畫主要是要研究登革病毒的 B 細 胞 抗 原 決 定 位 。 經 由 ELISA, immunofluorescence 和 immunoblotting 方 法,我們找出具專一性的第二型登革病毒 單株抗體(Ab4)。此抗體之抗原決定位的確 認,有助於病毒感染機制的暸解,疫苗及 偵檢試劑的研發。在此研究中,我們以噬 菌體顯現法來研究第二型登革病毒的抗原 決定位。我們也已經篩選出多株的噬菌體 可以專一性的與第二型登革病毒抗體結 合 。 這 些 專 一 性 噬 菌 體 所 攜 帶 的 外 來 peptide,都含有 His-Arg/Lys-Leu/Ile motif。 以 synthetic peptide 代替噬菌體所攜帶的外 來 peptide,可與第二型登革病毒抗體明顯 反應。第二型登革病毒感染之老鼠及兔子 血清,可以用此 epitope-based peptide 來確 認。另外,此 epitope-based peptide 可有效 的分辨登革病毒及日本腦炎病毒感染之老 鼠血清,這些抗原決定位的確認與研究, 將有助於 epitope-based peptide antigen 之研 發,及了解出血性登革之病理機制。關鍵詞:第二型登革病毒,噬菌體顯現法,
抗原決定位,登革偵檢試劑
ABSTRACT
Viral infection usually results in the production of antibodies directed against the epitopes of viral proteins through the
activation of the host’s humoral immunity. Identification of these epitopes on viral proteins is important for understanding the pathogenesis of viral infectious diseases as well as for developing vaccines and
diagnostic reagents. Serotype-specific
monoclonal antibody, Ab4, against dengue virus type 2 (DEN-2) was generated and identified by ELISA, immunofluorescence and immunoblotting analysis. The B-cell epitope of Ab4 was further identified from a random peptide library displayed on phage. The selected phage clones were specifically reacted with Ab4 and did not reacted with
other monoclonal antibodies. These
immunopositive phage clones displayed a consensus motif, His-Arg-Leu/Ile. Synthetic peptide corresponding to the phage-borne peptide can bind the antibody specifically. The epitope-based synthetic peptide could
detect serum samples from DEN-2
immunized mice and rabbits by ELISA assay. The peptide was also able to differential very clearly between serum samples from DEN-2 and Japanese encephalitis virus (JEV) immunized mice. The results show that this monoclonal antibody and its epitope-based peptide will be useful for serologic diagnosis of DEN-2. Furthermore, the identification of the epitope of DEN-2 will permit us to functionally dissect the antibody response and to address the role of antibody in the pathogenesis of primary or secondary DEN-2 infection.
KEY WORDS: monoclonal antibody; B-cell
epitope; synthetic peptide; serologic
INTRODUCTION
Dengue virus (DEN) causes serious febrile illness in humans, including dengue hemorrhagic fever (DHF) and dengue shock syndrome (DSS) (Henchal, et al. 1990; Halstead, 1988). Primary dengue virus
infection often results in a painful,
debilitating, but nonfatal dengue fever and appears to against reinfection with the same serotype. However, the more severe and sometimes fatal forms DHF and DSS have been frequently seen in region where more than one serotype of dengue virus is circulating (Halstead, 1988; Gubler, 1998). Secondary infection with a different serotype is associated with an increased risk for DHF and maybe caused by the uptake by monocytes/macrophages of virus complexes
to nonneutralizing antibodies,
sub-neutralizing cross-reactive antibodies or low-titer neutralizing antibodies (Halstead, 1988; Bielefeldt-Ohmann, 1997; Halstead, et al. 1984). Viral infection usually results in the production of antibodies directed against the epitopes of the viral proteins through the activation of the host’s humoral immunity. The epitopes have been divided into linear or continuous epitopes and conformational or discontinuous epitopes (Sela, 1969; Barlow et al., 1986). Linear epitopes are short stretches of the primary structure of the protein and are made up of some continuous amino acid residues of the primary sequence. Conformational epitopes consist of several amino acid residues which are discrete in the primary sequence but assemble to form an antigenic determinant on the tertiary structure of the native protein. Identification of these epitopes on viral proteins is important in understanding the pathogenesis of viral infectious diseases as well as in developing effective vaccines and diagnostic reagent.
Recent advance in peptide technology
has allowed the development of
combinatorial peptide libraries expressed either on a solid phase support or displayed on bacteriophages. The high molecular diversity displayed by these libraries provides the possibility to study B-cell epitope mapping (Scott and Smith 1990; Young et al.,
1997; Wu et al., 2001)
Recently, we have identified
serotype-specific B-cell epitope of DEN-1 (Wu et al., 2001). In this study, we used a phage-displayed peptide library to identify the serotype-specific B-cell epitope for DEN-2. Up to now, it is still not clear whether DHF/DSS is due to a primary or secondary infection of DEN or other immunopathologic mechanisms (Halstead,
1988; Gubler, 1998). Therefore, the
identification of B-cell epitopes for DEN can provide important information for the development of a safe and effective dengue vaccine and contribute to the understanding of the pathogenesis and immunological responses in DEN infection.
RESULTS
Identification of serotype-specific MAb against DEN-2
To show that the MAb Ab4 was specific for DEN-2 and did not cross-react with other serotype of DENs, the immunoblotting and ELISA assay was perform. The AB4 reacted only to the NS-1 of DEN-2 and did not cross-react with DEN-1, 3, and 4 (Fig 1).
Native gel NS12 NS1 NS1 Denature gel D1D2D3D4 Ab4 A O D4 9 0 n m C B D1D2D3D4 Ab4 0 0.1 0.2 0.3 0.4 0.5 D1 D2 D3 D4 Ab4 NM-IgG NMS Native gel NS12 NS1 NS1 Denature gel D1D2D3D4 Ab4 A O D4 9 0 n m C B D1D2D3D4 Ab4 0 0.1 0.2 0.3 0.4 0.5 D1 D2 D3 D4 Ab4 NM-IgG NMS
Fig. 1. Identification the specificity of DEN-2 monoclonal antibody (Ab4) by ELISA and immunoblotting assay.
Screening of phage displayed peptide library with DEN-2 serotype-specific antibody
To select the phage clones by DEN-2 specific monoclonal antibody (Ab4), the ascites were purified by protein G affinity column. The purified antibodies were immobilized on ELISA plate and the bound phage clones were selected after three times
of biopanning. For further screen of these selected phage clones, the single phage clones were isolated and amplified for Ab4 screening by ELISA assay. Selected phage clones showed significant enhancement of reactivity to antibody Ab4 and these phage clones did not bind to normal mouse serum or IgG (Fig. 2).
Fig. 2. Identification of DEN-2 specific monoclonal antibody (Ab4) selected phage clones by ELISA assay. Phage displayed random peptide library was screened by Ab4. After three rounds of screening, 17 phage clones from 20 selected phage clones showed were significant highly reactivity to antibody Ab4 but not to normal mouse serum (NMS) or PBS.
Characterization of the B cell epitope
Ten phage clones selected by Ab4 were amplified and the phage DNAs were isolated for DNA sequencing. The primer used for
phage DNA sequencing is
5’-CCCTCATAGTTAGCGTAA-3’. This
primer locates in antisense strand of gene III of the M13 phage and has 96 nucleotides separated from the inserted DNA. The inserted nucleotides of selected phage clones were sequenced and all of them contain 36
inserted nucleotides. The inserted
oligonucleotide sequences of phage DNAs were selected and translated to peptide sequences. The peptide sequences were aligned using a MacDNASIS software to analyze the epitopes of the antibody Ab4 and its binding motif. The binding motif for the Ab4 is H-R/K-L/I and all of the selected phage clones contain this motif.
Binding assay of synthetic peptide
In order to verify that the peptide
sequences identified by phage display were indeed recognized by antibody Ab4, synthetic peptide binding assays were carried out. The phage-displayed peptides were synthesized in multiple antigen peptide (MAP) form because the ELISA sensitivity of this
eight-chain MAP were more than
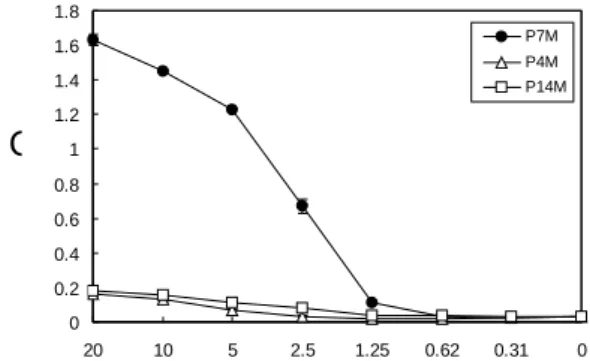
single-chain peptides (Tam and Zavala, 1989). As shown in Fig. 3, the synthetic peptide (P7M) XXXXXXTMPSES, selected with MAb Ab4 from phage displayed RPLs,
binds the antibody in a
concentration-dependent manner. An
unrelated control peptide KGTFDPLQEPRT (P4M) and EHKYSWKS (P14M) showed no reactivity.
Fig. 3. ELISA reactivity of synthetic peptide corresponding with selected phage clone. The phage-displayed peptide was synthesized and immobilized on a 96-well plate as an antigen for detection of Ab4. The synthetic peptide (P7M), corresponding with the selected phage clone, reacted with Ab4 specifically, but not the control peptide (P4M and P14M).
Detection of DEN-2-infected animal sera using synthetic peptide P7M
To test whether the antibodies produced from outbred rabbit sera that had been intravenously infected with the DEN-2 would react with synthetic peptide (P7M), we collected hyperimmune serum samples after four doses of DEN-2 for ELISA assay. The results revealed that all serum samples from DEN-2-hyperimmune rabbits demonstrated a higher ELISA antibody reactivity with P7M peptide than the preimmune serum samples (Fig. 4B). Furthermore, our additional results revealed that serum samples obtained from five JEV-hyperimmune and eight normal ICR
0 0.4 0.8 1.2 1.6 2 2.4 1 3 5 7 9 11 13 15 17 19 NMS O O 0 0.2 0.4 0.6 0.8 1 1.2 1.4 1.6 1.8 20 10 5 2.5 1.25 0.62 0.31 0 P7M P4M P14M
mice showed no ELISA reactivity with P7M peptide, but eight DEN-2-hyperimmue ICR mice showed significant ELISA reactivity with the peptide antigen (Fig. 4A), which
suggested its differentiation capability
between JEV and DEN-2 in mouse serum samples.
Fig. 4. (A). ELISA reactivities of DEN-2 synthetic peptide P7M with DEN-2 and JEV immunized mice sera (100-fold dilution). Eight DEN-2 immunized mice sera could be identified by the peptide P7M. (B). ELISA reactivities of DEN-2 synthetic peptide P7M with pre-immunized and DEN-2 immunized rabbit sera (100-fold dilution). Five DEN-2 immunized rabbit sera could be identified by the peptide P7M.
DISCUSSION
Identification of viral B-cell epitopes is of importance in the selection of peptides for
inclusion into subunit vaccines, the
development of virus-specific serological diagnosis and understanding the interaction of antibodies with viruses at a molecular level. This is the first study to characterize serotype-specific B-cell epitope of DEN-2 MAb (Ab4) and detect antibodies of DEN-2 from serum samples of DEN-2-immunized animal using the epitope-based peptide antigen. Our results found that the selected phage displayed 12mer peptide sequence
revealed a consensus motif,
His-Arg/Lys-Leu/Ile. It is feasible that our phage displayed epitope and epitope-based
peptide antigen can be used to develop laboratory diagnosis serologic tests for dengue viruses in the future.
Using our epitope-based peptide antigen to detect anti-DEN antibody is relatively simple and specific. Therefore, it will be promising to become routine procedures for viral diagnosis in clinical and public health laboratories. On the other hand, conventional IgM- and IgG-capture ELISA tests, which require the preparation of DEN antigen and antibody, have been primarily used in specialized virology centers. Several reliable dengue serologic diagnosis tests are now
available, they still display difficulty
distinguishing among the four serotypes of DEN and still show a 45-50% cross-reaction with antibody of the Japanese encephalitis virus (JEV) (Lam et al., 1998., Vaughn et al., 1998; Vaughn et al., 1999). Otherwise, in our results suggested the epitope-based synthetic
peptide P7M differentiation capability
between JEV and DEN-2 in mouse serum samples.
Moreover, our developed method can also be applied to detect future DF and DHF patients who had secondary infection with a heterologous serotype of DEN, which would minimize possible morbidity and mortality. Finally, our test will be very valuable for further development of a serotype-specific diagnostic reagent that can be used to serological distinguish four serotypes dengue patients and thus help combat dengue diseases.
REFERENCES
1. Barlow, D. J., M. S. Edwards, and J. M.
Thornton. (1986). Continuous and
discontinuous protein antigenic
determinants. Nature 322: 747-748.
2. Bielefeldt-Ohmann, H. (1997).
Pathogenesis of dengue virus diseases: missing pieces in the jigsaw. Trends Microbiol. 5: 409-413.
3. Folgori, A., R. Tafi, A. Meola, F. Felici, G. Galfre, R. Cortese, P. Monaci, and N. Alfredo. 1994. A general strategy to
identify mimotopes of pathological
antigens using only random peptide
0 0.1 0.2 0.3 0.4 0.5 0.6 0.7 0.8 0.9 1 DEN-2 JEV NMS O 0 0.1 0.2 0.3 0.4 0.5 0.6 0.7 0.8 322 323 325 326 327 D EN2 sera Pre-immune O
libraries and human sera. EMBO J. 13: 2236-2243.
4. Gubler D. J. 1998. Dengue and dengue
hemorrhagic fever. Clin. Microbiol. Rev.
11: 480-496.
5. Gubler, D. J., and G. G. Clark. 1995.
Dengue/dengue hemorrhagic fever: the emergence of a global health problem. Emerg. Infect. Dis. 1: 55-57.
6. Halstead, S. B. 1989. Antibody,
macrophages, dengue virus infection, shock, and hemorrhage: a pathogenetic cascade. Rev. Infect. Dis. 11: S830-839.
7. Halstead, S. B. 1988. Pathogenesis of
dengue: challenges to molecular biology. Science 239: 476-481.
8. Halstead, S. B., C. N. Venkateshan; M. K. Gentry; and L. K. Larsen. 1984.
Heterogeneity of infection enhancement of dengue 2 strains by monoclonal antibodies. J. Immunol. 312, 1529-1532.
9. Henchal, E. A., and J. R. Putnak. 1990.
The dengue viruses. Clin. Microbiol. Rev.
3: 376-396.
10. Lam, S. K., and P. L. Devine. 1998.
Evaluation of capture ELISA and rapid immunochromatographic test for the determination of IgM and IgG antibodies produced during dengue infection. Clin. Diagnostic Virol. 10: 75-81.
11. Scott, J. K., and G. P. Smith. 1990.
Searching for peptide ligands with an epitope library. Science 249: 386-390.
12. Scott, J. K., and G. P. Smith. 1990.
Searching for peptide ligands with an epitope library. Science 249: 386-390.
13. Sela, M. 1969. Antigenicity: Some
molecular aspects. Science 166:
1365-1374.
14. Vaughn, D. W., A. Nisalak, S. Kalayanarooj, T. Solomon, N. M. Dung, A. Cuzzubbo, and P. L. Devine. 1998.
Evaluation of a rapid
immunochromatographic test for
diagnosis of dengue virus infection. J. Clin. Microbiol. 36: 234-238.
15. Vaughn, D. W., A. Nisalak, T. Solomon, S. Kalayanarooj, M. D. Nguyen, R. Kneen, A. Cuzzubbo, and P. L. Devine.
1999. Rapid serologic diagnosis of
dengue virus infection using a
commercial capture ELISA that
distinguishes primary and secondary infections. Am. J. Trop. Med. Hyg. 60: 693-698.
16. Young, A. C., P. Valadon, A.
Casadevall, M. D. Scharff, and J. C. Sacchettini. 1997. The three-dimensional
structures of a polysaccharide binding antibody to Cryptococcus neoformans and its complex with a peptide from a phage display library: implications for the identification of peptide mimotopes. J. Mol. Biol. 274: 622-634.