Effects of Maternal Undernutrition on Lung Growth and
Insulin-like Growth Factor System Expression in Rat Offspring
CHUNG-MING CHEN1, LENG-FANG WANG2, YAW-DONG LANG3
Background: Maternal undernutrition may alter the development of the lung structure in rat offspring.
Methods: We investigated the effects of maternal undernutrition (50% rations of the control food intake) during the last week of gestation on the expression of the rat lung insulin-like growth factor (IGF) system in the postnatal period.
Results: Body weights of undernourished pregnant rats were significantly lower than those of control rats from gestational days 16 to 21. Rats subjected to intrauterine growth restriction (IUGR) exhibited significantly lower body weights and lower lung weights on postnatal days 1, 7, 14, and 28 and lower lung/body weight ratios on postnatal days 7 and 14 when compared with control rats. Lung IGF-I, IGF-II, IGF receptors types 1 (IGFR-1) and 2 (IGFR-2), and the IGF binding protein (IGFBP) mRNA expressions increased as rats aged and reached a peak on postnatal day 14. Maternal undernutrition significantly increased IGF-I and IGF-II mRNA expressions on postnatal days 1 and 28. Lung IGFR-1, IGFR-2, and IGFBP mRNA expressions were similar between control and IUGR rats during the study period.
Conclusions: This study presents a comprehensive overview of lung IGF system expression in control and IUGR rats and demonstrates the developmental regulation of each component. (Acta Paediatr Tw 2007; 48:62-7)
Key words: insulin-like growth factor, intrauterine growth restriction, receptor, rat
INTRODUCTION
Maternal undernutrition during late pregnancy may have significant effects on the developing fetal lung, which undergoes rapid cellular multiplication and differentiation shortly before birth. We previously found that inadequate maternal dietary intake during late gestation altered the development of the lung structure (reduced alveolar surface area and volume fraction) in the postnatal period.1 Those results indicated that the
nutritional status of the mother has a marked effect on postnatal lung growth and development. Several growth factors and their receptors play critical roles in cell proliferation, migration, and differentiation during this well-coordinated growth process.
Department of Pediatrics, Taipei Medical University Hospital1; Department of Biochemistry, College of Medicine, Taipei
Medical University2; Graduate Institute of Medical Sciences, College of Medicine, Taipei Medical University3, Taipei,
Taiwan.
Received: December 27, 2006. Revised: February 5, 2007. Accepted: April 19, 2007.
Address reprint requests to: Dr. Chung-Ming CHEN, Department of Pediatrics, Taipei Medical University Hospital, 252 Wu-Hsing Street, Taipei 110, Taiwan.
TEL : 886-2-2737-2181 ext. 3320 FAX : 886-2-2736-0399
E-mail: cmchen@tmu.edu.tw
Insulin-like growth factors (IGFs) are small peptides that modulate cell proliferation and differentiation during embryogenesis through paracrine or autocrine interactions with the cell-surface receptor, IGF receptor type 1 (IGFR-1).2-4 IGFR-1 belongs to a receptor tyrosine kinase family,
and previous studies demonstrated that both IGF-I and -II act through IGFR-1 for mitogenic signaling in murine embryonic development.5,6 IGFR-2 has no tyrosine kinase
activity and is important in internalizing IGF-II/receptor complexes and transporting IGF-II to lysozymes.7
IGF-II mRNA expression is decreased in hypoplastic lungs produced by transaction of the cervical spinal cord or tracheal drainage,8 and IGF-I and IGF-II mRNA
expressions are increased in the large lungs produced by tracheal ligation.9,10 We carried out this study because
experiments studying IUGR consequences on the lung IGF system (peptides, receptors, and binding proteins) in postnatal rats were limited in the literature. We hypothesized that IUGR would influence the lung IGF system in rat offspring, and thus we examined the mRNA expression of each component of the IGF system in the postnatal lung to determine its developmental regulation. The aims of this study were to define the potential roles and interactions of the IGF system during postnatal lung development.
MATERIALS AND METHODS Animals
This study was approved by the Institutional Animal Use Committee at Taipei Medical University and was performed using timed pregnant Sprague-Dawley rats (vaginal smear positive, day 0; term, day 22). All animals were individually caged at 22 , with a 12-h lig12-ht-dark cycle in an isolated room. T12-hree control rats received food (regular rat chow containing 23.5% protein, 4.5% fat, and 53% carbohydrates) and water ad libitum from hanging containers throughout their pregnancies. Their food consumption was measured daily by weighing the container after carefully collecting spilled chow. The four experimental animals (subjected to undernutrition) consisted of an age-and weight-matched group that received rations at 50% of the control food intake during the last trimester from days 14 to 21 of gestation.
The dams delivered spontaneously at term and were
then immediately switched back to standard rat chow. The offspring were nursed by their mothers until being weaned at 4 weeks of age, and then they too were switched to standard chow. On days 1, 7, 14, and 28 after birth, 6 to 8 rat pups from each of the 4 age groups were randomly selected from each of the control litters and undernourished litters, irrespective of sex, and anesthetized with an intraperitoneal injection of pentobarbital (50 mg/kg, Abbott Laboratories, North Chicago, IL, USA). Each newborn rat was weighed, a ventral midline incision was made, and the lungs were dissected free and weighed.
Insulin-like growth factor system gene expression by reverse transcription polymerase chain reaction (RT-PCR)
Lung tissue from the right lungs was ground into a powder in liquid nitrogen, and gene expressions of IGF-I, IGF-II, IGFR-1, IGFR-2, and IGF-BP were measured with RT-PCR. Total RNA was extracted using the TRIzol Reagent (Invitrogen Life Technologies, Paisley, UK) according to the manufacturer’s instructions. The yield and purity of the isolated RNA solution were determined by A260 and A280 readings on a spectrophotometer. Reverse transcription was performed on 3 g of RNA with an oligo-dT primer and avian myeloblastosis virus reverse transcriptase (Roche, Indianapolis, IN, USA). The PCR was carried out with the primers shown in Table 1. The numbers of cycles for the PCR for IGF-I, IGF-II, 1, IGFR-2, IGF-BP, and -actin were 29, 31, 30, 30, 31, and 28, respectively. The PCR products were analyzed
by electrophoresis on an agarose gel, stained with ethidium bromide, and photographed. Five samples on each postnatal day were analyzed in each group for each IGF system component.
Statistical analysis
Data are expressed as the mean ± SEM. Between-group comparisons at each age Between-group were made using Student’s t-test. The Spearman test was used for correlation analyses of relationships between lung and body weights and IGF- and- expressions. Differences were considered significant at P < 0.05.
RESULTS
There were 24 pups from 3 rats in the control group and 30 pups from 4 rats in the undernourished group. There was no significant difference in litter sizes and
sex between the two study groups. All rat pups were alive at the time of sacrifice.
Effects of undernutrition on maternal body weight during late gestation
Body weights of undernourished pregnant rats were kept at around 380 g during the last 8 gestational days, and the values were significantly lower than those of control rats from gestational days 16 to 21 (Fig. 1). Body weight, lung weight, and the lung/body weight ratio in control and IUGR rats
Effects of maternal undernutrition on postnatal body weight and lung weight are presented in Table 2. IUGR rats exhibited significantly lower body weights and lower lung weights on postnatal days 1, 7, 14, and 28 and lower lung/body weight ratios on postnatal days 7 and 14 when compared with control rats.
Insulin-like growth factor system gene expression in control and IUGR rats
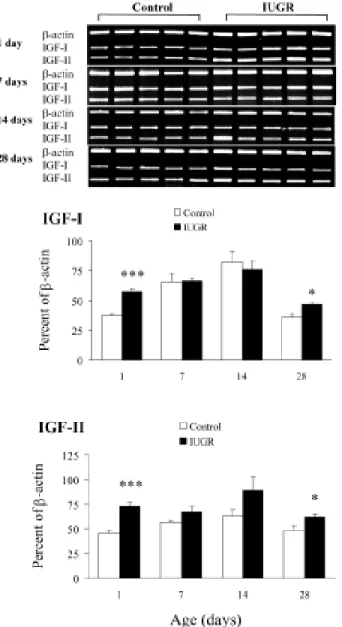
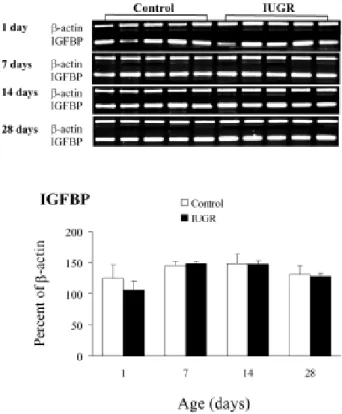
Effects of maternal undernutrition on the lung IGF-I, IGF-IIGF-I, IGFR-1, IGFR-2, and IGFBP mRNA expressions during the postnatal period are shown in Figs. 24. The results are expressed on the basis of -actin for each postnatal day. Lung IGF-I, IGF-II, IGFR-1, IGFR-2, and IGFBP mRNA expressions increased as rats aged, reaching a peak on postnatal day 14. Maternal undernutrition significantly increased IGF-I and IGF-IGF-IGF-IIGF-I mRNA expressions on postnatal days 1 and 28 (Fig. 2). Lung IGFR- , IGFR- , and IGFBP mRNA expressions were similar between control and IUGR rats during the study period (Figs. 3 and 4). There were no significant correlations between lung and body weights and IGF-I and –II RNA expressions.
DISCUSSION
The IGF system functions within a complex system composed of peptides, receptors, and binding proteins, Fig. 1. Body weights of control (open symbols) and
undernourished (filled symbols) pregnant rats during the last 8 gestational days. Body weights of undernourished rats were significantly lower than those of controls from gestational days 16 to 21 (*P < 0.05, **P < 0.01, ***P < 0.001 vs. the control group on each gestational day).
but the complete developmental profiles of the lung gene expression of the IGFs, IGF receptors, and IGFBP are lacking in postnatal rats from undernourished dams. The actions of IGF-I and IGF-II depend not only on the degree of their expression, but also on the expression and actions of the cell surface receptors and the IGFBP that interact with them. In the present study, late gestational exposure of rat fetuses to maternal Fig. 2. Effects of maternal undernutrition on mRNAs encoding IGF-I and -II in control and IUGR rat lungs. The results for each postnatal day are expressed and plotted as a percent of -actin. Maternal undernutrition significantly increased IGF-I and -II mRNA expressions on postnatal days 1 and 28 (*P < 0.05, ***P < 0.001 vs. the control group at each postnatal age).
Fig. 3. Effects of maternal undernutrition on mRNAs encoding IGFR-1 and -2 in control and IUGR rat lungs. Lung IGFR-1 and -2 mRNA expressions were similar between control and IUGR rats during the study period.
undernutrition was found to have produced changes in lung IGF-I and IGF-II expressions in the immediate and late postnatal period, while IGF receptor expressions were not affected. It is not known whether IGF-I and -II participated in the transduction of IUGR to alter lung development or whether the observed changes in these growth factors expression were merely secondary effects. Maternal undernutrition during late gestation is
Fig. 4. Effects of maternal undernutrition on mRNAs encoding the IGFBP in control and IUGR rat lungs. Lung IGFBP mRNA expressions were similar between control and IUGR rats during the study period.
associated with poor maternal weight gain and reduced fetal body weight in rats.11,12 In this study, we found
that maternal undernutrition during the last week of pregnancy reduced maternal body weight and neonatal body and lung weights in the postnatal period. When adjusted for body weight, the lung weight was significantly lower in IUGR rats than in control rats on postnatal days 7 and 14. Although the experimental dams were immediately switched back to standard rat chow after delivery, they nourished their offspring until 4 weeks of age. The consequence of this is that newborn rats received insufficient nutrition in the postnatal period, and this event may have partially contributed to the differences in body weight between older control and IUGR rats.
The regulation of cell proliferation in the developing lung is dependent on interactions between circulating endocrine agents and growth factors produced within the pulmonary environment. There is evidence suggesting that these endogenous factors and their receptors participate in signaling pathways between different cell types.13 IGFs are known to be produced in the perinatal
rat lung.14 The functional activity of IGF peptides was
modified by the IGFBP, which was also found in
developing lung tissues.14 Deletion of the genes for
IGF-I and -IGF-IIGF-I and their receptors in mice causes growth retardation and impairs lung function at birth.15,16 In
this study, we found increased IGF-I and -II mRNA expressions with comparable lung/body weight ratios on postnatal days 1 and 28 and decreased lung/body weight ratios on postnatal days 7 and 14 in IUGR rats. These data are in agreement with the findings of Nobuhara et al.,17 who found that IGF gene expression was
significantly increased both in liquid-based airway distension and postpneumonectomy models of accelerated postnatal lung growth. These results suggest that there is a close linking between IGF-I, -II and lung development.
Several limitations have to be kept in mind and need to be addressed in future experiments: In the present study, we primarily measured mRNA levels. The demonstrated alterations in IGF system mRNA expression might be due to an alteration in gene transcription and/ or in RNA stability. Although mRNA levels are important regulators of the protein levels, other regulatory elements downstream of mRNA levels might be operative, but it is still conceivable that this may also have affected to some extent the protein synthesis and secretion of these factors in the lungs in our study.
In conclusion, the results of this study showed that maternal undernutrition during late gestation significantly increased lung IGF-I and IGF-II mRNA expressions in the immediate and late postnatal period but that lung IGFR-1, IGFR-2, and IGFBP mRNA expressions did not change. This study presents a comprehensive overview of lung expression of the IGF system and demonstrates the developmental regulation of each component. REFERENCES
1. Chen CM, Wang LF, Su B. Effects of maternal undernu-trition during late gestation on the pulmonary surfactant system and morphology in rats. Pediatr Res 2004; 56:329-35. 2. Jetten AM. Growth and differentiation factors in
tracheobron-chial epithelium. Am J Physiol 1991; 260:361-73. 3. D’Ercole AJ. Somatomedin/insulin-like growth factors and
fetal growth. J Dev Physiol 1987; 9:481-95.
4. Kobayashi S, Clemmons DR, Venkatachalam MA. Colocal-ization of like growth factor binding protein with insulin-like growth development of lung vasculature. Am J Physiol 1991; 261:22-8.
5. Adams TE, Epa VC, Garrett TPJ, Ward CW. Structure and function of the type 1 insulin-like growth factor receptor. Cell Mol Life Sci 2000; 57:1050-93.
6. Rappolee DA, Strum KS, Behrendsten O, Schultz GA, Pedersen RA, Werb Z. Insulin-like growth factor II acts through an endogenous growth pathway regulated by imprint-ing in early mouse embryos. Genes Dev 1992; 6:939-52. 7. Kornfeld S. Structure and function of the mannose
6-phos-phate/insulinlike growth factor II receptors. Annu Rev Bio-chem 1992; 61:307-30.
8. Harding R, Hooper SB, Han VK. Abolition of fetal breath-ing movements by spinal cord transection leads to reductions in fetal lung liquid volume, lung growth, and IGF-II gene expression. Pediatr Res 1993; 34:148-53.
9. Hooper SB, Han VK, Harding R. Changes in lung expan-sion alter pulmonary DNA synthesis and IGF-II gene expres-sion in fetal sheep. Am J Physiol 1993; 265:403-9. 10. Joe P, Wallen LD, Chapin CJ, Lee CH, Allen L, Han VK,
Dobbs LG, Hawgood S, Kitterman JA. Effects of mechanical factors on growth and maturation of the lung in fetal sheep. Am J Physiol 1997; 272:95-105.
11. Lesage J, Blondeau B, Grino M, Bréant B, Dupouy JP. Maternal undernutrition during late gestation induces fetal overexposure to glucocorticoids and intrauterine growth retardation, and disturbs the hypothalamo-pituitary adrenal axis in the newborn rat. Endocrinology 2001; 142:1692-702. 12. Lin Y, Lechner AJ. Surfactant content and type II cell de-velopment in fetal guinea pig lungs during prenatal starvation.
Pediatr Res 1991; 29:288-91.
13. Minoo P, King RJ. Epithelial-mesenchymal interactions in lung development. Annu Rev Physiol 1994; 56:13-45. 14. Batchelor DC, Hutchins AM, Klempt M, Skinner SJ.
De-velopmental changes in the expression patterns of IGFs, type 1 IGF receptor and IGF-binding proteins-2 and -4 in perin-atal rat lung. J Mol Endocrinol 1995; 15:105-15.
15. Liu JP, Baker J, Perkins AS, Robertson EJ, Efstratiadis A. Mice carrying null mutations of the genes encoding insulin-like growth factor I (Igf-1) and type 1 IGF receptor (Igf1r). Cell 1993; 75:59–72.
16. Powell-Braxton L, Hollingshead P, Warbuton C, et al. IGF-I is required for normal embryonic growth in mice. Genes Dev 1993; 7:2609-17.
17. Nobuhara KK, DiFiore JW, Ibla JC, et al. Insulin-like growth factor-I gene expression in three models of accelerated lung growth. J Pediatr Surg 1998; 33:1057-60.