行政院國家科學委員會專題研究計畫 成果報告
大豆異黃酮代謝物致甲狀腺過氧化氫酵素失活現象---共價 結合研究
研究成果報告(精簡版)
計 畫 類 別 : 個別型
計 畫 編 號 : NSC 95-2320-B-468-001-
執 行 期 間 : 95 年 01 月 01 日至 95 年 10 月 31 日 執 行 單 位 : 亞洲大學生物科技與生物資訊學系
計 畫 主 持 人 : 張淳文
計畫參與人員: 碩士班研究生-兼任助理:魏士竣、張佑慈
處 理 方 式 : 本計畫可公開查詢
中 華 民 國 96 年 01 月 23 日
行政院國家科學委員會補助專題研究計畫
v 成 果 報 告
□期中 進度報告
大豆 異黃酮代謝物致甲狀腺過氧化氫酵素失活現象 ---共 價結合研究
計畫類別:v 個別型計畫 □ 整合型計畫 計畫編號:NSC 95-2320 -B-408-001
執行期間: 95 年 01 月 01 日至 95 年 10 月 31 日
計畫主持人:张淳文 共同主持人:N/A
計畫參與人員:魏士竣,张佑慈
成果報告類型(依經費核定清單規定繳交):v 精簡報告 □完整報告
本成果報告包括以下應繳交之附件:
□赴國外出差或研習心得報告一份
□赴大陸地區出差或研習心得報告一份
□出席國際學術會議心得報告及發表之論文各一份
□國際合作研究計畫國外研究報告書一份
處理方式:除產學合作研究計畫、提升產業技術及人才培育研究計畫、列管計畫及下列情形者 外,得立即公開查詢
□涉及專利或其他智慧財產權,□一年□二年後可公開查詢
執行單位:亚洲大学生物科技与生物资讯系
中 華 民 國 95 年 01 月 22 日
I.中文摘要
異 黃 與 類 豬 狀 關
由於大豆 酮 人 及 甲 腺的功能有 ,在推 大豆食品作 保健食品的同 ,必需薦 為 時
慮 對 類 產
考 它 人 可能 生的不良影 .響 大豆異 黃酮代 物,謝 如染料木 酮(genistein)及大豆甘黃 原(daidzein)已被 明 甲證 對 狀腺功能有不良的影 .響 本研究發 現染料木 酮,大豆甘原及雌黃 馬酚(equol)對泌乳 氧化 酵素(LPO),過 氫 豬甲 腺 氧化 酵素(pTPO)的活性的致失活效果狀 過 氫 隨著作用時 間而增加.酵素 力 的動 學 參 數 說明 LPO 及 pTPO被大豆異 黃酮代 物 致失活的抑謝 導
為 種
制 一 "機制因子型的致失活作用(mechanism-based inactivation). 染料木 酮及大豆黃 甘原對LPO的解 常 (離 數 Ki)為 0.2 及 0.5 mM,分配係 數(partition ratio)均 為 2 比 1。以
標 黃 與
放射性同位素氚 定的染料木 酮 LPO 作用,在 LPO失去活性失去後檢 測染料木 酮黃 與 LPO的共價 結合率.結果 示大顯 約500nmole的染料木 酮可黃 與40nmole 的 LPO結合(約 12.5:1). 以液相二極 質 譜 儀(LC-MS-MS)檢 測 LPO活化中心亞 鐵 紅素(heme)的 化,變 結果顯 示,雖然 LPO 或大鼠 TPO 的活性降低,總 亞 鐵 紅素卻 沒有減少,也 有被修 的 象. 以液相沒 飾 現
極 質 譜 儀
二 (LC-MS-MS)檢 測LPO活化中心亞 鐵 紅素(heme)的 化,變 結果顯示,雖然 LPO 或大鼠 TPO 的活性降低,總 亞 鐵 紅素卻 沒有 少,減 也 有被修 的 象.沒 飾 現 以放射性同位素稀 法釋 檢 測染料木 酮黃 與LPO的共價 結合 象, 以氚---現 染料木黃酮與LPO 作用,再以 HPLC 分析經 過 胰蛋白酵素分解的蛋白胜(peptide),發 現 約有 5~6個分離峰有氚---染料木 酮.再以不具黃
黃 與
放射性同位素的染料木 酮 LPO 作用, 根 氚---據 染料木 酮的分 峰保留黃 離 時 間
(retention time),收集對 應峰的分 液,離 濃 縮後以 MALDI-TOF MS 檢 測分子量,5~6個 對 應峰 分離液(保留時 間=24.5, 32, 34, 36 or 37.5 分)的分子量介於 820-3,500 Da. 保留時 間 為 24.5分的分 峰可以離 檢 測出 2,259, 2,663 及 3,350 Da 等代 物.謝 經由 算可以推計 測 2,259 = LVGYLDEEGVLDQNR (1,719) + 2 個染料木 酮 (270); 2,667(黃 分子量 算值;計 MALDI-TOFMS分子量檢 測值為 2,263) = TPDNIDIWIGGNAEPMVER (2,127為分子量 算值;計 MALDI-TOFMS分子量檢 測值為 2,123) + 2 個染料木黃酮 (270); 及 3,060 (為分子量 算計 值; MALDI-TOFMS分子量檢 測值為 3,063) =WLPAEYEDGLALPFGWTQR (2,250為分子量計算值;
MALDI-TOFMS分子量檢 測值為 2,253) + 3 個染料木黃酮 (270). .保留時 間 為 36分的分離 峰可以檢 測出 1,222 及 1,792 Da 等代 物.謝 經由 算可以推 : 1,792計 測 為 CDENPYR (982) + 3 分子的染料木 酮 (270).黃 推 染料木 酮可 上述蛋白胜片段中的論 黃 與 組氨酸(H), 苯丙 氨酸(F), 色氨酸(W) 或酪氨酸 (Y)產生共價 結合. 然而,在保留時 間 為 32, 34 或 37.5 分
離 離 無 檢 測
的分 峰分 液中 法 出蛋白胜. 以 LC-MS-MS 檢 測的 果結 發 現,保留時 間 為32, 34 或 37.5分的分 峰含有染料木離 黃酮的多聚合 ,體 這 個 結果可以解釋 這三 分 峰的放射性,基個 離 本上是由氚---染料木 酮的多聚合 所反 出 的 果.黃 體 應 來 結 本研究 一步 比進 將 對 LPO 及其他
狀 過 氫
甲 腺 氧化 酵素的氨基酸序列,探 染料木 酮 其他甲 腺 氧化 酵素共討 黃 與 狀 過 氫 價 結合的 關 係.
關 鍵 詞:大豆異 黃酮,泌乳過氧化 酵素,氫 甲 腺 氧化 酵素,狀 過 氫 共價 結合,蛋白胜片段,
氨基酸序列
Inactivation of thyroid peroxidase by isoflavone metabolites- a covalent binding study
II. ABSTRACT
The soybean has a long association with goiter in animals and humans and current promotion by soy as a nutritional aid requires a full understanding of potential adverse effects.
It was previously identified that isoflavone metabolites, genistein and daidzein, as the only anti-thyroid compounds present in soy. In this study, it was showed that genistein, daidzein and equol caused irreversible, time-dependent inactivation of bovine lactoperoxidase (LPO) and porcine thyroid peroxidase (TPO) that was dependent on H2O2-induced turnover. The kinetics was consistent with a mechanism-based inactivation and apparent dissociation constant for genistein and daidzein were 0.2 and 0.5 M, respectively. The partition ratios were estimated to be 2:1 for genistein:LPO and daidzein:LPO, respectively. Radiolabeled genistein was applied as a model to study the covalent bonding of genistein and LPO, which was concomitant to the loss of enzymatic activity and the binding was not recovered by gel filtration. The heme prothetic group of LPO and TPO was released by proteolysis and analyzed using LC with electrospray MS. Although it was observed that total heme decreased after isoflavone-mediated inactivation, no evidence for modification of LPO and rat TPO (rTPO) in which radical products derived from oxidative processing of genistein and daidzein inactivate the peroxidases by destruction of the prosthetic heme and/or effects of soy and prompted further examination of isoflavone-induced thyroid effects in vivo. Covalent binding study of3H-genistein and LPO showed that the covalent binding of genistein and LPO reached to 40 nmole/nmole in the presence of 500 nM genistein.
Peptide analysis showed that radilabeled genistein bound to 5~6 peptide fragments with molecular weights between 820 Da to 2,700 Da. MALDI-TOF MS analysis showed that fragments with molecular weight at 2259, 2663 and 3350 Daltons (peak at 24.5 min) were putatively recognized as: 2259= LVGYLDEEGVLDQNR (1719) + 2 genistein (270); 2667 (observed MW=2263) = TPDNIDIWIGGNAEPMVER (2127; OMW=2123) + 2 genistein (270);
and 3060 (OMW=3063) =WLPAEYEDGLALPFGWTQR (2250; OMW = 2053) + 3 genistein (270). Molecular weight at 1222 and 1792 Daltons were observed in the peak sample of retention time at 36 min. The fragment of 1792 Daltons was putatively recognized as: 1792 = CDENPYR (982) + 3 genistein (270). These results concluded that genistein might covalently bound to the aromatic amino acids such as histidine (H), phenylalanine (F), tryptophan (W) or tyrosine (Y). However, no major peptide related fragments were observed in the pooled samples collected from retention times at 32, 34, 36 or 37.5 min. LC-MS-MS analysis showed that these peaks might be the fractions containing genistein polymers for covalent binding study of3H-genistein and LPO showed that the these peaks were highly radioisotope labeled fractions due to the response from3H-genistein.
Key words: soy isoflavones, lactoperoxidase, thyroid peroxidase, covalent binding, peptide, amino acid sequence
III. INTRODUCTION
Previous studies considered soybean as goitrogenic in human and animals (1-6). The consumption of soy products in infant formula and vegetarian diets may become potential problems causing goiter and hypothyroidism (1-3). It was reported that genistein and daidzein inactivated TPO-catalyzed iodination and coupling reactions (6). The kinetics for inactivation of LPO by isoflavone metabolites was still not clarified. In this study, bovine LPO was used as a model to elucidate the inactivation mechanisms of bovine LPO by genistein, daidzein and equol. Thee results were applied to study the inactivation of porcine TPO and rat TPO microsomes by soy isoflavone metabolites.
IV. MATERIALS AND METHODS Chemicals.
Lactoperoxidase (LPO) (A412/A280=0.80;
412= 112 mM-1cm-1), genistein, hydrogen peroxide, guaiacol, iodoacetamide, potassium iodide were purchased from Sigma.
Tris-(2-carboxyethyl)-phosphine HCl (TECP-HCl) was purchased from PIERCE (Rockford, IL).
Pronase, trypsin, endoproteinasews Lys-C, Glu-C, and deglycosidase F were purchased from Boehringer Mannheim GmbH (Mannheim, Germany). 3H-genistein (25~30 Ci/mmol) was purchased from SibTech. Co. (Elmsford, NY). Daidzein was purchased from Toronto Research Chemical Co. (North York, Ontario, Canada), and equol was purchased from Indofine Chemical Co. (Somerville, NJ). Thyroid peroxidase was prepared with modification as described (6).
Inactivation of LPO by isoflavones and H2O2.
LPO (0.2 M) was incubated with various concentrations of genistein (10 nM to 1 M), daidzein (5 nM to 500 nM) or equol (10 nM to 1 M) in the presence of 100 M H2O2. This reaction was initiated by H2O2and incubated for 12 minutes. Aliquots of 100lwere removed at 15 seconds and 2 minutes intervals and were assayed for remaining guaiacol oxidation activity. The guaiacol assay contained 5 mM guaiacol, 500 M H2O2in 0.1 M potassium phosphate buffer, pH 7.0. Assays were monitored at 470 nm using HP 8452A diode array spectrophotometer (Hewlett Packard). LPO with same concentrations were also incubated with 1 M genistein, daidzein or equal for 12 minutes to compare the % remaining guaiacol oxidation activity in the presence of 100 M H2O2. KI (100 M) was also added to the mixtures contained 1M genistein and 100 M H2O2to study the recovery of oxidation rate by KI.
Inactivation of microsomal TPO by isoflavones and H2O2.
Microsomal TPO (0.2M) was incubated with 1 M genistein, daidzein or equol. The reactions were initiated by 250 nM H2O2. Percent of remaining activity was monitored by using guaiacol assay as mentioned above.
Determination of kinetic parameters for inactivation of LPO by genistein, daidzein or equol
LPO (0.2 µM) was incubated for 12 min at room temperature with 100 µM H2O2 and various concentrations of genistein (10 nM ~ 1 µM) or daidzein (5 nM ~ 500 nM) in a final volume of 1 ml to assess inactivation of LPO by genistein or daidzein during turnover.
Aliquots of 100 µl were removed at 15 seconds and 2-minute intervals and were assayed for
remaining activity using the guaiacol oxidation assay. Guaiacol assay mixtures contained 5 mM guaiacol, 500 µM hydrogen peroxide in 0.1 M phosphate buffer, pH 7.0. Assays were monitored at 470 nm using a HP 8452A diode array spectrophotometer. Extrapolation of the linear portion to the abscissa gives the approximate value of partition ratio. Half times were plotted against the reciprocal of genistein, daidzein or equol concentration. Kiis the negative reciprocal of the intercept on the abscissa; t
1/2is the intercept on the ordinate.
Covalent binding of 3H-labeled genistein to LPO.
LPO (0.26 M) was incubated with 10 M genistein (with 0.5 nmol 3H-genistein, ~60 pmol; 25~30 Ci/mmol), and various concentrations of H2O2(H2O2/LPO = 0, 2.5, 5, 10, 25, 50, and 100) for 30 minutes at room temperature. Aliquots of 100 µl were removed to measure radioactivity after gel filtration. The incubation mixtures were filtered by using PD-10 G 25 columns. Fractions from 1~6 ml were collected in 20 ml liquid scintillation vials. Twenty ml of scintillation fluid (Ultra Gold, Packard) was added and radioactivity was measured by liquid scintillation spectrometry. Radioactivity present was used to calculate the amount of 3H-genistein bound covalently bound to LPO.
Determination of heme by HPLC and LC/MS.
One mg of LPO was dissolved in 0.1 M potassium phosphate buffer, pH 7.0, with or without 650M genistein. The reaction was initiated by adding various concentrations of H2O2
(H2O2/ LPO = 0, 1,5,10, and 50) and the reaction mixture was incubated at room temperature for 30 minutes. The remaining activity of each sample was monitored by guaiacol assay after gel filtration. Following chromatography on a PD-10 column equilibrated in potassium phosphate buffer, pH 7.0, pronase (0.25 mg/mg enzyme) was added to protein containing fractions which were then incubated at 37 oC overnight. Heme and heme derivatives were analyzed by HPLC using a Vydac C18, 4.6 x 250 mm column. The solvent system consisted of 0.1% formic acid in acetonitrile (solution A) and 0.1% formic acid in water (solution B).
Elution was initiated using a mobile phase consisting 20% A and 80% B for 2 minutes followed by a linear gradient to 50% A and 50% B. Isocratic elution in 50% A and 50% B continued for 3 minutes. The flow rate was 1 ml/min. Elution was monitored at 400 nm.
Samples were also analyzed by LC/MS using the same column and mobile phase. Mass determinations were performed using a platform single quadrupole mass spectrometer (Micromass, Altrincham, U.K.) equipped with an APCI interface. The mass spectrometer was operated at capillary voltage 3.50 kV, HV lens voltage 0.45 kV, cone voltage 40~110 V. The source temperature was 150oC and scanning was performed over the range of m/z 300-1150 at a rate of 1.49 sec/scan for positive ion acquisition.
LPO (0.26M) was also incubated with 10 M genistein (with 0.5 nmol3H-genistein), and various concentrations of H2O2(H2O2/LPO = 0, 2.5, 5, 10, 25, 50, and 100) in a total volume of 1 ml for 30 minutes at room temperature. Aliquots were removed to measure radioactivity and guaiacol oxidation activity after gel filtration. Radioactivity present was used to calculate the amount of 3H-genistein bound covalently bound to LPO. Inactivated LPO was calculated according to the loss of guaiacol oxidation activity.
Covalent binding of heme and3H-genistein were also analyzed by incubating 1 mg LPO with 100M genistein and ~60 nM3H-genistein in the presence of 750M H2O2for 30 minutes at room temperature. The mixtures were then digested with pronase for overnight at 37oC after gel filtration by using PD-10 G 25 column. Samples were analyzed by HPLC mentioned
above. Fractions were collected at one-minute intervals in scintillation vials containing 8ml scintillation fluid (Ultra Gold, Packard) and radioactivity was determined by Liquid Scintillation Spectrometry.
Peptide digestion and covalent binding of3H-genistein with peptides.
One mg of LPO was incubated with or without 650 M genistein in the presence of 650
M H2O2in 0.1 M phosphate buffer, pH 7.0 at room temperature for 30 minutes. The reaction mixtures were passed through PD-10 columns equilibrated with the same buffer. One-ml fractions were collected. Fractions from 4~6 ml containing the large molecules, were collected and pooled. Two ml of a buffer containing 0.1 M Tris-HCl, 6M guanidine, 0.2 mM EDTA, pH 8.0 and 1.0 mg TECP-HCl were added to the collected fractions which were then incubated at 45oC. After one hr incubation, 100 mg iodoacetamide was added to the mixture and incubation was continued for another one hr at 45oC. The mixture was then dialyzed (Spetra/Por MWCO: 6-8,000; The Spectrum Co., Gardena, CA) against double deionized water for 4 hr and then against 0.1 M Tris-acetate buffer, pH 8.0 overnight. The dialyzed solution was incubated with one unit of deglycosidase F at 37oC for 4 hr then with trypsin (trypsin: protein 1:30), 0.5 units endoproteinase Lys-C, and 5 units Glu-C at 37oC overnight.
After enzymatic digestion, the peptide solution was analyzed by HPLC.
HPLC of digested native or inactivated enzyme (injection volume = 200l alquots) was achieved on an Aquapore BU-300 7 µ, 0.4 x 25 cm column (Perkin Elmer). The solvent system contained solvent A (0.1% formic acid in H2O) and solvent B (0.1% formic acid in acetonitrile). Chromatography was initiated with a mobile phase of 90% A and 10% B for 2 minutes followed by a linear gradient to 25% A and 75% B over 50 minutes. This was followed by isocratic elution for 5 minutes in 25% A and 75% B. The flow rate was 1.0ml/min. Elution was monitored at 215 nm and 255 nm.
LPO (1 mg) was incubated with 650M genistein, ~60 nM3H-genistein and 650M H2O2
in 0.1 M phosphate buffer, pH 7.0 at room temperature for 30 minutes as described above.
The mixture was digested and analyzed by HPLC also as described above. Fractions were collected at one-minute intervals in scintillation vials containing 8 ml scintillation fluid (Ultra Gold, Packard) and radioactivity was determined by Liquid Scintillation Spectrometry
MALDI-TOF MS analysis for genistein-bound LPO fragments after digestion
LPO (1mg) was incubated with 650 M genistein and 650 M H2O2 in 0.1 M phosphate buffer, pH 7.0 at room temperature for 30 minutes as described above. The mixture was digested and then aliquots of 200 l sample preparations were analyzed by HPLC and the digested fragments were collected according to the co-elution peaks showed on previous study. The peaks with retention times at 24.5, 32, 34, 36, 37.5 and 40 min were collected and pooled after 20 injections. The pooled samples were then dried with a speed vacuum system.
The dried samples were applied in a Vestec model YM200 (Vestec Inc., Houston, TX, USA) MALDI-TOF MS for analysis (7). Mass assignments were made using a commercial software package (Gram 386, Galactic, Salem, NH). The mass accuracy was estimated to be + 5 Da.
Only the major peaks that corresponded to peptide fragments were assigned. Not all peaks were interpreted.
V.RESULTS AND DISCUSSION
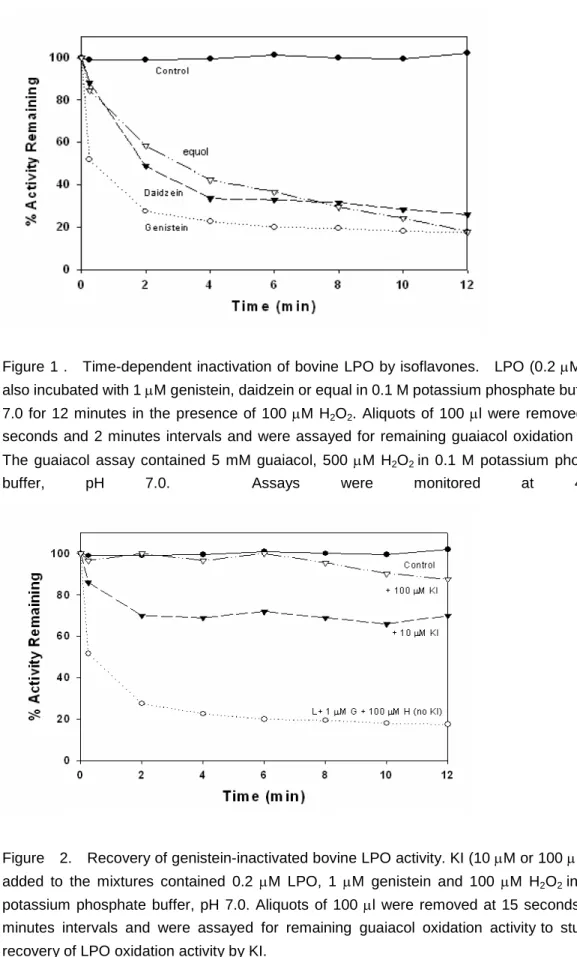
The inactivation of bovine lactoperoxidase (LPO) by genistein, daidzein and equol were
time-dependent (Fig. 1). The inactivation of LPO mediated by 1 M genistein was ~90%
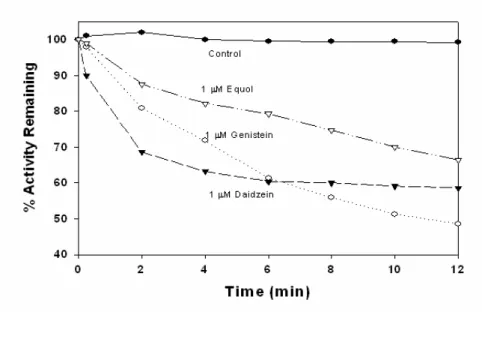
recovered by 100 M KI after 12 min incubation (Fig. 2). These data showed that in the presence of KI which might be a competitor for genistein for LPO inactivation. In the presence of 250 M H2O2, LPO activity was reduced to ~60% by 10 nM genistein, 1 M daidzein or M equol after 12 min incubation. However, 1M genistein had only ~50%
inactivation for porcine TPO under the same condition (Fig. 3). Partition ratio studies for genistein, daidzein and bovine LPO showed that the Ki of genistein and daidzein for LPO were 200 nm and 500 nM. The kinact of genistein and daidzein for LPO were 1.38 sec-1and 0.92 sec-1 respectively. The heme prothetic group of LPO and TPO was released by proteolysis and analyzed using LC with electrospray MS. Although it was observed that % of total heme did not decrease after isoflavone-mediated inactivation, no evidence for modification of LPO (Fig. 4) in which radical products derived from oxidative processing of genistein and daidzein inactivate the peroxidases by destruction of the prosthetic heme and/or effects of soy and prompted further examination of isoflavone-induced thyroid effects in vivo (7-10). Titration covalent binding study of3H-genistein and LPO showed that the covalent binding of genistein and LPO reached to 40 nmole/nmole in the presence of 500 nM genistein (data not shown).
These results implied that inactivation of LPO by genistein was not in the heme moiety as other LPO inactivator (12)
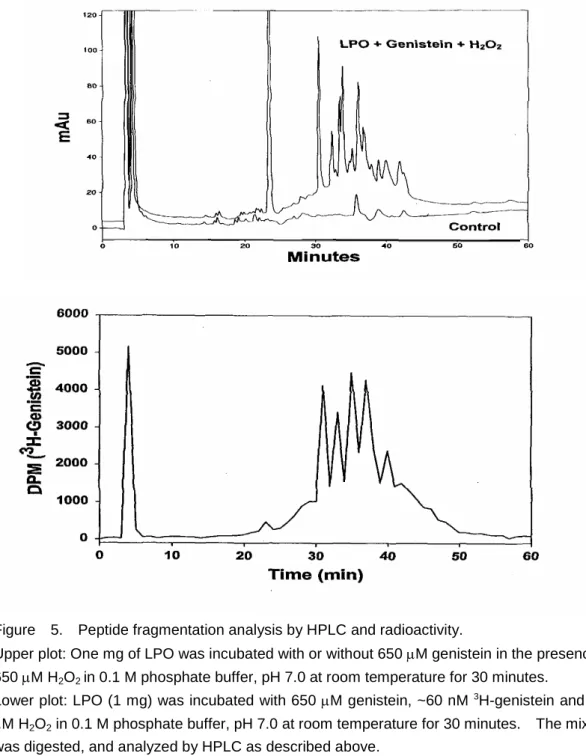
Peptide fragmentation analysis by HPLC and radioactivity showed that after enzymatic digestion with trypsin, endoproteinase Lys-C, and Glu-C at 37oC overnight, five peaks (RT=24.5, 32, 34, 36, 37.5 and 40 min) after peptide fragmentation were found with
3H-genistein bound (Fig. 5). LC/MS/MS analysis also confirmed that various molecules of genistein were found in these peaks (data not shown). MALDI-TOF MS analysis showed that fragments with molecular weight at 2259, 2663 and 3350 Daltons (peak at 24.5 min). These fragments were putatively recognized as: 2259= LVGYLDEEGVLDQNR (1719) + 2 genistein (270); 2667 (observed MW=2263) = TPDNIDIWIGGNAEPMVER (2127; OMW=2123) + 2 genistein (270); and 3060 (OMW=3063) =WLPAEYEDGLALPFGWTQR (2250; OMW = 2053) + 3 genistein (270). Molecular weight at 1222 and 1792 Daltons were observed in the peak sample of retention time at 36 min. The fragment of 1792 Daltons was putatively recognized as:
1792 = CDENPYR (982) + 3 genistein (270) (Table 1). However, no major peptide related fragments were observed in the pooled samples collected from retention times at 32, 34, 36 or 37.5 min. LC-MS-MS analysis showed that these peaks might be the polymers of genistein (data not shown). From these results observed in this study, it is concluded that genistein and daidzein are suicide inactivators for bovine LPO and porcine TPO. Genistein is more potent than daidzein and equol for the inactivation of bovine LPO and porcine TPO. KI is able to reverse genistein-inactivated LPO guaiacol oxidation activity. Loss of LPO guaiacol oxidation activity is due to covalent binding of genistein to LPO. Bis-hydroxymethyl heme of LPO was not affected by genistein during turnover, also no evidence to show the covalent binding of genistein adducts with heme adducts. 3H-genistein covalently bound to peptide fragments of LPO during turnover, thus the covalent binding of genistein to LPO is responsible for LPO inactivation. Polymers of genistein may be formed in the incubation of genistein and LPO in the presence of hydrogen peroxide.
Figure 1 . Time-dependent inactivation of bovine LPO by isoflavones. LPO (0.2M) were also incubated with 1M genistein, daidzein or equal in 0.1 M potassium phosphate buffer, pH 7.0 for 12 minutes in the presence of 100 M H2O2. Aliquots of 100 l were removed at 15 seconds and 2 minutes intervals and were assayed for remaining guaiacol oxidation activity The guaiacol assay contained 5 mM guaiacol, 500 M H2O2in 0.1 M potassium phosphate
buffer, pH 7.0. Assays were monitored at 470nm.
Figure 2. Recovery of genistein-inactivated bovine LPO activity. KI (10M or 100 M) was added to the mixtures contained 0.2 M LPO, 1 M genistein and 100 M H2O2in 0.1 M potassium phosphate buffer, pH 7.0. Aliquots of 100 l were removed at 15 seconds and 2 minutes intervals and were assayed for remaining guaiacol oxidation activity to study the recovery of LPO oxidation activity by KI.
Figure 3. Inactivation of porcine TPO (pTPO) by isoflavones. pTPO (0.2M) was incubated with 1M genistein, daidzein or equol, and 250 nM H2O2. Aliquots of 100lwere removed at 15 seconds and 2 minutes intervals to analyze the inactivation rate of porcine TPO by isoflavones. Percent of remaining LPO oxidation activity was monitored by using guaiacol assay as mentioned in Figure 1.
Figure 4. Analysis of percent heme content, and percent guaiacol activity for genistein-inactivated LPO. LPO (13 M) was incubated with or without 650 M genistein, and various concentrations of H2O2 (H2O2/ LPO = 0, 1, 5, 10, and 50). After incubation at room temperature for 30 minutes, reaction mixtures were analyzed for guaiacol oxidation activity (as mentioned in Figure 1) after gel filtration. Following gel filtration, protein fractions were digested by pronase, and heme and heme derivatives were analyzed by HPLC
Figure 5. Peptide fragmentation analysis by HPLC and radioactivity.
Upper plot: One mg of LPO was incubated with or without 650M genistein in the presence of 650M H2O2in 0.1 M phosphate buffer, pH 7.0 at room temperature for 30 minutes.
Lower plot: LPO (1 mg) was incubated with 650M genistein, ~60 nM 3H-genistein and 650
M H2O2in 0.1 M phosphate buffer, pH 7.0 at room temperature for 30 minutes. The mixture was digested, and analyzed by HPLC as described above.
Table 1. Assignments of peptide fragments from MALDI-TOF MS analysis
Retentiom time (min)
Peptide fragments (Da)
Assignments
(amino acid sequences + genistein) 2,259 LVGYLDEEGVLDQNR (1719) + 2 genistein (270) 2,267 (OMW=2,263) TPDNIDIWIGGNAEPMVER (2,127; OMW=2,123) + 2
genistein (270) 24.5
3,060 (OWM=3,063) WLPAEYEDGLALPFGWTQR (2,250; OMW = 2,053) + 3 genistein (270)
36 1,222 N/A
1,792 1792 = CDENPYR (982) + 3 genistein (270) 32, 34 or
37.5 min.
N/A N/A
OWM:Molecular weight observed under MALDI-TOF MS analysis
VII. REFERENCES
1. C. Irvine, M, Fitzpatrick, Robertson, I, and Woodhams, D. 1993. The potential adverse effects of soybean phytoestrogens in infant feeding (letter). N Z Med J 108:208-209.
2. R. McCarrison. 1933. The goitrogenic action of soybean and ground nut. Indian J. Med. Res.
21:179-181.
3. S. Kimura, J. Suwa, M Ito,.and H. Sato. 1976. Development of malignant goiter by defatted soybean with iodine-free diet in rats. Gann. 67:763-769.
4. D. Van Dyke, M.B. Arnold, and J. P. Wynn. 1959. The effects of a soybean product on thyroid function in humans. Pediatrics 24:752-760.
5. T. H. Sherpard, G. E. Ryne, J. P. Kirschvink, and M. Mclean. 1960. Soybean goiter. N. Engl. J.
Med. 262:1099-1103.
6. R. L. Divi, H.C. Chang and D. R. Doerge. 1997. Anti-thyroid isoflavones from soybean: isolation, characterization, and mechanisms of action. Biochem. Pharm. 54:1087-1096.
7. H. C. Chang, M. I. Churchwell, K. B. Delclos, and D. R. Doerge. 2000. Mass spectrometric determination of genistein tissue distribution in Sprague Dawley rats from dietary exposure. J.
Nutrition 130:1963-1970.
8. D. R. Doerge, H. C. Chang, M. I. Churchwell, and C. L. Holder. 2000. Analysis of soy isoflavone conjugation in vitro and in human blood using LC/MS. Drug Metab. D. 28(3):298-307.
9. D. R. Doerge, H. C. Chang and R. L. Divi. 1998. Inhibition of thyroid peroxidase-catalyzed reactions by the major metabolite of the antifungal dye, malachite green. Chem. Res. Toxicol. 11: 1098-1104.
10. D. R. Doerge and H. C. Chang. 2002. Inactivation of thyroid peroxidase by soy isoflavones, in vitro and in vivo. J Chroma. B 777:269-279.
11. B. L. Dillimngham, B. L. McVeigh, J. W. Lampe and A. M. Duncan. 2005. Soy protein isolates of varying isoflavone content exert minor effects on serum reproductive hormones in healthy young men. J. Nutr. 135:584-591.
12. H. C. Chang, R. Holland, M. I. Churchwell, J. A. Bumpus and D. R. Doerge. 1999. Inactivation of Coprinus cinereus peroxidase by 4-chloroaniline during turnover: Comparison with horseradish peroxidase and bovine lactoperoxidase. Chem.-Bio. IN. 123(3):197-217.