國立臺灣大學醫學院臨床藥學研究所 碩士論文
Graduate Institute of Clinical Pharmacy College of Medicine
National Taiwan University Master Thesis
靜脈血栓栓塞症於癌症病人之流行病學與治療現況 Epidemiology and Clinical Profile of Venous
Thromboembolism in Cancer Patients
周丹薇 Tan-Wei Chew
指導教授:蕭斐元 博士 高純琇 博士
Advisor: Fei-Yuan Sharon Hsiao, Ph.D.
Churn-Shiouh Gau, Ph.D.
中華民國 101 年 7 月
July, 2012
謝辭
兩年的臨藥所生活就這樣過去了,到現在還是覺得有點難以置信,過去常常 說很累很煩不想寫論文的研究生生活現在倒開始懷念起來了。
能安然地渡過這兩年,要感謝的人實在太多了。感謝凍齡美鵲娘-蕭斐元老 師花了那麼多心力和時間指導我們,不管在學術上還是生活上老師都不遺餘力地 給予我們支持與幫助,在我們徬徨無助時幫我們導向正途。老師當新手媽媽已經 夠忙了,還要照顧我們這兩個天然呆的學生,真的辛苦了。感謝高純琇老師在忙 碌的業務之餘,還給予我研究方法上的指導,讓我的論文架構與分析結果更加全 面。如果沒有老師當初的建議,我現在就要擔心我的結果是不是就沒有甚麼發表 的價值了。感謝溫有汶老師在背後的支持,讓我可以無後顧之憂地專注於研究上。
感謝沈麗娟老師抽空來參加我的論文口試,提點我在研究上應該注意的關鍵與盲 點。感謝老師們的建議與指導,讓我可以順利地完成我的碩士研究。
感謝我的最佳夥伴林婉婷,你真的太重要了!當初你在和信抄病歷的時候,
我一個人在 1222 每天都在期待你抄完病歷回來台大。在寫論文的過程中,因為有 你在身邊一起努力,我才能靜下心來繼續奮鬥。謝謝妳常常聽我 murmur 有的沒的,
跟我分享生活中的大小事,當我遇到瓶頸時總是放下手邊的事情來幫助我。感謝 小喵總是讓我們隨 call 隨問,還提醒了我們很多論文與口試中需要注意的事項,
即使你在被期中報告轟炸的時候還擠出時間幫我們審稿和 rehearsal,真的太感謝你 了。也要感謝新加入天然呆家族的欣諄,在口試前夕給了我們很多寶貴的意見,
還請我們吃好吃的手工餅乾,陪我們渡過口試前的焦慮期。
感謝同窗兩年的阿邵、笑點、阿穴、小花、品慧、Joke、以雯和小餅乾,真的 很開心可以跟你們成為同學。跟你們一起吃飯、講垃圾話、看電視、旅遊的時光 是我研究所中珍貴的回憶,也是這之中最重要的調劑!因為有你們在,我的研究 生生活在苦悶之餘也能充滿歡笑。記得欣諄也說過我們在口試前還能嘻嘻哈哈的 真的蠻難得的。感謝經常幫我們處理大小事務的翊吟。謝謝你們所有人這麼照顧 常常犯呆的我,我會很想念你們的!真的很感謝你們在我離開前送我的卡片,我 看到後來感動地都快哭了,我會把它當作寶貝好好珍惜的。最後也要感謝我的家 人和權峰,謝謝你們在這段時間給我的支持和鼓勵,這是我繼續努力下去的動力。
短短兩年的臨藥所生活真的是充滿了苦痛與歡笑,雖然一開始在台北生活真 的很不習慣覺得很寂寞,但是我很慶幸自己可以成為臨藥所的一員,認識這麼棒 的你們。謝謝你們!
i
中文摘要
研究背景 靜脈血栓栓塞症(venous thromboembolism)對癌症病人的死亡率和疾病 狀況的顯著影響,已是重要的臨床的問題。以往的研究顯示,不同種族的靜脈血 栓栓塞症發生率不同。雖然在西方國家對靜脈血栓栓塞症的預防已有完整的治療 指引可遵循,但是在亞洲國家,有關靜脈血栓栓塞症的流行病學資料卻仍然有限。
研究目的 本群體觀察性研究(population-based observational study)的目的乃為建 立臺灣癌症病人發生靜脈血栓栓塞症的流行病學資料,分析發生靜脈血栓栓塞症 的危險因子,並探討其臨床特徵和目前國內的治療現況。
研究方法 本研究以臺灣健保資料庫 2000 年、2005 年和 2010 年三套百萬人承保 抽樣歸人檔為資料來源,找出 2001 年至 2008 年住院主診斷為癌症的新診斷癌症 病人,並以初次住院主診斷為癌症的入院日期為 index date。在 index date 的住院 主診斷有兩個(含)以上的癌症的病人,則被排除在研究之外。本研究的研究終點為 index date 當天或之後因靜脈血栓栓塞症而住院。本研究共有兩個靜脈血栓栓塞症 的定義:定義一為住院檔中出現靜脈血栓栓塞症的診斷,定義二則為住院檔中同 時有靜脈血栓栓塞症診斷及靜脈血栓栓塞症的治療。本研究針對所有癌症病人和 不同癌症部位的靜脈血栓栓塞症的發生率,及針對發生靜脈血栓栓塞症和沒有發 生靜脈血栓栓塞症的病人在年齡、性別、共病症和可能危險因子,連續變項使用 t-test,類別分項使用 Chi-square 或 Fisher’s exact test 進行分析。針對以定義二而住 院的病人,則使用邏輯迴歸模型(logistic regression model)進行分析,找出發生靜脈 血栓栓塞症的危險因子,並探討靜脈血栓栓塞症長期治療的狀況、復發率與發生 出血性副作用的機率。
研究結果 在 43,855 名新診斷癌症病人中,1388 名(3.2%) (定義一)和 473 名(1.1%) (定義二)病人在 index date 當天或之後因靜脈血栓栓塞症而入院。靜脈血栓栓塞症 的發生率分別為 9.9 每 1,000 人年(1.0 – 68.2 每 1,000 人年) (定義一)和 3.4 每 1,000 人年(0.0 – 16.1 每 1,000 人年) (定義二)。靜脈血栓栓塞症發生率較高的癌症包括:
ii
肝臟、胰臟、肺臟、多發性骨髓瘤(multiple myeloma)、肉瘤(sarcoma)和非何杰金 氏淋巴瘤(non-Hodgkin’s lymphoma)。靜脈血栓栓塞症(定義一)在 index date 30 天、
90 天、180 天和 365 天內的累積發生率(cumulative occurrence)分別為 42.9%、53.5%、
61.7%和 70.8%。靜脈血栓栓塞症(定義二)在 index date 30 天、90 天、180 天和 365 天內的累積發生率分別為 25.2%、39.8%、47.8%和 59.4%。靜脈血栓栓塞症的顯著 危險因子包括癌症部位、之前有發生靜脈血栓栓塞症的病史、動脈栓塞症(arterial embolism)、肥胖(obesity)、高血壓、風濕性疾病、接受化學治療、合併治療和胸腔、
腹部或泌尿生殖道大手術。三個月內的輸血治療與靜脈血栓栓塞症風險的降低有 關。在 1,388 位因靜脈血栓栓塞症(定義一)住院的病人中,只有 33.6%的病人(n=467) 在當次住院中有接受抗凝血劑的治療或接受栓塞切除術。在 473 位因靜脈血栓栓 塞症(定義二)住院的病人中,1.5%的病人(n=7)接受栓塞切除術,其他病人則接受 肝素(heparin)或低分子量肝素(low molecular weight heparin)作為靜脈血栓栓塞症的 初期治療。81 個病人(19.5%)在第一次靜脈血栓栓塞症後有復發現象。在 415 個存 活病人中,266 個病人(64.1%)有接受靜脈血栓栓塞症的長期治療,其中 72.2%的病 人(n=192)接受 warfarin 作為長期治療靜脈血栓栓塞症的藥物。長期治療的時間長 度中位數為 66 天,其中大約三分之二(58.7%, n=156)的病人的治療時間長度小於或 等於 3 個月。
結論 雖然臺灣的癌症相關靜脈血栓栓塞症的發生率比高加索族群的發生率來的 低,但其發生率比一般亞洲族群(general population)要高得多,尤其是在特定的癌 症如:多發性骨髓瘤、胰臟癌、肝癌和肺癌。大部分的靜脈血栓栓塞症發生在癌 症新診斷後一年內。在臺灣,目前的治療現狀與國外的臨床治療指引並不一致。
靜脈血栓栓塞症的治療和預防可能需要更加完善,特別是針對發生靜脈血栓栓塞 症危險性較高的病人。
關鍵詞 靜脈血栓栓塞症、癌症、流行病學、治療現狀、危險因子、全民健康保 險研究資料庫
iii
Abstract
Background Venous thromboembolism (VTE) is an increasing clinical problem in
cancer patients that results in significant mortality and morbidity. Reports indicated that
the incidence of VTE varies among different ethnic populations. Although the clinical
guidelines for the prevention of VTE have been suggested in Western countries, the
understanding of the epidemiology of VTE in Asian countries remains limited.
Objectives The goal of this population-based observational study is to explore the
epidemiology of VTE among cancer patients in Taiwan, analyze the risk factors for
VTE and describe the clinical characteristics and treatment pattern of VTE
Methods Using three sets of longitudinal health insurance database (LHID 2000, LHID
2005 and LHID 2010), we identified newly diagnosed cancer patients who have been
hospitalized with a primary diagnosis of malignant disease between 2001 and 2008. The
date when the patient was first hospitalized with a primary diagnosis of malignant
disease was defined as the index date. Patients had more than one primary diagnosis of
malignant diseases at index date were excluded. Primary endpoint of our study was
hospital admission for VTE during or after index date. Two definitions of VTE were
adopted in our study. VTE definition 1 was based on VTE diagnosis codes in the
inpatient medical claims. VTE definition 2 was based on both the VTE diagnosis codes
and management of VTE. The incidence rates of VTE for the entire study cohort and
iv
subgroups of patients categorized by sites of cancer were estimated. Differences in age,
gender, comorbidities, and potential risk factors for VTE between patients with and
without VTE events were analyzed. We use t-test for continuous variables and
Chi-square analysis or Fisher’s exact test for discrete variables. Only patients who
hospitalized with VTE (definition 2) were included in the logistic regression model to
identify the risk factors for VTE. We also describe the long-term treatment pattern of
VTE and incidence rates of recurrent VTE and bleeding complications.
Results Among 43,855 newly diagnosed cancer patients, 1388 (3.2%) (definition 1) and
473 (1.1%) (definition 2) patients were hospitalized for VTE during or after index date.
The incidence rates of VTE (definition 1 and definition 2) were 9.9 per 1,000
person-years (1.0-68.2 per 1,000 person-years) and 3.4 per 1,000 person-years (0.0-16.1
per 1,000 person-years), respectively. The incidence rates were higher in certain cancers,
particularly cancer of liver, pancreas, lung, multiple myeloma, sarcoma, and
non-Hodgkin’s lymphoma. The cumulative occurrence of VTE (definition 1) within 30,
90, 180, and 365 days after index date were 42.9%, 53.5%, 61.7%, and 70.8%,
respectively. Cumulative occurrence of VTE (definition 2) within 30, 90, 180, and 365
days after index date were 25.2%, 39.8%, 47.8% and 59.4%, respectively. Significant
risk factors for VTE were site of cancer, prior history of VTE, arterial embolism, obesity,
hypertension, rheumatologic diseases, chemotherapy, combination therapy and major
v
thoracic, abdominal or urogenital surgery. In contrast, blood transfusion within 3
months was significant associated with reduced risk of VTE. Among 1388 patients who
hospitalized with VTE (definition 1), only 33.6% of patients (n=467) received
anticoagulant therapy or thromboectomy during the hospitalization. Among 473 patients
who hospitalized with VTE (definition 2), 1.5% of patients received thromboectomy,
other patients received heparin or low molecular weight heparin for initial treatment of
VTE. Eighty-one patients (19.5%) had recurrent VTE after the first VTE event. Of 415
survived patients, long-term anticoagulant therapy was initiated in 266 patients (64.1%),
72.2% of them (n=192) received warfarin alone. The median duration was 66 days.
Approximately two-thirds of patients (58.7%, n=156) received ≤ 3 months of long-term
anticoagulant therapy.
Conclusions Although the incidence of cancer-related VTE among Taiwanese is lower
than Caucasians populations, it is much higher than Asian general populations,
particularly in patients with certain cancers such as multiple myeloma, pancreas, liver,
and lung cancer. Most VTE occurred within 1 year after cancer diagnosis. Adherence to
treatment guidelines was poor in Taiwan. Treatment and prophylaxis of VTE should be
optimized, especially in patients with higher-risk of VTE.
Keywords Venous thromboembolism, cancer, epidemiology, clinical profile, risk factors,
National Health Insurance Research Database
vi
Contents
Chapter 1 Introduction ... 1
Chapter 2 Literature Review ... 3
2.1 Venous Thromboembolism ... 3
2.1.1 Overview of Venous Thromboembolism ... 3
2.1.2 Epidemiology of Venous Thromboembolism ... 3
2.1.3 Risk Factors for Venous Thromboembolism ... 5
2.1.4 Complications of Venous Thromboembolism ... 7
2.1.5 Clinical Presentations of Venous Thromboembolism ... 8
2.1.6 Diagnosis of Venous Thromboembolism ... 8
2.2 Venous Thromboembolism in Patients with Cancer ... 10
2.2.1 Overview ... 10
2.2.2 Epidemiology of Cancer-related VTE ... 10
2.2.3 The Pathogenesis of VTE in Cancer ... 11
2.2.4 Risk Factors for Cancer-related VTE ... 14
2.2.5 Consequences of Cancer-related VTE ... 17
2.2.6 Treatment of Venous Thromboembolism in Cancer Patients ... 18
2.2.7 Prevention of Venous Thromboembolism in Cancer Patients ... 25
2.2.8 Venous Thromboembolism in Asian Patients with Cancer ... 29
vii
Chapter 3 Study Objective ... 32
Chapter 4 Materials and Methods ... 33
4.1 Data Source ... 33
4.2 Study Population and Study Outcomes ... 34
4.2.1 Study Cohort ... 34
4.2.2 Primary Endpoint – Hospital Admission for VTE ... 36
4.2.3 Baseline Characteristics and Comorbid Diseases ... 39
4.2.4 Potential Risk Factors for VTE ... 39
4.2.5 Treatment Pattern of VTE ... 41
4.2.6 Recurrence of VTE and Bleeding Complications ... 43
4.3 Statistical Analysis ... 44
4.3.1 Incidence Rate of VTE ... 44
4.3.2 Descriptive Analysis ... 44
4.3.3 Logistic Regression Analysis ... 45
4.3.4 Statistical Software... 45
Chapter 5 Results ... 47
5.1 Study Cohort ... 47
5.2 Patient Characteristics of Study Cohort... 49
5.3 Incidence Rate and Clinical Characteristics of VTE ... 50
viii
5.3.1 Incidence Rate of VTE ... 50
5.3.2 Clinical Characteristics of VTE Events ... 54
5.4 Risk Factors for VTE ... 57
5.4.1 Baseline Characteristics, Comorbid Diseases and Potential Risk Factors ... 57
5.4.2 Multivariate Logistic Regression – Risk Factors for VTE ... 65
5.5 Treatment Pattern of VTE ... 67
5.5.1 Initial Treatment of VTE ... 67
5.5.2 Long-term Treatment of VTE ... 69
5.6 Recurrence of VTE and Bleeding Complications ... 71
5.6.1 Recurrence of VTE ... 71
5.6.2 Bleeding Complications ... 72
Chapter 6 Discussion ... 73
6.1 Baseline Characteristics of Study Cohort ... 73
6.2 Incidence Rate of VTE among Cancer Patients ... 74
6.3 Clinical Characteristic of VTE ... 79
6.4 Risk Factors for VTE ... 80
6.5 Initial Treatment Pattern of VTE ... 84
6.6 Long-term Treatment of VTE ... 86
6.7 Recurrence of VTE and Bleeding Complications ... 88
ix
6.8 Strengths of Our Study ... 91
6.9 Limitations ... 91
Chapter 7 Conclusions and Suggestions ... 93
References ... 94
Appendix ... 103
x
List of Figures
Figure 4.1 Initial and long-term treatment for VTE ... 42
Figure 4.2 Study framework - risk factors for VTE development among cancer patients
... 46
Figure 5.1 Flowchart of the population-based study ... 48
xi
List of Tables
Table 2.1 Incidence of venous thromboembolism reported in different populations ... 4
Table 2.2 Risk factors for venous thromboembolism ... 6
Table 2.3 Cohort studies of incidence of venous thromboembolism in patients hospitalized with cancer ... 12
Table 2.4 Cohort studies of incidence of venous thromboembolism in ambulatory cancer patients receiving active therapy ... 13
Table 2.5 Risk factors for venous thromboembolism in patients with malignant disease ... 16
Table 2.6 Regimens for prophylaxis/treatment of VTE in patients with cancer ... 22
Table 2.7 Recommendations for treatment of VTE in cancer patients ... 23
Table 2.8 Recommendations for prophylaxis of VTE in cancer patients ... 28
Table 2.9 Cohort studies of incidence of venous thromboembolism in patients of specified cancer site among Asian population ... 31
Table 4.1 ICD-9-CM codes of inclusion diagnosis ... 35
Table 4.2 ICD-9-CM diagnosis codes of venous thromboembolism ... 37
Table 4.3 Anatomic distribution of VTE and relevant ICD-9-CM codes ... 38
Table 4.4 List of hormone therapy ... 41
Table 4.5 Bleeding complications and relevant ICD-9-CM codes ... 43
xii
Table 5.1 Site of cancer and associated incidence rate of VTE (VTE definition 1) ... 51
Table 5.2 Site of cancer and associated incidence rate of VTE (VTE definition 2) ... 53
Table 5.3: Time-to-VTE after cancer diagnosis ... 56
Table 5.4 Anatomic distribution of VTE ... 56
Table 5.5: Baseline characteristics of the study population (VTE definition 1) ... 59
Table 5.6 Potential risk factors for development of VTE (VTE definition 1) ... 60
Table 5.7: Baseline characteristics of the study population (VTE definition 2) ... 63
Table 5.8: Potential risk factors for development of VTE (VTE definition 2) ... 64
Table 5.9 Multivariate analysis of risk factors for VTE... 66
Table 5.10 Initial treatment of VTE during the hospital admission for VTE ... 69
Table 5.11 Anticoagulants administered during long-term treatment ... 70
Table 5.12 Duration of long-term anticoagulant therapy ... 70
Table 5.13 Cumulative rates of VTE recurrence ... 71
Table 5.14 Recurrence of VTE and bleeding complications during long-term anticoagulant treatment ... 72
Table 6.1 Sensitivity analysis of risk factors for VTE ... 83
Table 6.2 Clinical studies of long-term treatment of VTE among cancer patients ... 87
1
Chapter 1 Introduction
Venous thromboembolism (VTE), including deep vein thrombosis (DVT) and
pulmonary embolism (PE), is an increasing clinical problem in cancer patients that
results in significant mortality and morbidity. Cancer patients have 4- to 7- folds higher
risk for VTE than general population, occurring in 4% to 20% of patients.1,2 Use of
anticoagulants, increased risk of VTE recurrence, bleeding complications during
anticoagulant therapy, post-thrombotic syndrome and reduced lung function due to
chronic thrombotic pulmonary hypertension further complicate the clinical management
of cancer and worsen patients’ quality of life.3,4 Besides, cancer patients who develop
VTE have a significant worse survival and this effect is more pronounced in patients
with loco-regional disease rather than distant metastatic disease.5-7
Solid tumors of gastrointestinal (GI) tract, lungs, pancreas, ovaries, and
hematological malignancies are associated with high risk of VTE, with the highest risk
occurring during the initial period after cancer diagnosis.5,9-11 Other risk factors for VTE
among cancer patients including older age, metastatic stage, prior history of VTE,
presence of indwelling central venous catheter (CVC), chemotherapy or hormone
therapy, major surgery, inherited or acquired thrombophilia, and elevated
pre-chemotherapy platelet count.1
2
The incidence of VTE in Asian population has been perceived to be lower than
Caucasian population. However, the incidence of VTE in Asian population increased
rapidly by 56% from 2004 to 2008.8 Increased awareness of physicians, easier diagnosis
of VTE, growing elderly population, and a Westernized lifestyle may contribute to this
increasing trend.9 While several nationwide epidemiologic studies had been performed
using Taiwan’s and Korea’s National Health Insurance (NHI) database to understand the
incidence and risk factors for VTE among the general population, only few in cancer
patients. 8,10,11
In addition, existing studies among Asian populations only focused on patients with
colorectal, gastric, and pancreatic cancers, diffuse large B cell lymphoma, and multiple
myeloma.12-18 Furthermore, very few Small numbers of patients were included in these
studies. Most existing studies only included patients from one medical center.12-18 Up to
know, the clinical significance of VTE among cancer patients, and the epidemiological
study of VTE across various cancer subtypes have never been conducted in Asian
populations. Given the growing incidence of cancer and elderly population, burden of
VTE is expected to be increased. An epidemiological study will help us to understand
the incidence and treatment of VTE among cancer patients and optimize the clinical
practice. Therefore, a population-based study was performed using the Taiwan’s NHI
database to understand the epidemiology and clinical profile of VTE in cancer patients.
3
Chapter 2 Literature Review
2.1 Venous Thromboembolism
2.1.1 Overview of Venous Thromboembolism
VTE refers to all forms of pathologic thrombosis occurring within the venous
circulation, represents a spectrum from simple superficial thrombophlebitis to fatal
pulmonary embolism. Most venous thrombosis occurs at the deep veins of the lower
extremities, giving rise to deep vein thrombosis (DVT). They also can occur in other
parts of body, including the veins of the upper extremities, pelvis, abdomen, and
cerebral venous sinuses. Pulmonary embolism (PE) is the most life-threatening
manifestation of VTE, which occurs when a clot dislodges from the site of formation
and embolizes into pulmonary arteries. Death from PE can occur within minutes after
the onset of symptoms, before effective treatment is given.19,20
2.1.2 Epidemiology of Venous Thromboembolism
The actual incidence of VTE is unknown because the disease is often clinically
silent. The annual incidence rate of VTE is reported to be 104-183 events per 100,000
persons in the Caucasian populations (Table 2.1).21-25 The annual incidence of VTE
increases markedly with age, from less than 5 cases per 100,000 persons under 15
year-old to 149 events per 100,000 persons over the age of 80.21
4
The prevalence of VTE varies among different ethnic cohorts. Compared to
Caucasian populations, the incidence of VTE is significantly higher among African-
American and significantly lower among Asian populations.22,26,27 Among Asian
populations, the estimated annual incidence of VTE is 14-57 per 100,000 persons (Table
2.1).8,10,28,29 The incidence ranges from 2.5 events per 100,000 person-years in those
younger than 30 years to 100 events per 100,000 person-years in those aged over 80
years.10 Although the annual incidence of VTE among Asian populations has been
perceived to be lower than Caucasian populations, it appears to be rapidly increasing.8,30
Along with rapid aging of the population, VTE is a major healthcare problem which
causing significant mortality, morbidity and healthcare resource expenditure in our
aging society.
Table 2.1 Incidence of venous thromboembolism reported in different populations Incidence per 100,000
Location Study design VTE DVT PE
America
Minnesota (Silverstein et al. 1998)21 California (only Caucasian)22 (White et al. 2005)
Worcester (Spencer et al. 2009)31
Population-based study Population-based study
Population-based study
117 104
114
48
95
69
34 Europe
French (Oger et al. 2000)24 Norway (Naess et al. 2007)25
Population-based study Cohort-study
183 143
124 93
60 50 Asia
Hong Kong (Cheuk et al. 2004)28 Singapore (Molina et al. 2009)29 Taiwan (Lee et al. 2010)10 Korea (Jang et al. 2011)8
Population-based study Population-based study Population-based study Population-based study
21 57 15.9 13.8
17
5.31
3.9
7.01
5
2.1.3 Risk Factors for Venous Thromboembolism
VTE is a multifactorial condition involving genetic and both constant and transient
acquired risk factors. In 1884, Virchow’s triad first described three primary factors
contribute to the formation of thrombosis: abnormalities in blood flow (venous stasis),
abnormalities in blood constituents (hypercoagulability), and abnormalities in the vessel
wall (vascular endothelial injury). Risk factors for VTE, include increasing age,
malignancy, prolonged immobility, major surgery, major trauma, prior VTE, chronic
heart failure, and inherited or acquired thrombophilia, alter one or more of the
components of the triad (Table 2.2).32 There is convincing evidence that the risk of VTE
increases in proportion to the number of predisposing factors.32,33
Compared with residents in the community, hospitalization without surgery or
nursing home confinement is associated with 8-folds increased risk of VTE.34
Hospitalization and nursing home residence together account for almost 60% of incident
VTE events occurring in the community, with hospitalization for medical illness and
hospitalization for surgery accounted for 22% and 24% of VTE, respectively.2
6
Table 2.2 Risk factors for venous thromboembolism Strong risk factors (odd ratio > 10)
Fracture (hip or leg) Hip or knee replacement Major general surgery Major trauma
Spinal cord injury
Moderate risk factors (odd ratio 2-9) Arthroscopic knee surgery
Central venous lines Chemotherapy
Congestive heart or respiratory failure Hormone replacement therapy
Malignancy
Oral contraceptive therapy Paralytic stroke
Pregnancy (postpartum)
Previous venous thromboembolism Thrombophilia
Weak risk factors (odd ratio < 2) Bed rest > 3 days
Immobility due to sitting (e.g. prolonged car or air travel) Increasing age
Laparoscopic surgery (e.g. cholecystectomy) Obesity
Pregnancy (antepartum) Varicose veins
Adapted from Anderson FA, Jr., Spencer FA. Risk factors for venous thromboembolism.
Circulation 2003;107:I9-16.
7
2.1.4 Complications of Venous Thromboembolism
VTE is an important worldwide healthcare burden associated with significant
morbidity and mortality. It is reported to be the third common cardiovascular causes of
death after myocardial infarction and stroke.35 The 1-week survival rate after a PE is
only 71%, and almost 25% of all cases of PE essentially present as sudden death.36 In
USA, 100,000-300,000 VTE-related deaths occur every year and PE had been declared
to be the most common preventable cause of hospital death and the significant target to
improve patient safety in hospitals.37
Recurrence of VTE is common. Despite anticoagulant therapy, about 30% of
patients develop recurrent VTE within the next ten years, with the highest recurrence
rate within the first year after their first VTE event.38,39 Men have a higher rate of
recurrence than women (relative risk of recurrent VTE: 1.6).40,41 In addition, survivors
of VTE always suffer from long-term complications, including post-thrombotic
syndrome and chronic thrombotic pulmonary hypertension. One-third to one-half of
patients with lower extremity DVT develop post-thrombotic syndrome during 20 years
of follow-up, characterized by pain and swelling, and in severe cases with venous
ulceration. These conditions can be disabling for patients and have great impact on
healthcare costs. Subsets of VTE patients require long-term anticoagulation to prevent
additional clots, which also decreases their quality of life and places them at an
8
increased risk for adverse bleeding episodes.37,38
2.1.5 Clinical Presentations of Venous Thromboembolism
The signs and symptoms of VTE are nonspecific. Furthermore, many patients with
VTE were asymptomatic. A leg DVT commonly presents with pain, erythema, and
swelling of the affected limb. Physical examination may show palpable cord, warmth,
and unilateral edema.42,43 Patients with upper extremity or neck DVT often complain
with upper extremity or head or neck swelling, erythema, and/or discomfort.44
Symptoms associated with PE depend on the degree of vascular obstruction, the
magnitude of inflammatory response, and the patient’s cardio-pulmonary reserve.
Patients may present with dyspnea, hypoxemia, tachycardia, pleuritic chest pain,
hemoptysis or even collapse with shock or pulseless electrical activity cardiac arrest.20,43
2.1.6 Diagnosis of Venous Thromboembolism
Duplex ultrasonography remains the test of choice in the investigation and
diagnosis of clinically suspected DVT. Although ultrasound is highly sensitive for the
detection of proximal DVT, it is less accurate for isolated DVT of the calf. The ideal
method, invasive venography, is used when a definitive answer is required. Newer
image modalities such as magnetic resonance venography and computerized
9
tomography (CT) scan can detect thrombosis of vessels proximal to the inguinal
ligament and intra-abdominal vessels. Another advantage of magnetic resonance
venography and CT scan is their ability to provide information about surrounding
structures that may lead to alternative diagnosis.20,42-44
Gold standard for the diagnosis of PE is pulmonary angiography, but it is an
invasive procedure that involves injection of contrast dye into pulmonary artery and
associated with 0.5% of mortality. Nowadays, CT scan has become the most commonly
used imaging test to diagnose PE. Before CT scan, ventilation-perfusion (V/Q) scan was
the first-line imaging modality of PE. Spiral CT scan can detect emboli in the
pulmonary arteries whereas V/Q scan measures the distribution of blood and air flow in
the lungs.20,42,43
10
2.2 Venous Thromboembolism in Patients with Cancer
2.2.1 Overview
VTE is a major complication of cancer and is one of the leading causes of death in
patients with cancer.1,4,45 The risk of VTE is higher in cancer patients than the general
population, especially hospitalized patients with cancer and those receiving active
therapy. In a population-based study, malignancy alone was associated with a 4-folds
increased risk of VTE, whereas the use of chemotherapy increased the risk to
6.5-folds.34 Overall, approximately 20% to 39% of VTE cases were attributable to
active malignant disease.33,46
2.2.2 Epidemiology of Cancer-related VTE
The reported incidences of VTE in cancer patients have varied widely, with
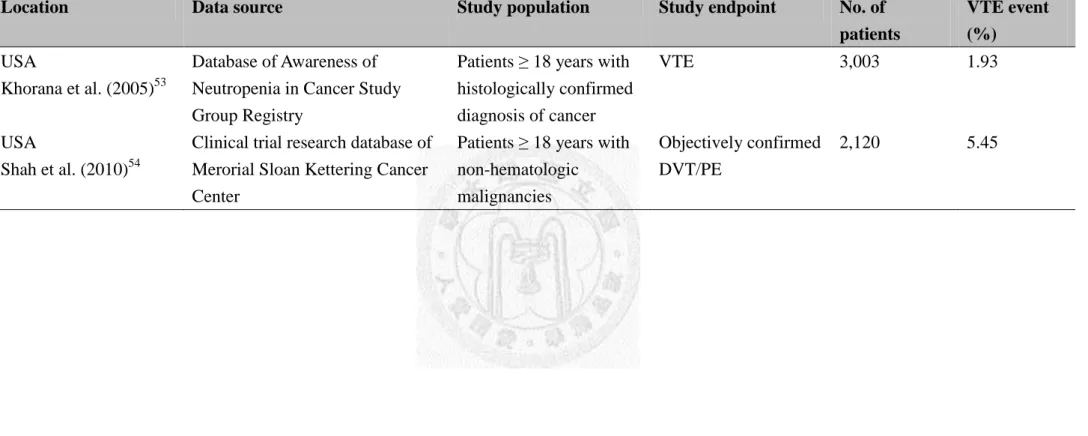
reported incidences ranging from 0.6% to 12.1% (Table 2.3 and 2.4).47-54 However, the
reported rates of VTE in cancer patients are believed to be underestimated, given that
autopsy rates of VTE can be as high as 50% compared with clinical rates of 4% to
20%.1 In addition, the burden of VTE in cancer patients is increasing. In previous study,
the rate of VTE event in cancer patients increased by 28% over the period 1995–2003 (p
< 0.0001 for the trend).51
The risk of postoperative VTE in cancer patients also exceeds that of non-cancer
11
surgical patients by 2- to 3-folds. Without anticoagulant prophylaxis, the incidence of
postoperative DVT ranges from 40% to 80%.55 In an observation study using
administrative database, the rate of VTE in cancer patients within 30 days
post-admission after major surgery was 3.5%, with ranging by procedure from 1.8 to
13.2%.56 Another prospective study, focused on postoperative clinical overt VTE,
reported an incidence of 2.1% even when in-hospital prophylaxis was given in 81.6% of
the patients. The 30-day mortality was 1.7% and VTE was adjudicated as the most
common of death (19 of 41 cases, 46.3%) in the study.57
2.2.3 The Pathogenesis of VTE in Cancer
Cancer cell may induce thrombosis by triggering several complex prothrombotic
pathways, including procoagulant effects of tissue factors expressed by tumor cells, the
release of cytokines, the inhibition of fibrinolysis, and the overexpression of membrane
adhesion molecules. Furthermore, solid tumor-mediated extrinsic vascular compression
and invasion can obstruct venous return, resulting in blood flow stasis, endothelial cell
injury, and coagulation activation. Malignancy-associated inflammation can also result
in increased concentrations of acute-phase proteins such as factor VIII, fibrinogen, and
von Willebrand factor. Elevations of these acute-phase proteins are associated with an
increased risk of thrombosis.3,58
12
Table 2.3 Cohort studies of incidence of venous thromboembolism in patients hospitalized with cancer
Location Data source Study population Study endpoint No. of
patients
VTE event (%)
USA
Levitan et al. (1999)47
Medicare database Patients ≥ 65 years with malignant disease
Hospital admission for DVT and/or PE
1,211,944 0.6
(0.16-1.20) USA
Sallah et al. (2002)48
Medical records of University of Tennessee Health Science Center, University of North Carolina at Chapel Hill, and East Carolina University
Patients with solid tumor Objectively confirmed DVT and/or PE
1,041 7.8
USA
Stein et al. (2006)49
National Hospital Discharge Survey
Patients with malignant disease
Hospital admission for DVT and/or PE
40,787,000 2.0
(0.60-4.30) USA
Khorana et al. (2006)50
Discharge database of the University Healthsystem Consortium
Adult cancer patients with febrile neutropenia
Hospital admission for VTE
5,272 5.4
(2.74-12.10)
USA
Khorana et al. (2007)51
Discharge database of the University Healthsystem Consortium
Adult patients with solid tumor
Hospital admission for VTE
1,015,598 4.1
(1.90-8.10)
Denmark
Cronin-Fenton et al.
(2010)52
Database of Danish National Registry of Patients, Danish Cancer Registry and Danish Civil Registration System
Patients ≥ 15 years with malignant disease
Hospital admission for VTE
57,591 1.8
(0.80-4.00)
13
Table 2.4 Cohort studies of incidence of venous thromboembolism in ambulatory cancer patients receiving active therapy
Location Data source Study population Study endpoint No. of
patients
VTE event (%)
USA
Khorana et al. (2005)53
Database of Awareness of Neutropenia in Cancer Study Group Registry
Patients ≥ 18 years with histologically confirmed diagnosis of cancer
VTE 3,003 1.93
USA
Shah et al. (2010)54
Clinical trial research database of Merorial Sloan Kettering Cancer Center
Patients ≥ 18 years with non-hematologic
malignancies
Objectively confirmed DVT/PE
2,120 5.45
14
2.2.4 Risk Factors for Cancer-related VTE
Despite the overall increased risk of VTE among cancer patients, VTE risk is
especially high among certain subgroups, such as hospitalized patients, those receiving
active neoplastic therapy or hormone therapy, those undergoing major surgery, and
those with metastatic disease. The risk of VTE differs across various cancer subgroups
and over the natural history of cancer. Sites of cancer with highest rates of VTE include
pancreas, stomach, brain, ovary, kidneys, lungs, and hematologic malignancies, such as
multiple myeloma and non-Hodgkin’s lymphoma. The risk of VTE is highest in the
initial period after the cancer diagnosis.1,4,5,51
Cancer patients receiving active therapy are at greater risk of VTE. In a
retrospective cohort study of 1,015,598 cancer patients, use of chemotherapy is
identified to be an independent risk factor for VTE.51 Recent study among
chemotherapy-treated patients with lung cancer found that use of chemotherapy is
associated with 30% greater risk of VTE compared with patients not receiving
chemotherapy.59 Hormone therapy (such as tamoxifen) and antiangiogenic drug (such as
bevacizumab) have been associated with an increased risk of VTE.1,60,61 Among patients
with multiple myeloma, the risk of VTE is higher in whom receiving thalidomide or
lenalidomide in combination with dexamethasone or chemotherapy. Besides,
erythropoietin-stimulating agents (ESA) are also associated with an increased risk of
15
VTE.1
Other risk factors for VTE among cancer patients include increasing age, prior
history of VTE, pre-chemotherapy platelet count ≥ 350,000 μL, the presence of
prothrombotic mutation, placement of CVC, and concomitant comorbid conditions.
Race is also a significant factor associated with VTE risk. Compared with Caucasian
patients with cancer, Asian patients with cancer have significant reduced risk of
VTE.6,7,51 A comprehensive list of risk factors associated with VTE in cancer patients is
summarized in Table 2.5.1
16
Table 2.5 Risk factors for venous thromboembolism in patients with malignant disease Patient-related factors
Older age
Race (higher in African Americans; lower in Asian-Pacific Islanders)
Comorbid conditions (obesity, infection, renal disease, pulmonary disease, arterial thromboembolism)
Prior history of VTE
Elevated pre-chemotherapy platelet count Heritable prothrombotic mutations
Cancer-related factors
Primary site of cancer (GI, brain, lung, gynecologic, renal, hematologic) Initial 3-6 months after diagnosis
Current metastatic disease Treatment-related factors
Recent major surgery Current hospitalization Active chemotherapy Active hormonal therapy
Current or recent antiangiogenic therapy (thalidomide, lenalidomide, bevacizumab*)
Current erythropoiesis-stimulating agents Presence of central venous catheters
* Bevacizumab is clearly associated with an increased risk of arterial thrombotic events; an association with venous thrombosis is not fully established.
Adapted from Lyman GH, Khorana AA, Falanga A, et al. American Society of Clinical Oncology guideline: recommendations for venous thromboembolism prophylaxis and treatment in patients with cancer. J Clin Oncol 2007;25:5490-505.
17
2.2.5 Consequences of Cancer-related VTE
VTE is associated with significant morbidity and mortality among cancer patients.
In a retrospective analysis based on California cancer registry, development of VTE
after cancer diagnosis was a significant predictor of mortality within 1 year, with hazard
ratios (HR) ranging from 1.6 to 4.2 (p < 0.01). The strongest predictor of death in that
study was metastatic disease at the time of cancer diagnosis, with an HR ranging from
1.8 to 49.0 (p<0.001).7 Similar finding was shown in another retrospective study
included hospitalized neutropenic cancer patients. Patients with VTE had 2-folds greater
risk of mortality than patients without diagnosis of VTE.50 Studies in patients with
colorectal, lung and breast cancer in California also found that VTE is a significant
predictor of death within 1 year of cancer diagnosis. The effect was more pronounced in
patients with loco-regional stage rather than patients with advanced and metastatic
disease.6,7,62 Similar finding was found in other studies in patients with pancreatic,
gastroesophageal, bladder, and ovarian cancer.54,63-65
VTE is also associated with hospitalization, anticoagulants use, reduced pulmonary
function, and post-thrombotic syndrome. The occurrence of VTE may interfere with
planned chemotherapy and causes treatment delay.4 Furthermore, compared with
general population, the risk of recurrent VTE and bleeding complications during
anticoagulant treatment in cancer patients was increased about 4 times and 2 times,
18
respectively.66 The occurrence of VTE worsens patients’ quality of life and lead to
increased consumption of healthcare resource. In a retrospective study of medical
records of 529 cancer patients using medical records as the data source, the mean
hospitalization cost for DVT was $20,065 per episode (2002 US$) compared with a cost
of $7712 to $10,804 per episode in a general medical population with VTE.67
2.2.6 Treatment of Venous Thromboembolism in Cancer Patients
In response to the increasing concern regarding VTE in cancer patients, several
international cancer organizations have recently issued guidelines regarding its
treatment and prevention. These include the Italian Association of Medical Oncology
(AIOM),68 the National Comprehensive Cancer Network (NCCN),69 the American
Society of Clinical Oncology (ASCO),1 the European Society of Medical Oncology
(ESMO),70 and the French National Federation of the League of Centers Against Cancer
(FNCLCC).71,72 Anticoagulant therapy remains the cornerstone of VTE treatment.
Anticoagulant therapy is divided into two phases: initial treatment to minimize the risk
of thrombus extension and subsequent fatal PE, and long-term treatment to prevent
recurrent VTE, thereby reducing the risk of post-phlebitic syndrome.73
19
2.2.6.1 Initial Treatment of Venous Thromboembolism
When VTE is objectively confirmed, parenteral anticoagulants should be initiated.
Treatment is started with unfractionated heparin (UFH), low molecular weight heparin
(LMWH) or fondaparinux. Agent selection should be individualized according to
characteristics of the individual agents (ease of administration, reversibility, half-life,
and cost) and patient’s clinical situation (inpatient or outpatient status, renal function,
medical or surgical patient). In most circumstances, LMWH is preferred because it is
recommended for the long-term treatment of VTE in patients with cancer and facilitates
the transition to outpatient management. 1,44,69-71
Furthermore, LMWH and fondaparinux
provide additional advantages over UFH, including better bioavailability after
subcutaneous administration, longer half-life, more predictable anticoagulant response
and lower incidence of heparin-induced thrombocytopenia.74 LMWH should be used
cautiously in patients with creatinine clearance (CCr) < 30 mL/min and fondaparinux is
contraindicated in these patients.1,69,70
2.2.6.2 Long-term Treatment of Venous Thromboembolism
After initial treatment for 5 to 10 days, LMWH is the preferable approach for
long-term treatment lasting at least 3 to 6 months for patients with VTE. Vitamin K
antagonists (VKA) with a target international normalized ratio (INR) of 2 to 3 are
20
considerable choice for long-term treatment when LMWH is not available or in patients
with severe renal insufficiency. Indefinite therapy may be required for patients with
active cancer, such as those with metastatic disease and those receiving chemotherapy
or hormonal therapy. 1,69,70,75 Although enoxaparin and tinzaparin also have been studied
in open-label randomized controlled trials in cancer patients, the efficacy of dalteparin
is supported by the highest quality evidence and it is the only LMWH approved by the
FDA for long-term treatment of VTE in cancer patients.69 Randomized controlled trials
indicate that LMWH is more effective than VKA in long-term treatment for preventing
VTE recurrence with similar bleeding risk.76-79 The CLOT (Randomized Comparison of
LMWH versus Oral Anticoagulant for The Prevention of Recurrent VTE in Patients
with Cancer) study demonstrated a relative risk reduction of 49% with LMWH versus a
VKA.77 In patients with contraindications to anticoagulation, inferior vena cava filter is
an alternative but anticoagulant therapy should be resumed once the bleeding risk is
resolved. Dosage regimens and recommendations for treatment of VTE in patients with
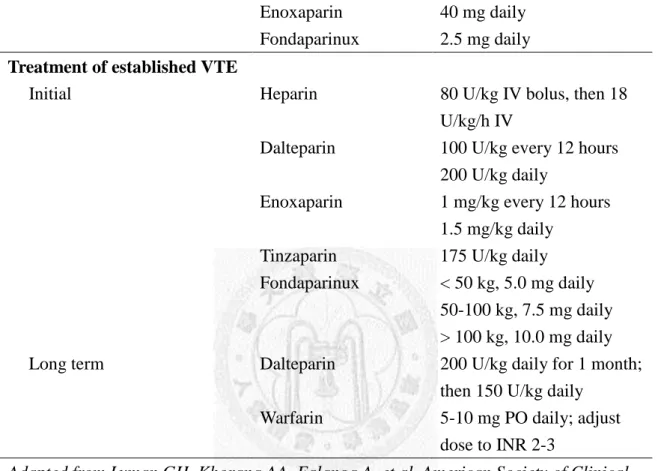
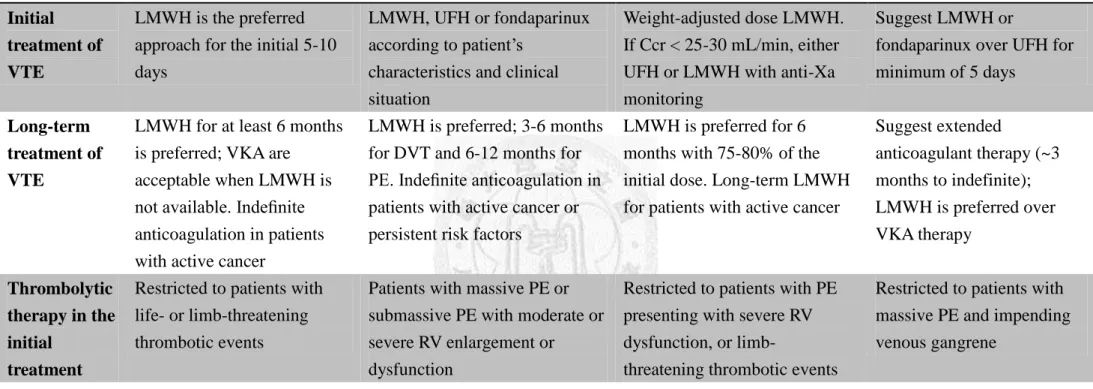
cancer are provided in Table 2.6 and 2.7.1,69,70,75
On the other hand, cancer patients are more likely to have thrombosis in
uncommon sites such as the veins of upper extremities, vena cava, visceral, portal, or
cerebral circulation.80 There are no specific guidelines and randomized controlled trials
focus on the treatment of abdominal DVT in cancer patients or non-cancer patients.
21
Recently, the 9th American College of Chest Physicians (ACCP) guidelines published in
2012 recommend anticoagulation over no anticoagulation in patients with symptomatic
splanchnic vein thrombosis.75
Treatment of VTE in cancer patients is more challenging than general population.
Compared with those without malignancy, VTE recurs 3-folds more frequently in
cancer patients and they are more prone to bleeding complications during long-term
treatment of VKA therapy despite a stable INR between 2 to 3. Interactions between
VKA and chemotherapeutic agents may cause elevation of INR and clinically relevant
bleeding. Tendencies toward thrombocytopenia, osteopenia, malnutrition, brain
metastasis and hepatic metastasis all further complicate thrombosis care in cancer
patients.3,55
22
Table 2.6 Regimens for prophylaxis/treatment of VTE in patients with cancer
Management Drug Regimen
Prophylaxis
Hospitalized medical or surgical cancer patients
Unfractioned heparin Dalteparin
Enoxaparin Fondaparinux
5,000 U every 8 hours 5,000 U daily
40 mg daily 2.5 mg daily Treatment of established VTE
Initial
Long term
Heparin
Dalteparin
Enoxaparin
Tinzaparin Fondaparinux
Dalteparin
Warfarin
80 U/kg IV bolus, then 18 U/kg/h IV
100 U/kg every 12 hours 200 U/kg daily
1 mg/kg every 12 hours 1.5 mg/kg daily
175 U/kg daily
< 50 kg, 5.0 mg daily 50-100 kg, 7.5 mg daily
> 100 kg, 10.0 mg daily 200 U/kg daily for 1 month;
then 150 U/kg daily 5-10 mg PO daily; adjust dose to INR 2-3
Adapted from Lyman GH, Khorana AA, Falanga A, et al. American Society of Clinical Oncology guideline: recommendations for venous thromboembolism prophylaxis and treatment in patients with cancer. J Clin Oncol 2007;25:5490-505.
23
Table 2.7 Recommendations for treatment of VTE in cancer patients
ASCO1 NCCN69 ESMO70 ACCP75
Initial treatment of VTE
LMWH is the preferred approach for the initial 5-10 days
LMWH, UFH or fondaparinux according to patient’s
characteristics and clinical situation
Weight-adjusted dose LMWH.
If Ccr < 25-30 mL/min, either UFH or LMWH with anti-Xa monitoring
Suggest LMWH or
fondaparinux over UFH for minimum of 5 days
Long-term treatment of VTE
LMWH for at least 6 months is preferred; VKA are
acceptable when LMWH is not available. Indefinite anticoagulation in patients with active cancer
LMWH is preferred; 3-6 months for DVT and 6-12 months for PE. Indefinite anticoagulation in patients with active cancer or persistent risk factors
LMWH is preferred for 6 months with 75-80% of the initial dose. Long-term LMWH for patients with active cancer
Suggest extended
anticoagulant therapy (~3 months to indefinite);
LMWH is preferred over VKA therapy
Thrombolytic therapy in the initial
treatment
Restricted to patients with life- or limb-threatening thrombotic events
Patients with massive PE or submassive PE with moderate or severe RV enlargement or dysfunction
Restricted to patients with PE presenting with severe RV dysfunction, or limb-
threatening thrombotic events
Restricted to patients with massive PE and impending venous gangrene
Inferior vena cava filters
Restricted to patients with contraindications to
anticoagulation or recurrent VTE despite adequate long- term LMWH
Contraindications to failure of anticoagulation; cardiac or pulmonary dysfunction severe enough to make any new PE life-threatening or multiple PE with chronic pulmonary hypertension
Contraindications to
anticoagulation or recurrent PE despite adequate long- term LMWH. Resume
anticoagulation on the risk of bleeding is reduced
Contraindications to
anticoagulation or recurrent PE despite adequate long- term LMWH. Start
anticoagulation if the risk of bleeding is resolved
24
Table 2.7 Recommendations for treatment of VTE in cancer patients (continued)
ASCO1 NCCN69 ESMO70 ACCP75
Treatment of catheter- related thrombosis
NA Anticoagulation for as long
as catheter is in place and for at least 3 months after catheter removal
NA 3 months of anticoagulation
if the catheter is removed;
continue anticoagulation as long as the CVC remains Abbreviations: VTE, venous thromboembolism; DVT, deep vein thrombosis; PE, pulmonary embolism; ASCO, American Society of Clinical Oncology; NCCN, National Comprehensive Cancer Network; ESMO, European Society of Medical Oncology; UFH, unfractionated heparin;
LMWH, low molecular weight heparin; VKA, vitamin K antagonist; RV, right ventricular; Ccr, creatinine clearance; NA, not addressed.
25
2.2.7 Prevention of Venous Thromboembolism in Cancer Patients
2.2.7.1 Prevention of VTE in Cancer Patients Undergoing Surgery
Cancer patients who undergo surgery are at high risk of developing VTE. Post-
operative VTE occurs 2- to 3-folds more frequent in cancer patients compared with
non-cancer patients. Prophylaxis of VTE with low-dose UFH, LMWH or fondaparinux
is recommended in all patients undergoing major surgery (such as laparotomy,
laparoscopy, or thoracotomy lasting > 30 minutes). Pharmacological prophylaxis should
be started as soon as possible and continued for at least 7 to 10 days postoperatively
unless contraindicated. Extended prophylaxis up to 4 weeks may be considered in
patients undergoing major abdominal or pelvic surgery for cancer patients with
high-risk features such as residual malignant disease after operation, obese patients, and
those with a previous history of VTE. 1,69,70
LMWH has shown to be as effective and safe as low dose UFH in preventing
post-operative VTE.81-83 In a double-blind randomized trial of fondaparinux versus
dalteparin in high-risk abdominal surgery patients, postoperative fondaparinux was at
least as effective as perioperative dalteparin.84 However, after pooling the results of
studies in patients undergoing elective hip replacement, elective knee replacement, and
hip fracture surgery, it is suggested that compared to LMWH, fondaparinux does not
reduce clinically significant VTE events but leads to more major bleeding events.75
26
Mechanical prophylaxis such as pneumatic calf compression (IPC) may be added
to pharmacological prophylaxis but should not be used as monotherapy for VTE
prevention unless pharmacological prophylaxis is contraindicated because of active
bleeding.1,70 Mechanical prophylaxis alone can reduce the rate of DVT by 66% but
achieve only a non-statistical 33% risk reduction in the rate of PE.55 Combination of
mechanical and pharmacological prophylaxis may improve efficacy in the very high
risk patients.1 Dosage regimens and recommendations for prevention of VTE in patients
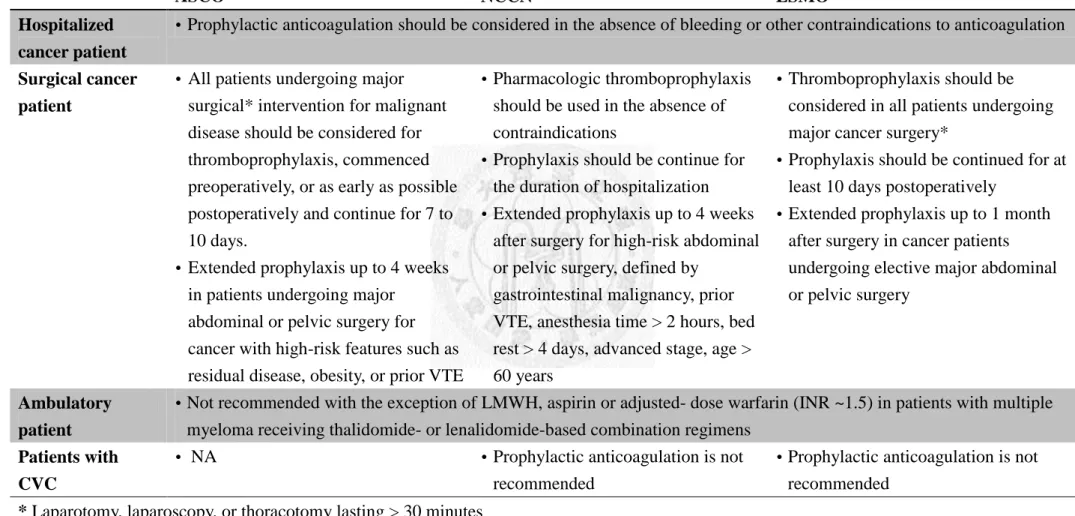
with cancer are provided in Table 2.6 and 2.8.1,69,70
2.2.7.2 Prevention of Hospitalized and Ambulatory Cancer Patients
Hospitalized cancer patients should be considered to receive anticoagulation for
prevention of VTE.1,69,70 Among patients hospitalized for cancer, chronic heart disease,
severe infectious diseases, or lung disease, cancer patients had the highest incidence of
VTE (7.6%) compared to the average rate of all patients being 5.6%.85 Previous
randomized clinical trials have demonstrated that pharmacological prophylaxis leads to
a lower VTE incidence compared with placebo, without increasing major bleeding.86-88
Routine prophylaxis with anticoagulants is not recommended in ambulatory
patients with cancer except patients with multiple myeloma receiving thalidomide or
lenalidomide with chemotherapy or dexamethasone. ESMO guideline recommended
27
LMWH, aspirin, or adjusted-dose warfarin (INR ~1.5) while ASCO guideline
recommended LWMH or adjusted-dose warfarin (INR ~1.5). Prophylaxis in cancer
patients receiving chemotherapy and/or hormone therapy is not recommended.1,70 For
cancer patients with indwelling CVC, benefit of pharmacological prophylaxis had not
been shown. Current guidelines do not support the use of anticoagulants for VTE
prophylaxis.70,72
28
Table 2.8 Recommendations for prophylaxis of VTE in cancer patients
ASCO1 NCCN69 ESMO70
Hospitalized cancer patient
Prophylactic anticoagulation should be considered in the absence of bleeding or other contraindications to anticoagulation
Surgical cancer patient
All patients undergoing major surgical* intervention for malignant disease should be considered for thromboprophylaxis, commenced preoperatively, or as early as possible postoperatively and continue for 7 to 10 days.
Extended prophylaxis up to 4 weeks in patients undergoing major
abdominal or pelvic surgery for cancer with high-risk features such as residual disease, obesity, or prior VTE
Pharmacologic thromboprophylaxis should be used in the absence of contraindications
Prophylaxis should be continue for the duration of hospitalization
Extended prophylaxis up to 4 weeks after surgery for high-risk abdominal or pelvic surgery, defined by
gastrointestinal malignancy, prior VTE, anesthesia time > 2 hours, bed rest > 4 days, advanced stage, age >
60 years
Thromboprophylaxis should be considered in all patients undergoing major cancer surgery*
Prophylaxis should be continued for at least 10 days postoperatively
Extended prophylaxis up to 1 month after surgery in cancer patients undergoing elective major abdominal or pelvic surgery
Ambulatory patient
Not recommended with the exception of LMWH, aspirin or adjusted- dose warfarin (INR ~1.5) in patients with multiple myeloma receiving thalidomide- or lenalidomide-based combination regimens
Patients with CVC
NA Prophylactic anticoagulation is not
recommended
Prophylactic anticoagulation is not recommended
* Laparotomy, laparoscopy, or thoracotomy lasting > 30 minutes
Abbreviations: VTE, venous thromboembolism; ASCO, American Society of Clinical Oncology; NCCN, National Comprehensive Cancer Network; ESMO, European Society of Medical Oncology; LMWH, low molecular weight heparin; NA, not addressed.
29
2.2.8 Venous Thromboembolism in Asian Patients with Cancer
Previous cohort studies had demonstrated that Asian patients with cancer have
significant reduced risk of VTE compared with Caucasian patients with cancer.6,7,51
However, population-based epidemiological studies in Asian demonstrated a yearly
increased incidence of VTE in Asians.8 Recently, several studies focused on VTE
among cancer patients in Asian populations had been published. Incidence of VTE in
multiple myeloma, gastric cancer, colorectal cancer, advanced pancreatic cancer, diffuse
large B-cell lymphoma, and cholangiocarcinoma ranged from 3.7% to 10.6% (Table
2.9).12-18
VTE incidence among patients with multiple myeloma receiving thalidomide- or
lenalidomide- based combination therapy was 5.3%, which is lower than that reported
in Western countries (10 to 20%).13 In a retrospective study among patients with
colorectal cancer based on database of Seoul National University Bundang Hospital
found that the 2-year cumulative incidence of VTE was 3.6%. The 2-year cumulative
incidence of DVT/PE ranged from 0.3%, 0.9%, 1.4% and 6.4% in stages 0-1, 2, 3, and 4,
respectively.15 Development of DVT/PE but not intra-abdominal venous thrombosis was
related to increased mortality in both patients with loco-regional and metastatic disease.
Similar finding was shown in another study in patients with gastric cancer.14 Although
Asian patients with loco-regional malignant disease had lower VTE incidence than
30
Western populations, the VTE incidence is similar among patients with distant
malignant disease. The incidence of VTE among Japanese patients with DLBCL was
reported to be comparable with that in Caucasian populations.16 On the other hand, the
risk of postoperative VTE was much lower than in Caucasian patients (~0.2%) but
further investigations are needed to confirm this finding.14,15
31
Table 2.9 Cohort studies of incidence of venous thromboembolism in patients of specified cancer site among Asian population
Location Data source Study population Study endpoint No. of
patients
VTE event (%) Korea
Oh et al. (2008)12
Electronic medical records database of Seoul National University Bundang Hospital
Patients with advanced pancreatic cancer
Objectively confirmed VTE 132 5.3
Korea
Koh et al. (2010)13
Korean multiple myeloma registry
Patients received thalidomide for multiple myeloma
Objectively confirmed symptomatic VTE
360 3.9
Korea
Lee et al. (2010)14
Electronic medical records database of Seoul National University Bundang Hospital
Patients with gastric cancer
Objectively confirmed extremity venous thrombosis, PE and intra-abdominal thrombosis
2,085 3.8*
Korea
Choi et al. (2011)15
Electronic medical records database of Seoul National University Bundang Hospital
Patients with colorectal cancer
Objectively confirmed extremity venous thrombosis, PE and intra-abdominal thrombosis
2,006 3.6*
Japan
Yokoyama et al. (2011)16
Medical records database of Keio University Hospital
Patients with diffuse large B-cell lymphoma
Objectively confirmed symptomatic VTE
142 10.6
Korea
Kang et al. (2012)17
Medical records database of Asan Medical Center, Seoul
Patients with advanced gastric cancer
Objectively confirmed DVT/PE 3,095 4.9*
Korea
Jeon et al. (2012)18
Medical records database of Pusan National University Hospital
Patients with
cholangiocarcinoma
Objectively confirmed VTE (including intra-abdominal thrombosis)
273 3.7
* 2-year cumulative incidence
32
Chapter 3 Study Objective
Using the administrative claims data from the NHI database, the goal of this
population-based cohort study were:
(1) to explore the incidence date and timing of VTE,
(2) to identify the risk factors for VTE,
(3) to describe the clinical characteristics and treatment pattern of VTE,
(4) to examine the incidence of recurrent VTE and bleeding complications during
long-term treatment of VTE among cancer patients.
33
Chapter 4 Materials and Methods
4.1 Data Source
This population-based cohort study was based on Longitudinal Health Insurance
Database (LHID) of National Health Insurance research database (NHIRD) in Taiwan.
The NHI program was organized by the government and operated by Taiwan’s Bureau
of the NHI. This mandatory, single-payer health insurance was launched in 1995, and
has covered over 99% of Taiwan’s population (approximately 23 million residents) and
contracted with 97% of hospital as well as clinics throughout the nation. It provides comprehensive benefits, including inpatient care, ambulatory care, dental care, and
prescription drug coverage, to all beneficiaries. Registration datasets and claims
databases of all beneficiaries in the NHI program have been maintained since 1997 and
offer an excellent opportunity to conduct studies.89,90
This study uses LHID 2000, LHID 2005 and LHID 2010 as data source. LHID
2000, LHID 2005 and LHID 2010 contain all the original claim data of 1,000,000
beneficiaries randomly sampled from the year 2000, 2005, and 2010 Registry for
Beneficiaries (ID), respectively. All traceable personal identifiers are removed from the
database to protect patient confidentiality.90 The databases used in this study included
all inpatient and outpatient medical claims from January 1, 1999 to December 31, 2009.
34
4.2 Study Population and Study Outcomes
4.2.1 Study Cohort
Our study population included newly diagnosed cancer patients who have been
hospitalized with a primary diagnosis of malignant disease, identified by International
Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) code
(ICD-9-CM codes: 140.x-208.xx) between January 1, 2001 and December 31, 2008.
Diagnoses in NHI research databases are coded using the ICD-9-CM coding scheme
since 2000.89 This time frame allowed for retrieving at least 1 year of baseline data prior
to cancer diagnosis and up to 1 year of follow up after cancer diagnosis.
Major categories of malignant diseases that were included and their ICD-9-CM
diagnosis codes were shown in Table 4.1. We used the year 1999 as a run-in year to
identify incident cases of malignant diseases. The date when the patient was first
hospitalized with a primary diagnosis of malignant disease was defined as the index
date. Patients were excluded from this study if their genders were unknown or they had
more than one primary diagnosis of malignant diseases at index date, for their cancer
sites cannot be categorized.
35
Table 4.1 ICD-9-CM codes of inclusion diagnosis
Cancer site ICD-9-CM codes
Head & neck cancer 140.x-149.x, 160.x-161.x
Esophageal caner 150.x
Stomach cancer 151.x
Colorectal cancer 153.x-154.x
Liver cancer 155.x
Pancreas cancer 157.x
Other abdominal cancers 152.x, 156.x, 158.x, 159.x
Lung cancer 162.x-163.x
Sarcoma 170.x-171.x
Skin cancer 172.x-173.xx
Breast cancer 174.x-175.x
Endometrial cancer and cervical cancer 179-182.x
Ovarian cancer 183.x
Prostate cancer 185
Testicular cancer 186.x
Bladder cancer 188.x
Renal cancer 189.x
Brain cancer 191.x-192.x
Thyroid cancer 193
Non-Hodgkin’s lymphoma 200.xx, 202.xx
Hodgkin’s lymphoma 201.xx
Multiple myeloma 203.xx
Leukemia 204.xx-208.xx