Other uses, including reproduction and distribution, or selling or licensing copies, or posting to personal, institutional or third party
websites are prohibited.
In most cases authors are permitted to post their version of the article (e.g. in Word or Tex form) to their personal website or institutional repository. Authors requiring further information
regarding Elsevier’s archiving and manuscript policies are encouraged to visit:
http://www.elsevier.com/copyright
ContentslistsavailableatScienceDirect
Journal of Ethnopharmacology
j ou rn a l h o m e pa g e:w w w . e l s e v i e r . c o m / l o c a t e / j e t h p h a r m
Steady-state pharmacokinetics and tissue distribution of anthraquinones of Rhei Rhizoma in rats
Chi-ShengShiaa,Shang-YuanTsaia,Jung-ChihLinb,Ming-LiLic,Miau-HwaKod,Pei-DawnLeeChaoa, Yu-ChingHuanga,Yu-ChiHoua,e,∗
aSchoolofPharmacy,CollegeofPharmacy,ChinaMedicalUniversity,Taichung,Taiwan
bDepartmentofIntegratedChineseandWesternMedicine,ChungShanMedicalUniversityHospital,Taichung,Taiwan
cCardiovascularDivision,SurgeryDepartment,ChinaMedicalUniversityHospital,Taichung,Taiwan
dDepartmentofAnatomy,CollegeofMedicine,ChinaMedicalUniversity,Taichung,Taiwan
eDepartmentofMedicalResearch,ChinaMedicalUniversityHospital,Taichung,Taiwan
a r t i c l e i n f o
Articlehistory:
Received1March2011
Receivedinrevisedform2August2011 Accepted2August2011
Available online 9 August 2011
Keywords:
Anthraquinone Tissuedistribution Pharmacokinetics Polyphenol
a b s t r a c t
Aimofthestudy:RheiRhizoma,therhizomeofRheumpalmatumL.(RP),isapopularherbinclinical Chinesemedicine.RPisabundantinpolyphenolicanthraquinones,whichhavebeenreportedtoshow variousbeneficialbioactivities.Thisstudyinvestigatedthepharmacokineticsandtissuedistributionof anthraquinonesfollowingseven-doseadministrationofRPdecoctiontorats.
Materialsandmethods:SixSprague-Dawleyratsweregiven2.0g/kgofRPtwicedailyforsevendosesand bloodsampleswerecollectedatdesignatedtimeafterthe7thdose.Anothersixratsweresacrificedat 30minafterthe7thdoseandorgansincludingliver,kidney,lungandbrainwerecollected.Serumand tissuespecimenswereassayedbyHPLCbeforeandafterhydrolysiswith-glucuronidaseandsulfatase, respectively.
Results:Pharmacokineticanalysisindicatedthattheanthraquinonesinserummainlypresentedasglu- curonides/sulfatesandcontainedhigherratioofsulfateswhencomparedwithsingle-doseadministration ofRP.Contrarytothefindinginserum,tissueanalysisdiscoveredmainlyfreeformofanthraquinonein mostorgansassayed,suchasaloe-emodinandrheininkidney,liver,lung;emodininliver,lung;trace ofchrysophanolinkidneyandliver.Inallbrains,neitherfreeformsnortheirglucuronides/sulfateshave beendetected.
Conclusions:Theglucuronides/sulfatesofanthraquinoneswerethemajorformsinbloodstream,whereas thefreeformsofmostanthraquinoneswerepredominantinkidneyandliver.
© 2011 Elsevier Ireland Ltd. All rights reserved.
1. Introduction
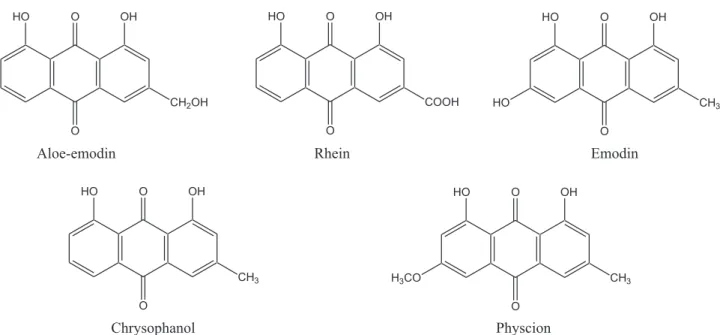
Anthraquinones are a group of polyphenolicconstituents in RheiRhizoma (the rhizome of Rheum palmatumL., RP), which is commonly usedin clinical Chinese medicineas potent laxa- tive,antibacterial, anti-inflammatory, anticancer,antispasmodic andcholereticagents(Shenand Li,1997;Shiaetal.,2011).The anthraquinonesofRPincludealoe-emodin,rhein,emodin,chryso- phanoland physcion(chemicalstructuresshown in Fig.1)and theirglycosides(Li,2002).Numerousstudiesreportedthatthese anthraquinones exhibited beneficial bioactivities such as anti- cancer(Shietal.,2001;Pecereetal.,2003;Kuoetal.,2004;Guo etal.,2007;Huangetal.,2007),antivirus(Shuangsuoetal.,2006),
∗ Correspondingauthorat:SchoolofPharmacy,ChinaMedicalUniversity,91 Hsueh-ShihRd,Taichung,Taiwan.Tel.:+886422053366x5107;
fax:+886422031028.
E-mailaddress:hou5133@gmail.com(Y.-C.Hou).
antioxidation (Caiet al., 2004) and anti-diabetes (Zheng et al., 2008).Despitethesepositiveresults,itremainsunclearwhether thoseactivitiesfoundinvitrocanexistinvivo.
Our previous study documenting the pharmacokinetics of anthraquinones after single dosing of RP has pointed out that the free formsof aloe-emodin,emodin and chrysophanolwere notdetectedinbloodbyHPLC/UVdetection,whereas theirglu- curonides/sulfateswerepredominant.The onlyfreeformfound inthecirculationwasrhein,which alsopresentedmainlyasits glucuronides/sulfates(Shiaetal.,2009).Later,anotherourstudy reportingthetissuedistributionofflavonoidsofScutellariaeRadix (root of Scutellariaebaicalensis)disclosed thatthefree forms of baicaleinandwogoninwerethemajorformsinmanyorgans,in contradictiontothefindinginblood(Houetal.,2011).Therefore, inordertoelucidatetherealmolecularformsandconcentrations ofrhubarbanthraquinonesinthecirculationandinvariousorgans atthesteadystate,pharmacokineticandtissuedistributionstud- iesfollowingrepeateddosingofRPdecoctionwereconductedin rats.
0378-8741/$–seefrontmatter © 2011 Elsevier Ireland Ltd. All rights reserved.
doi:10.1016/j.jep.2011.08.003
O O
HO OH
CH3
Aloe-emodin Rhein Emodin
Chrysophanol Physcion
O O
HO OH
CH3
H3CO O
O
HO OH
CH2OH
O O
HO OH
COOH
O O
HO OH
CH3
HO
Fig.1.Chemicalstructuresofanthraquinones.
2. Materialsandmethods 2.1. Chemicals
Aloe-emodin (purity95%),rhein(purity95%),emodin(purity 90%), chrysophanol (purity 98%), physcion (purity 95%), - glucuronidase (type B 1,666,400units/g, from bovine liver), sulfatase(typeH1,14,400units/g,fromHelixpomatia,containing 300,000units/gof-glucuronidase)and2-methlylanthraquinone (purity95%)werepurchasedfromSigmaChemicalCo.(St.Louis, MO, U.S.A.). Acetonitrile, methanol and ethyl acetate were LC grade and purchased from Mallinckrodt Baker, Inc. (Phillips- burg, NJ, U.S.A.). l(+)-Ascorbic acid was obtained from RdH Laborchemikalien GmbH & Co. KG (Seelze, Germany). Other reagentswereHPLCgradeoranalyticalreagentgrade.Milli-Qplus water(Millipore,Bedford,MA,U.S.A.)wasusedthroughout this study.
2.2. Instrumentation
The HPLC apparatus included a pump (LC-10AT, Shimadzu, Japan),anUVspectrophotometricdetector(SPD-10AVP,Shimadzu, Japan), a fluorescence detector (RF-10A, Shimadzu, Japan) and chromatopac(C-R6A,Shimadzu,Japan)withanautomaticinjec- tor(SIL-10A,Shimadzu,Japan)andanAlltechApolloC18column (5m,4.6mm×150mm,Alltech,Deerfield,IL,USA).
2.3. PreparationofRPdecoction
TherhizomesofRheumpalmatumwaspurchasedfromaChinese drugstoreinTaichung,Taiwan.Theoriginofthecrudedrugwas identifiedmicroscopicallybythecorrespondingauthorDr.Yu-Chi Hou.Water(5l)wasaddedto250gcrudedrugsandheatingon agasstove.Afterboiling,gentleheatingwascontinueduntilthe volumereducedtoabout2.5landthenthemixturewasfiltered whilehot.Thefiltratewasgentlyboileduntilthevolumereduced tolessthan500mlandsufficientwaterwasaddedtomake500ml, whichwasimmediatelydividedintoaliquotsandfrozenat−20◦C forlateruse.
2.4. QuantitationofRPdecoctionanditsacidhydrolysate
Rhubarbdecoction(3.0ml)wasmixedwith7.0mlofmethanol and centrifuged to remove polysaccharide. Then the properly diluted rhubarb decoction (100l) was added with 100l of 2-methylanthraquinone solution (50.0g/ml in methanol) as internalstandardand20lweresubjectedtoHPLCanalysis.The mobilephaseconsistedofacetonitrile(A)–0.1%phosphoricacid (B)andprogramedin agradientmannerasfollows:A/B: 50/50 (0–10min),85/15(15–22min)and50/50(27min).Thedetection wavelengthwassetat250nmandtheflowratewas1.0ml/min.
Acid hydrolysis of 1ml of the decoction was conducted by adding1mlof1.2NHClandthehydrolysiswasconductedat80◦C inawaterbathfor6h.Thehydrolysatewasthenaddedwithsuffi- cientmethanoltomake2.0mlbeforeHPLCanalysis.Thecontents of glycosides of aloe-emodin, rhein, emodin, chrysophanoland physcionweredeterminedbysubtractingtheconcentrations of eachaglyconeinthedecoctionfromthoseofcorrespondentagly- coneinthedecoctionhydrolysate.
2.5. Animals
MaleSprague-DawleyratsweresuppliedbyNationalLabora- toryAnimal Center(Taipei, Taiwan) and keptinAnimal Center ofChineseMedicalUniversity.Theanimalstudyadheredto“The Guidebook forthe Careand Useof LaboratoryAnimals(2002)”
(PublishedbytheChineseSocietyfortheAnimalScience,Taiwan, ROC).TheanimalprotocolwasapprovedbyInstitutionalAnimal CareandUseCommittee(IACUC)ofChinaMedicalUniversity.Rats werehousedina12-hlight–darkcycle,constanttemperatureenvi- ronmentpriortostudy.
2.6. Drugadministration,bloodandtissuecollection
Forpharmacokineticstudy,sixratsweighing390–440gwere fastedfor12hbeforedrugadministration.Afterfasting,ratswere administeredorallywith2.0g/kgofRPdecoctiontwicedailyfor7 dosesviagastricgavageinordertoreachsteady-state.Inthe4th daymorning,bloodsamples(0.6ml)werewithdrawnfromrats underanesthesiawithisofluraneat15,30,60,120,240,480,720 and1440minafterthe7thdoseviacardiopuncture.Thebloodsam- pleswerecollectedin microtubesand centrifugedat10,000×g
for15mintoobtaintheserum,whichwasstoredat−20◦Cuntil analysis.
For tissue distribution study, six rats weighing 350–450g wereadministeredRPdecoctionwiththesamedosageregimen describedaboveinpharmacokineticstudy.Inthe4thdaymorning, ratsweresacrificedat30minafterthe7thdoseandabloodsam- plewascollectedviacadiopuncture.Followingimmediatesystemic perfusionwithcoldsaline,organsincludingliver,lung,kidneyand brainwereremoved,washedwithcoldsaline, blotteddry with filterpaperandthenaccuratelyweighed.Thetissueswerehomog- enizedwithcoldsaline(3g/5ml)andthehomogenateswerestored at−30◦Cuntilanalysis.
2.7. Quantitationofanthraquinonesandtheir glucuronides/sulfatesinserumandtissues
Thedeterminationofanthraquinoneconcentrationsinserum andtissuehomogenatesfollowedapreviousmethodwithminor modification(Shiaet al.,2009).For theassayofanthraquinone aglycones, 100l of serum or tissue homogenate was mixed with 50l of pH 5 acetate buffer and 50l of ascorbic acid (100mg/ml). Themixture wasacidifiedwith50lof 0.1N HCl andpartitionedwith250lofethylacetate(containing1g/ml of2-methylantraquinoneasinternalstandard).Theethylacetate layerwasevaporatedunderN2 gastodrynessandreconstituted with50lofmethanol,then20lwassubjectedtoHPLCanalysis.
Forthequantitationofglucuronidesandsulfates,100lofserum ortissuewasmixedwith50lof-glucuronidaseandsulfatase, respectively,inpH5acetatebuffer(1000units/ml)and 50lof ascorbicacid(100mg/ml),thenincubatedat37◦Cfor10min,which hadbeendeterminedbyapreliminarytimestudy.Afterhydroly- sis,theserumortissuehomogenatewasacidifiedwith50lof 0.1NHClandthelaterprocessesfollowedthosedescribedabove.
Themobilephaseconsistedofacetonitrile(A)and0.1%phospho- ricacid(B),andagradientelutionprogramwasrunasfollows:
A/B:50/50(0–10min),85/15(15min),80/20(20min)and50/50 (25min).Forserumassay,thewavelengthofUVdetectorwasset at250nm.Fortissueassay,fluorescencedetectionwasperformed withwavelengthsof435and515nmforexcitationandemission, respectively.Theflowratewas1.0ml/min.
For calibrator preparation, 100l of serum or tissue homogenate each spiked with various concentrations of anthraquinones was added with 50l of pH 5 buffer. The laterprocedurewasthesameasthatdescribedaboveforserum sample.Thecalibrationgraphwasplottedbylinearregressionof thepeakarearatios(anthraquinonestointernalstandard)against concentrationsofanthraquinones.
The systemsuitability wasevaluated through intra-day and inter-dayanalysisofprecisionandaccuracy.Recoverywasassessed bycomparingthepeakareaobtainedfromextractedsamplespiked withstandards tothat obtainedfrom unextractedstandards in extracted sample matrix, which represents100% recovery.The lowerlimitofquantitation(LLOQ)representsthelowestconcentra- tionofanalysisinasamplethatcanbedeterminedwithacceptable precisionand accuracy,whereaslimit ofdetection(LOD) repre- sentsthelowestconcentrationofanalysisinasamplethatcanbe detected(withS/N>3).
2.8. Dataanalysis
Thepeakserum ortissueconcentration(Cmax)wasobtained fromexperimentalobservation.Thepharmacokineticparameters were analyzed by noncompartmentmodel withthe aidof the program WinNonlin (version 1.1 SCI software, Statistical Con- sulting, Inc., Apex, NC, U.S.A.). The area under the serum or tissue concentration–time curve (AUC0–t) was calculated using
Table1
Contents(g/ml)ofaloe-emodin,rhein,emodin,chrysophanolandphyscioninRP decoctionbeforeandafteracidhydrolysis.
Constituents Beforehydrolysis Afterhydrolysis
Aloe-emodin 119.5 613.6
Rhein 577.9 910.1
Emodin 114.4 552.6
Chrysophanol 90.5 471.1
Physcion 38.9 199.5
trapezoidal rule to the last point. The concentrations of anthraquinone sulfates in tissue were obtained by subtract- ing theconcentration of anthraquinoneglucuronides from that ofcorrespondent anthraquinoneglucuronides/sulfates.Unpaired Student’st-testwasusedforstatisticalcomparisontakingp<0.05 assignificant.
3. Results
3.1. QuantitationofanthraquinonesinRPdecoction
ThequantitationofanthraquinonesinRPdecoctionwasper- formed by HPLC analysis before and after acid hydrolysis. The concentrationsofeachanthraquinoneintheRPdecoctionbefore andafteracidhydrolysiswereshowninTable1.Themostabun- dant anthraquinone in RP decoction was rhein and the least wasphyscioneitherbeforeorafteracidhydrolysis.Aloe-emodin, emodin,chrysophanolandphyscionwerepredominantlypresent inglycosideforms,whereasrheinmainlyexistedinfreeform.
3.2. Quantitationofanthraquinonesandtheir glucuronides/sulfatesinserumandtissues
The quantitation of anthraquinones and their glu- curonides/sulfates in serum essentially employed a method published previously (Shia et al., 2009). The serum analysis revealedthatrhein freeformwaspresentin allspecimens, and emodinfreeformexistedonlytransientlyintheearlyphaseafter dosing,whereasthefreeformsofaloe-emodin,chrysophanoland physcion were not detected.The glucuronides/sulfates of aloe- emodin,rhein,emodinandchrysophanolpredominantlyexisted inbloodstream,whilenotraceofphyscionglucuronides/sulfates had been detected. The mean serum concentration–time pro- files of each anthraquinone afterthe 7th dose of RPdecoction in six rats are shown in Fig. 2. All profiles revealed a second peak, a clear indication of enterohepatic circulation for these anthraquinones. The pharmacokinetic parameters of rhein free form, glucuronides/sulfates (G/S) and glucuronides (G) of each anthraquinonearelistedinTable2.Thesystemicexposures(area under the concentration–time curve, AUC) of anthraquinones ranked as follows: rhein G/S>rhein>emodin G/S>aloe-emodin G/S>chrysophanolG/S.
Theassaymethodoftissuehomogenateswasestablishedand validated in this study. The calibration ranges of aloe-emodin (0.04–4.0g/ml), rhein (0.01–20.0g/ml), emodin and chryso- phanol(0.01–2.0g/ml)invarioustissuehomogenateswerewith goodlinearities.Themethodvalidationindicated thatallcoeffi- cientsofvariation inintra-runandinter-runanalysisofvarious tissuehomogenateswerebelow9.9%,andtherelativeerrorswere below 19.2%. For liver homogenate, theLLOQs and LODs were 0.04and0.01g/mlforaloe-emodinandchrysophanol,0.08and 0.02g/ml for rhein, 0.06 and 0.01g/ml for emodin, respec- tively. For kidney homogenate,the LLOQsand LODswere0.01 and0.006g/mlforaloe-emodin,0.06and0.04g/mlforrhein, 0.08and0.005g/mlforemodin,0.06and0.01g/mlforchryso- phanol.Forlunghomogenate,theLLOQsandLODswere0.008and
Table2
Pharmacokineticparametersofanthraquinonesandtheirglucuronides/sulfates(G/S)andglucuronide(G)after7thdoseofRP(2g/kg)insixrats.
Constituents Metabolites Cmax(nmol/ml) AUC0–720(nmolmin/ml) MRT0–720(min)
Aloe-emodin G/S 2.1±0.3 288.0±63.4 147.5±19.4
G 1.5±0.2 151.7±30.9 105.5±20.1
Rhein Freeform 5.8±0.7 1250.2±167.0 243.1±23.2
G/S 17.1±1.7 3209.4±398.0 236.8±13.1
G 9.3±1.3 1725.1±294.3 215.5±28.2
Emodin G/S 3.7±0.3 717.0±62.8 235.9±15.2
G 2.1±0.3 391.5±70.7 203.6±24.1
Chrysophanol G/S 1.8±0.2 161.6±47.6 93.5±22.4
G 1.4±0.2 117.6±27.6 136.5±44.2
Cmax,themaximumconcentration(nmol/ml).
AUC0–t,theareaunderconcentration–timecurvetothelasttime(nmolmin/ml).
MRT0–t,themeanresidencetime(min).
Dataexpressedasmean±S.E.
0.002g/mlforaloe-emodin,0.04and0.01g/mlforrhein,0.006 and0.002g/mlforemodin,0.004and 0.001g/ml forchryso- phanol,respectively.Therecoveriesofanthraquinonesfromliver, kidneyandlungwere68.6–84.2%,78.0–111.1%and76.9–109.4%, respectively,attestedconcentrations.
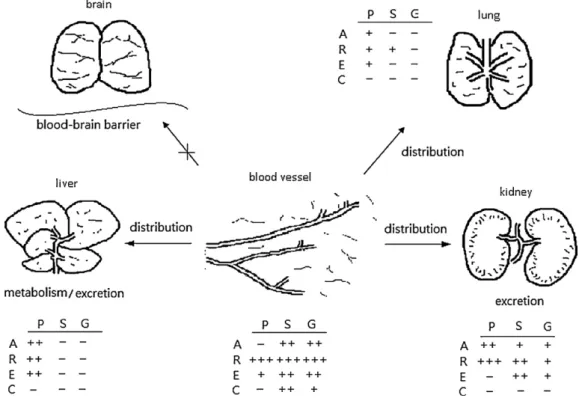
Tissue analysis had detectedthe free forms of aloe-emodin, rhein,emodinandchrysophanolinmanyorgansasshowninFig.3.
Thetissuecontentsofeachanthraquinonefreeformwererankedas follows:kidney,liver>lungforaloe-emodin;kidney>liver>lung forrhein;liver>lungforemodin;onlytracesofchrysophanolin
kidney andliver. Amongthe assayedorgans, theconcentration ofrheinfreeforminkidneywasthehighest(24g/g)andeven muchhigherthanserumconcentration(14g/ml). Withregard totheconcentrationsofglucuronides/sulfates,notracesofthem werefoundinliver.OnlytracesofSofaloe-emodin,rhein,emodin werefoundinlung.BothGandSofaloe-emodin,rhein,emodinand chrysophanolwerepresentinkidney,butinmuchlowerconcen- trationsthantheirfreeforms.NeitherphyscionnoritsG/Shadbeen detectedinalltissuesassayed.Inaddition,thefreeformsandthe G/Sofeachanthraquinonewerenotdetectedinallbrains(Fig.4).
Time (min)
0 200 400 600 800
serum concentration(nmol/ml)
0.0 0.5 1.0 1.5 2.0
Chrysophanol G/S Chrysophanol G Time (min)
0 200 400 600 800
serum concentration(nmol/ml)
0.0 0.5 1.0 1.5 2.0 2.5
Aloe-emodin G/S Aloe-emodin G
Time (min)
0 200 400 600 800
serum concentration(nmol/ml)
0 2 4 6 8 10 12 14 16 18
Rhein Rhein G/S Rhein G
Time (min) 2
0 00 400 6 00 800
serum concentration (nmol/ml)
0 1 2 3 4 5
Emodin G/S Emodin G
a b
c d
Fig.2.Mean(±S.E.)serumconcentration–timeprofilesofanthraquinonesandtheirglucuronides/sulfates(G/S)andglucuronides(G)afterthe7thdoseofRP(2g/kg)insix rats.(a)Aloe-emodin,(b)rhein,(c)emodin,and(d)chrysophanol.
Organs
serum liver lung kidney brain
Concentration (nmol/mL)
0.0 0.5 1.0 1.5 2.0 2.5
Concentration (nmol/g)
0.0 0.5 1.0 1.5 2.0 Aloe-emodin 2.5
Aloe-emodin G Aloe-emodin S
Organs
serum liver lung kidney brain
Concentration (nmol/mL)
0 5 10 15 20 25 30
Concentration (nmol/g)
0 5 10 15 20 25 Rhein 30
Rhein G Rhein S
Organs
serum liver lung kidney brain
Concentration (nmol/mL)
0 1 2 3 4 5 6
Concentration (nmol/g)
0 1 2 3 4 5 Emodin 6
Emodin G Emodin S
Organs
serum liver lung kidney brain
Concentration (nmol/mL)
0.0 0.5 1.0 1.5 2.0 2.5 3.0
Concentration (nmol/g)
0.0 0.5 1.0 1.5 2.0 2.5 Chrysophanol 3.0
Chrysophanol G Chrysophanol S
a b
c d
Fig.3. Concentrationsofanthraquinonesandtheirsulfates(S)andglucuronide(G)inserum(nmol/ml)andvarioustissues(nmol/g)afterthe7thdoseofRP(2g/kg)insix rats.(a)Aloe-emodin,(b)rhein,(c)emodin,and(d)chrysophanol.
Fig.4. Tissuedistributionsofanthraquinonesafterthe7thdoseofRPinrats(P,parentform;S,sulfates;G,glucuronides;A,aloe-emodin;R,rhein;E,emodin;andC, chrysophanol).