行政院國家科學委員會補助專題研究計畫成果報告
※※※※※※※※※※※※※※※※※※※※※※※※※
※ 類固醇對 capsaicin 受體的調控作用:結構與活性間 ※
※ 之關係及痛覺行為之測試 ※
※ Modulation of the Capsaicin Receptor by Ster oids: ※
※ Str uctur e-Activity Relationships and ※
※ Nociceptive Behavior al Tests ※
※※※※※※※※※※※※※※※※※※※※※※※※※
計畫類別:ˇ個別型計畫 □整合型計畫
計畫編號:NSC 90-2320-B-006-075-
執行期間:90 年 8 月 1 日至 91 年 7 月 31 日
計畫主持人:吳豐森
共同主持人:
本成果報告包括以下應繳交之附件:
□赴國外出差或研習心得報告一份
□赴大陸地區出差或研習心得報告一份
□出席國際學術會議心得報告及發表之論文各一份
□國際合作研究計畫國外研究報告書一份
執行單位:國立成功大學生理學研究所
中 華 民 國 91 年 5 月 20 日
行政院國家科學委員會專題研究計畫成果報告
類固醇對 capsaicin 受體的調控作用:結構與活性間之
關係及痛覺行為之測試
Modulation of the Capsaicin Receptor by Ster oids:
Str uctur e-Activity Relationships and
Nociceptive Behavior al Tests
計畫編號:NSC 90-2320-B-006-075
執行期限:90 年 8 月 1 日至 91 年 7 月 31 日
主持人:吳豐森 國立成功大學生理學研究所
計畫參與人員:陳淑貞、張翠容 國立成功大學生理學研究所
中文摘要
本研究計畫的主要目的在探討類固醇 對 capsaicin 受體調控作用的分子機制。先 前的研究顯示,神經性類固醇還原雄性素 (DHEA) 對 分 佈 在 腦 部 神 經 細 胞 膜 上 之
NMDA 受體和 GABAA受體具有調節的作
用。在本研究中,我們採用快速分離出來 的成年大白鼠背根神經節細胞為材料,利 用全細胞膜電位固定的電生理記錄方法,
探討 DHEA 及相關類固醇對 capsaicin 受體 所媒介電流反應的調控作用。結果顯示 DHEA 快速且可逆地抑制 capsaicin 所誘發 的全細胞內向電流。DHEA 對 capsaicin 所 誘發反應的抑制作用呈現劑量依賴效應,
最大抑制作用為 100%,而達到最大抑制作 用 一 半 所 需 的 DHEA 濃 度(EC50) 為 6.7 µM。DHEA 對 capsaicin 所誘發反應的抑制 作用隨著 capsaicin 濃度的增加而遞減,表 示 DHEA 對 capsaicin 受體所媒介反應的抑 制作用是屬於競爭型。並非所有的類固醇 對 capsaicin 所誘發的反應都有抑制作用。
黃體激素對 capsaicin 所誘發的電流幾乎沒 有作用,表示 DHEA 對 capsaicin 受體的抑 制作用具專一性。此外,DHEA 的立體異 構物 3α-DHEA 對 capsaicin 所誘發的反應 不但沒有抑制作用,反而有增強的作用,
顯示類固醇對 capsaicin 受體的調控作用具 立體專一性。
關鍵詞:Capsaicin 受體、類固醇、還原雄 性素、背根神經節細胞、全細胞膜電位固 定記錄法、劑量依賴效應、競爭型抑制作
用、立體專一性
1. Abstr act
The major goal of this proposal is to explore the molecular mechanisms whereby steroids modulate the capsaicin receptor.
Previous studies have shown that the neurosteroid dehydroepiandrosterone (5- androsten-3β-ol-17-one; DHEA) modulates both N-methyl-D-aspartate (NMDA) receptor- and γ-aminobutyric acid type A (GABAA) receptor-mediated responses in brain. In the present study, we investigated the effects of DHEA and related steroids on the capsaicin receptor-mediated current in acutely dissociated rat dorsal root ganglion neurons using the whole-cell voltage-clamp recording technique. DHEA rapidly and reversibly inhibited the capsaicin-induced current. Inhibition of the capsaicin response by DHEA was concentration-dependent, with an EC50 of 6.7 µM and a maximal inhibition of 100%. Inhibition by DHEA of the capsaicin response was significantly reduced when capsaicin was increased to higher concentrations, indicating that the blocking action of DHEA is competitive. Not all steroids inhibited the capsaicin response.
Progesterone did not exert any significant effect on the capsaicin response, suggesting that inhibition of the capsaicin-induced current by DHEA is a specific effect.
Furthermore, the stereoisomer of DHEA, 5- androsten-3α-ol-17-one (3α-DHEA), failed to inhibit the capsaicin-induced current,
producing instead a potentiating effect on the capsaicin response, demonstrating that the interaction of steroids with the capsaicin receptor is stereospecific.
Keywor ds: Capsaicin Receptor, Steroids, Dehydroepiandrosterone (DHEA), Dorsal Root Ganglion Neurons, Whole-Cell Voltage-Clamp Recordings, Concentration- Dependent, Competitive Inhibition,
Stereospecific
2. Backgr ound and Aim
Capsaicin, the main pungent ingredient in hot chili peppers [1], activates a distinct subpopulation of primary sensory neurons, with somata in dorsal root, trigeminal as well as nodose ganglia [2, 3]. Capsaicin exerts its effects by binding to distinct cell-surface receptors. Electrophysiological studies from dorsal root ganglion neurons demonstrate that capsaicin generates an inward current that results in the depolarization of the neuron [4]. This inward current results from the opening of a nonselective cationic channel that is largely permeable to Na+ and Ca2+ [5]. Capsaicin receptors have attracted particular attention by virtue of their proposed role in nociception [6].
Steroid hormones are known to influence profoundly the neuronal excitability [7]. Although the effects of steroids have been thought to be mediated by genomic steroid response elements [8], recent evidence indicates that many steroids influence the neuronal excitability via direct effects on excitatory and inhibitory amino acid receptors [9-12]. In particular, the neurosteroid dehydroepiandrosterone (5- androsten-3β-ol-17-one; DHEA) which has been claimed to be a “super hormone”
modulates both N-methyl-D-aspartate (NMDA) receptor- and γ-aminobutyric acid type A (GABAA) receptor-mediated responses in brain [13]. Compared to the extensive studies of DHEA effects on amino acid receptors, very little is known of interaction of DHEA with the capsaicin
receptor.
In the present study, we examined electrophysiologically the effects of DHEA and related steroids on the capsaicin receptor- mediated current in acutely dissociated male rat dorsal root ganglion neurons using whole- cell voltage-clamp technique [14]. We report that DHEA competitively inhibits the capsaicin-induced current in a specific and concentration-dependent manner. Moreover, structure-activity studies indicate that the interaction of DHEA and related steroids with the capsaicin receptor is stereospecific.
3. Results and Discussion
At a holding potential of –50 mV, capsaicin produced inward currents, which reversed at or near 0 mV in standard recording solutions, as expected for Na+, K+ and Ca2+-mediated currents (data not shown).
The current induced by 100 nM capsaicin reached slowly to a steady level and did not decline with repeated capsaicin applications.
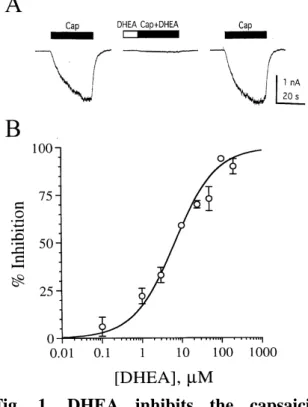
The effect of DHEA on the current induced by 100 nM capsaicin is illustrated in Fig. 1.
Pressure application of 100 µM DHEA rapidly and reversibly inhibited the capsaicin response by 94 ± 1.3% (n = 6) (Fig. 1A).
DHEA alone produced little or no direct response. To quantitatively evaluate the potency and efficacy of DHEA for capsaicin receptors, pooled data were used to construct the dose-response curve for inhibition of the 100 nM capsaicin response by DHEA.
DHEA inhibited the capsaicin-induced current in a concentration-dependent manner, with an EC50 of 6.7 µM, a maximal inhibition of 100%, and a Hill coefficient of 0.83 (Fig. 1B).
To investigate the mechanism of DHEA action on the capsaicin receptor, we examined the effect of 50 µM DHEA on currents induced by higher concentrations of capsaicin. In this experiment, KCl was replaced with CsCl in the pipet solution and the cell was held at –20 mV to reduce the amplitude of the capsaicin-induced current.
As illustrated in Fig. 2, the percentage of
inhibition by DHEA of the capsaicin-induced current was significantly attenuated by increasing capsaicin from 0.2 µM (81 ± 1.8%, n = 7) to 0.5 µM (70 ± 4.0%, n = 10), 1 µM (44 ± 7.0%, n = 7) or to 3 µM (26 ± 7.4%, n
= 5). This result suggests that antagonism of the capsaicin response by DHEA is competitive in nature and that DHEA inhibits the capsaicin response by acting through the capsaicin recognition site.
We also examined the effects of other chemically related steroids, including progesterone and some reduced metabolites of DHEA (Table 1). Not all steroids inhibited the capsaicin response. Progesterone (100 µM) did not exert any significant effect on the capsaicin response (Fig. 3 and Table 1), suggesting that inhibition of the capsaicin- induced current by DHEA is a specific effect.
5α-Androstan-3β-ol-17-one (5α3β-DHEA), the reduced metabolite of DHEA, had activity similar to that of DHEA, whereas DHEA sulfate (DHEAS) and pregnenolone sulfate (PS) produced lesser effect. Thus, reduction at C-5 in the DHEA structure does not change its effect on the capsaicin response, whereas addition of sulfate group at C-3β results in weaker activity.
Interestingly, the stereoisomer of DHEA, 5- androsten-3α-ol-17-one (3α-DHEA), failed to inhibit the capsaicin-induced current, producing instead a potentiating effect on the capsaicin response (Fig. 4 and Table 1).
These findings indicate that the interaction of steroids with the capsaicin receptor is stereospecific.
4. Self-Evaluation of Gr ant Repor t
This study demonstrates that the neurosteroid DHEA dose-dependently inhibits the capsaicin-induced current in a specific and competitive manner.
Furthermore, its stereoisomer 3α-DHEA produces an opposite modulatory effect, potentiating the capsaicin response. This result is consistent with the idea that interaction of steroids with the capsaicin
receptor is stereospecific. Capsaicin receptor activation has been proposed to play an important role in nociception. The inhibitory action of DHEA on the capsaicin receptor may provide a basis for a novel approach to reduce the pain.
The manuscript is in preparation and will be submitted for publication in near future. However, before submitting our manuscript for publication, we will perform further experiments to address the following questions: a) Does DHEA act intracellularly to inhibit the capsaicin-induced current? b) What is the effect of DHEA on kinetic properties of single-channel activity induced by capsaicin? c) Does DHEA inhibit capsaicin-induced nociceptive response in rats in vivo?
5. Refer ences
[1] Green, B.G. and Shaffer, G.S., The sensory response to capsaicin during repeated topical exposures: differential effects of itching and pungency, Pain, 53 (1993) 323-334.
[2] Holzer, P., Capsaicin: cellular targets, mechanisms of action, and selectivity for thin sensory neurons, Pharmacol. Rev., 43 (1991) 144-201.
[3] Szallasi, A. and Blumberg, P.M., Vanilloid (capsaicin) receptors and mechanisms, Pharmacol. Rev., 51 (1999) 159-211.
[4] Heyman, I. and Rand, H.P., Depolarizing responses to capsaicin in a subpopulation of rat dorsal root ganglion cells, Neurosci.
Lett., 56 (1985) 69-75.
[5] Oh, U., Hwang, S.W. and Kim, D., Capsaicin activates a nonselective cation channel in cultured neonatal rat dorsal root ganglion neurons, J. Neurosci., 16 (1996) 1659-1667.
[6] Szolcsanyi, J., Capsaicin, irritation and desensitization. In: Chemical senses (Green, B.G., Mason, J.R. and Kare, M.R., ed.), New York: Dekker, (1990) 141-168.
[7] Majewska, M.D., Steroids and brain
activity: essential dialogue between body and mind, Biochem. Pharmacol., 36 (1987) 3781-3788.
[8] McEwen, B.S., Non-genomic and genomic effects of steroids on neural activity, Trends Pharmacol. Sci., 12 (1991) 141-147.
[9] Majewska, M.D., Harrison, N.L., Schwartz, R.D., Barker, J.L. and Paul, S.M., Steroid hormone metabolites are barbiturate-like modulators of the GABA receptor, Science, 232 (1986) 1004-1007.
[10] Wu, F.-S., Gibbs, T.T. and Farb, D.H., Inverse modulation of γ-aminobutyric acid- and glycine-induced currents by progesterone, Mol. Pharmacol., 37 (1990) 597-602.
[11] Wu, F.-S., Gibbs, T.T. and Farb, D.H., Pregnenolone sulfate: a positive allosteric modulator at the N-methyl-D-aspartate receptor, Mol. Pharmacol., 40 (1991) 333-336.
[12] Park-Chung, M., Wu, F.-S., Purdy, R.H., Malayev, A.A., Gibbs, T.T. and Farb, D.H., Distinct sites for inverse modulation of N-methyl-D-aspartate receptors by sulfated steroids, Mol.
Pharmacol., 52 (1997) 1113-1123.
[13] Baulieu, E.-E. and Robel, P., Dehydroepiandrosterone and dehydroepiandrosterone sulfate as neuroactive neurosteroids, J. Endocrinol., 150 (1996) S221-S239.
[14] Hamill, O.P., Marty, A., Neher, E., Sakmann, B. and Sigworth, F.J., Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches, Pfluegers Arch. Eur. J. Physiol., 391 (1981) 85-100.
[15] Lean, A.P., Munson, P.J. and Rodbard, D., Simultaneous analysis of families of sigmoidal curves: application to bioassay, radioligand assay, and physiological dose-response curves, Am. J. Physiol., 235 (1978) E97-E102.
Fig. 1. DHEA inhibits the capsaicin r esponse. (A) DHEA (100 µM) inhibits rapidly and reversibly the current induced by 100 nM capsaicin (Cap). Horizontal bar above each trace, period of drug application.
(B) Dose-response curve for inhibition of the capsaicin (100 nM) response by DHEA. Data points, percentage change in peak current in the presence of DHEA (mean of 4-7 experiments). Error bars, standard errors.
DHEA dose-response curve is fitted with the logical equation (15). (% inhibition)/(%
inhibition)max = [DHEA]nH/([DHEA]nH + EC50nH) where [DHEA] is the concentration of DHEA, and nH is the Hill coefficient.
Curve-fit analysis reveals an EC50 of 6.7 µM, a maximal inhibition of 100%, and nH of 0.83.
Fig. 2. Antagonism of the capsaicin r esponse by DHEA is competitive.
Inhibition of the capsaicin-induced current by 50 µM DHEA is significantly reduced by increasing capsaicin from 0.2 µM to 0.5 µM, 1 µM or to 3 µM. Error bars, stndard errors.
Number of cells is indicated in parentheses.
*** P < 0.001 and * P < 0.05 when compared to 0.2 µM capsaicin.
Fig. 3. Pr ogester one does not have an inhibitor y effect on the capsaicin r esponse.
Application of 100 µM progesterone (P) produces no significant effect on the 100 nM capsaicin-induced current.
Fig. 4. 3α-DHEA enhances the capsaicin r esponse. Application of 50 µM 3α-DHEA enhances rapidly and reversibly the current induced by 100 nM capsaicin.
Table 1. Effects of ster oids on the 100 nM capsaicin-induced cur r ent. Holding potential is –50 mV. Values are means ± standard errors. Number of cells is indicated in parentheses. Abbreviations: DHEA, 5- androsten-3β-ol-17-one; 5α3β-DHEA, 5α- androstan-3β-ol-17-one; DHEAS, 5- androsten-3β-ol-17-one sulfate; PS, pregnenolone sulfate; 3α-DHEA, 5- androsten-3α-ol-17-one.