Rimantadine and 2-adamantanamine elicit local anesthesia against cutaneous nociceptive stimuli in a rat model
Ching-Hsia Hung1, Ph.D., Chin-Chen Chu2,3, M.D., Ph.D., Yu-Chung Chen4, M.S., Yu-Wen Chen5,6,*, Ph.D., Jhi-Joung Wang5, M.D., Ph.D.
1 Institute & Department of Physical Therapy, National Cheng Kung University, Tainan, Taiwan
2 Department of Anesthesiology, Chi Mei Medical Center, Tainan, Taiwan
3 Department of Recreation and Health-Care Management, Chia Nan University of Pharmacy and Science, Tainan, Taiwan
4 Division of Physical Therapy, Department of Physical Medicine and Rehabilitation, Cheng Hsin General Hospital, Taipei, Taiwan
5 Department of Medical Research, Chi Mei Medical Center, Tainan, Taiwan
6 Department of Physical Therapy, China Medical University, Taichung, Taiwan
Conflicts of interest: There is no conflict of interests for all authors.
Running Head (Limit 6 words): Cutaneous anaesthesia of rimantadine and 2- adamantanamine
*Corresponding Author:
Yu-Wen Chen, PhD.
Department of Physical Therapy China Medical University
No.91 Hsueh-Shih Road, Taichung 40402, Taiwan Phone: 886-4-22053366 ext 7327
FAX: 886-4-22065051
Email: cywhwok@mail.cmu.edu.tw
ABSTRACT
The aim of this study was to investigate infiltrative cutaneous anesthesia of 2-
adamantanamine and rimantadine. After subcutaneous injections of drugs in rats, the blockade of cutaneous trunci muscle reflex by 2-adamantanamine and rimantadine were evaluated. Lidocaine, a common local anesthetic, was used as control. We showed that rimantadine and 2-adamantanamine, as well as the local anesthetic lidocaine produced infiltrative anesthesia of skin in a dose-dependent fashion. Saline (vehicle) group displayed no cutaneous anesthesia. The relative potency of these drugs was rimantadine [23.8 (21.1 – 26.8)] = lidocaine [26.4 (22.7 – 30.6)] > 2- adamantanamine [64.6 (55.0 – 75.9)] (P < 0.01). On an equianesthetic basis [25%
effective dose (ED25), ED50, and ED75], rimantadine and 2-adamantanamine had longer duration of action than lidocaine (P < 0.05). Neither local injection of saline nor intraperitoneal administration of a large dose of drugs elicited cutaneous anesthesia (data not shown). These data demonstrated for the first time that rimantadine had a similar potent and longer duration of skin infiltrative anesthesia than did lidocaine, whereas 2-adamantanamine had a less potency but longer duration of cutaneous
anesthesia than did lidocaine.
Key Words: 2-adamantanamine; rimantadine; lidocaine; infiltrative cutaneous
anesthesia
INTRODUCTION
2-Adamantanamine (2-adamantylamine) has been shown to be a possible
candidate for a new class of insulin secretagogues in the in vitro experiments [1], though it is still not recognized as a drug for treatment of Parkinson’s disease. Also, rimantadine [1-(1-adamantyl) ethylamine] and memantine (1-amino-3,5-
dimethyladamantane), which are derivatives of 1-adamantanamine (amantadine), have potent abilities to treat Parkinson’s disease [2, 3]. Currently, subcutaneous injection of memantine shows skin local anesthesia, is more potent than lidocaine, and has a longer duration of action [4]. Furthermore, rimantadine exhibits equal efficacy and fewer adverse reactions than amantadine, and it is used to treat that infections of influenza virus [5, 6]. In adults, the efficacy and safety of rimantadine for relieving or
treating symptoms of influenza A has been reported [7, 8].
The most commonly used local anesthetics share the similar molecular
structures, and likewise cause the similar central nervous system and cardiovascular system toxicity [9, 10]. Though local anesthetics have been known to apply clinically for more than 150 years, there has been minimal exploration of new local anesthetics whose chemical structures totally differ from the traditional local anesthetics. In the present study, we examined whether the adamantine derivatives, in particular 2- adamantanamine and rimantadine (Fig. 1), possess the local anesthetic effects, although it has been known that memantine elicits cutaneous anesthesia [4]. The aim of this study was to evaluate the cutaneous (local) anesthetic effect of 2-
adamantanamine and rimantadine. Lidocaine, a known local anesthetic, was used as control.
MATERIALS AND METHODS
Animals
The experimental protocols were approved by the Institutional Animal Care and
Use Committee of China Medical University (Taichung, Taiwan), and conformed to the recommendations and policies of the International Association for the Study of Pain (IASP). Male Sprague-Dawley rats weighting 200-250 g were purchased from the National Laboratory Animal Centre (Taipei, Taiwan), and then housed in a climate controlled room maintained at 21 ℃ with approximately 50% relative humidity in Animal Center of China Medical University (Taichung, Taiwan). The lights were on a 12-h light/dark cycle (light on at 6:00 AM), with food and water
available ad libitum up to time of experiments.
Drugs
2-Adamantanamine (2-adamantylamine) HCl, 1-(1-adamantyl)ethylamine (rimantadine) HCl, and lidocaine HCl were obtained from Sigma-Aldrich Chemical Co. (St. Louis, MO, USA). All drugs were dissolved in 0.9% NaCl (saline) before the
experiment.
Experimental designs
The rats were divided into three experiments. In experiment 1, infiltrative cutaneous anesthesia of 2-adamantanamine (160, 120, 80, 40, 20 μmol/kg),
rimantadine (53, 40, 20, 10, 5 μmol/kg), lidocaine (53, 33, 23, 13 μmol/kg), and saline (vehicle) were evaluated (n=8 rats for each dose of each drug). In experiment 2, on an equipotent basis (ED25, ED50 and ED75), the duration of 2-adamantanamine or
rimantadine was compared with that of lidocaine (n=8 rats for each dose of each
drug). In experiment 3, one group (n=8 rats for each dose of each drug), subcutaneous injection of saline combined with intraperitoneal injection of each drug (2-
adamantanamine, rimantadine or lidocaine) at a dose of 2ED75 was performed to rule out the possibility of systemic effect of these drugs as infiltrative cutaneous
anesthesia.
Injections of drugs
Before subcutaneous injections, the hair on the dorsal surface of the
thoracolumbar region (6×10 cm2) of rats was mechanically shaved. Subcutaneous injections of drugs were carried out as previously reported [11, 12]. In brief, the 0.6 mL of drugs was subcutaneously injected by using a 30-gauge needle in
unanesthetized rats at the dorsal surface of the thoracolumbar region. In order to decrease the numbers of experimental animals used, the back of the rat was further divided into right and left parts, either of which, after a washout period of 1 week, received one drug injection. Subcutaneous injection elicited a circular skin wheal, approximately 2 cm in diameter. Then the wheal was marked with ink within one minute after subcutaneous injection [13, 14]. For consistency, an experienced
investigator, who was blinded to the identity of the injected drugs, was responsible for
assessing the local/cutaneous anesthetic effect.
Neurobehavioral evaluation
Infiltrative cutaneous anesthesia elicited by various drugs was evaluated
according to the cutaneous trunci muscle reflex (CTMR), characterized via the reflex movement of the skin over the back elicited by twitches of the lateral thoracispinal muscle in response to local dorsal cutaneous stimulus [4, 15]. The cut end of an 18- gauge needle (a fresh regular bevel needle) was affixed to a Von Frey filament (No.15; Somedic Sales AB, Stockholm, Sweden) to produce a standardized noxious mechanical stimulation (19±1 g). After observing the animal’s normal reaction to stimuli applied outside the wheal and on the contralateral side, we applied 6 stimuli at 6 different points within each wheal, with a frequency of 0.5-1 Hz, and scored the number to which the rat failed to react [16]. Each drug’s cutaneous anesthesia was quantitatively evaluated as the number of times the stimuli failed to elicit a response.
For instance, the absence of any response after 6 stimuli was defined as complete nociceptive/sensory block (100% of possible effect; 100% PE), which was calculated
as follows:
% PE = ((number of stimuli that provoked no response)/6) x 100
Stimulus testing was applied 10 min before injection of drugs to confirm normal responses of rats, then every 5 min after injection for the first 30 min and every 10-15
min thereafter, until the CTMR fully recovered from the block (no more than 3 h).
The duration and potency of drugs for cutaneous anesthesia
Each drug’s duration of action was defined as the time from drug injection (i.e., time=0) to full recovery of CTMR (no analgesic effect, i.e. 0% PE). The maximum
blockade in a time course of cutaneous anesthesia of drugs was described as the percent of maximal possible effect (% MPE). In order to measure 50% effective doses (ED50),as well as ED25 and ED75, rats were subcutaneously injected with different doses of each drug (n = 8 rats for each dose of each agent), and dose-response curves were constructed from the % MPE of each dose of each drug. The curves were then fitted via SAS nonlinear (NLIN) procedures (version 9.1, SAS Institute, Cary, NC), and this value of ED50, defined as the dose that caused 50% cutaneous anesthesia, was gained [17, 18]. Furthermore, the area under curve (AUC) of cutaneous anesthesia of drugs was estimated using Kinetica version 2.0.1 (InnaPhase Corporation,
Philadelphia, PA).
Statistical analysis
Values are presented as mean ± S.E.M. or ED25, ED50, and ED75 values with 95%
confidence interval (95% CI). The differences in Table 1 between drugs were
assessed by two-sided Student's t with unequal variances. The differences in potencies (ED50s in Table 2) between medications were evaluated using one-way analysis of variance (ANOVA) and Tukey's honestly significant difference (HSD) test for paired comparisons. In the control groups, a one-way ANOVA followed by the Dunnett test was used to evaluate the effects of medications. The differences in durations (Fig. 3) among drugs were evaluated by two-way ANOVA followed by pairwise Tukey's
HSD test. SPSS for Windows (version 17.0) was used for all statistical analyses.
Statistical significance was set at P < 0.05.
RESULTS
Dose-dependent effects of rimantadine, 2-adamantanamine, and lidocaine as
infiltrative cutaneous anesthesia
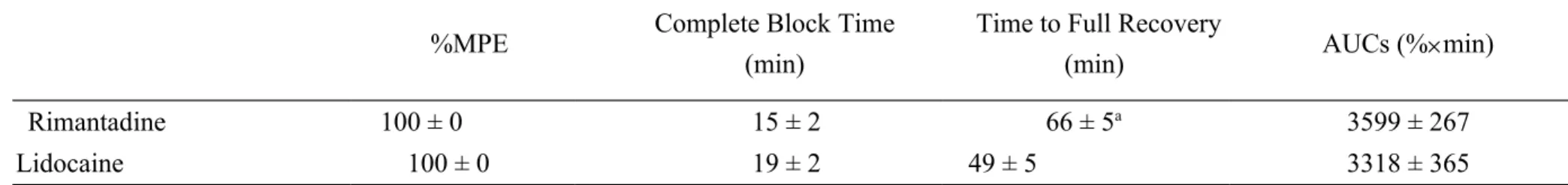
The chemical structures of rimantadine and 2-adamantanamine differ from that of lidocaine (Fig. 1). Both 2-adamantanamine and rimantadine, as well as the local anesthetic lidocaine elicited a dose-dependent cutaneous anesthesia in rats (Fig. 2).
Furthermore, rimantadine at a dose of 53 µmol/kg reached its peak effect (100%
MPE) with time to complete block about 15±2 min and had a more prolonged time to
full recovery than lidocaine (P = 0.042; Table 1). Lidocaine dose-dependently (13-53 µmol/kg) produced cutaneous anesthesia (Fig. 2) and reached its peak effect (100%
MPE) with time to complete block about 19±3 min after subcutaneous injection (Table 1). The AUCs between rimantadine and lidocaine were not significantly
different (Table 1). Saline injection produced no cutaneous anesthesia.
Durations of rimantadine, 2-adamantanamine, and lidocaine as infiltrative
cutaneous anesthesia
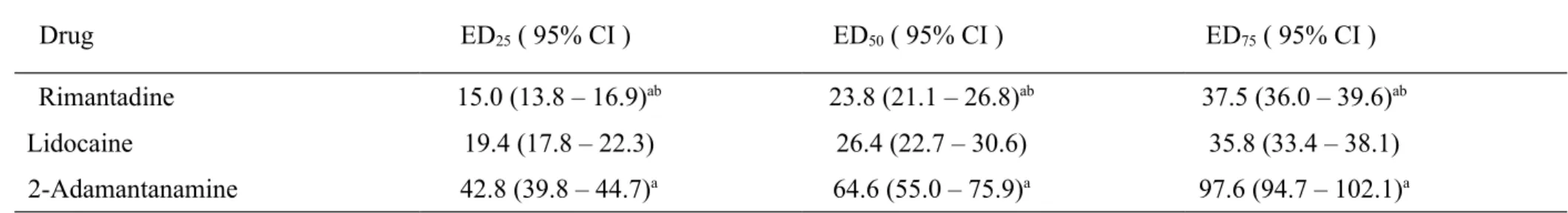
The ED25s, ED50s, and ED75s were obtained from the dose-response curves of 2- adamantanamine, rimantadine, and lidocaine (Table 2). On the ED50 basis, the relative potency of these drugs was shown to be rimantadine = lidocaine > 2-adamantanamine (P < 0.01; Table 2). On an equianesthetic basis (ED25, ED50, and ED75), the
nociceptive/sensory block duration caused by 2-adamantanamine or rimantadine was longer than that caused by lidocaine (P < 0.05; Fig. 3). All animals recovered
completely after subcutaneous injections of these drugs.
DISCUSSION
In this study, we reported for the first time that 2-adamantanamine and rimantadine displayed a dose-dependent local anesthetic effect as infiltrative
cutaneous analgesia in rats. Rimantadine had more potent cutaneous anesthesia than 2-adamantanamine. Another important finding in this work is that when compared with lidocaine, both rimantadine and 2-adamantanamine produced a longer duration
of infiltrative anesthesia of skin.
That rimantadine and 2-adamantanamine could produce cutaneous anesthesia in a dose-dependent fashion is not entirely unexpected. We suggest that most
adamantine derivatives, in particular memantine [4], rimantadine, and 2-
adamantanamine hold the local anesthetic effects. Furthermore, we demonstrated that
rimantadine displayed almost 2.7-folds greater potency than 2-adamantanamine for infiltrative cutaneous anesthesia. Although rimantadine had a similar potency of cutaneous anesthesia when compared with lidocaine, it produced a longer duration of
sensory/nociceptive blockade than lidocaine.
Injection of long-lasting local anesthetics is known to for, for example,
infiltration anesthesia of skin incision sites for laparoscopic surgery [19] and also to supply postoperative pain relief after inguinal hernia repair or vaginal hysterectomy [20]. In this present study, subcutaneous injection of rimantadine produced longer duration of action than lidocaine (Table 1) at the same dose of 53 μmol/kg. Besides, on an equianesthetic basis (ED25, ED50 and ED75), the block duration caused by rimantadine or 2-adamantanamine was longer than that of lidocaine (Fig. 3). It has been known that, in comparison with bupivacaine, the local anesthetic effect of lidocaine was relatively short action. According to our present and previous
experiments, the duration caused by 2-adamantanamine was similar to that caused by the long-acting local anesthetic bupivacaine [21]. Protein binding is to correlate well with the local anesthetic duration of action, but other factors also have a significant effect such as dose administered, potency, vascularity of the tissue, addition of vasoconstrictors, and rate of metabolism [9].
A paper by Muth-Selbach et al. reported that systemically administered lidocaine
had a central, not a peripheral antinociceptive effect in both acute and chronic pain model [22], hence we performed that one control study via systemic (intraperitoneal) administration of a large dose of these drugs caused no infiltrative cutaneous
anesthesia (data not shown). These results support our finding that rimantadine and 2-
adamantanamine as well as lidocaine elicit local anesthesia of skin.
This study has some limitations. Although memantine displays use-dependent inhibition of tetrodotoxin-resistant Na+ currents in small dorsal root ganglion neurons [23] and causes skin infiltrative anesthesia in rats [4], future study of whether
rimantadine and 2-adamantanamine block Na+ currents should be considered. Due to their similar chemical structures, conventional local anesthetics may induce central nervous system toxicity and cardiovascular toxicity. However, the chemical structure of rimantadine (or 2-adamantanamine) is totally different and systemic use has proved well-tolerated and safe [7, 8]. We did not evaluate whether these drugs caused local toxicity at the injection site, and further studies on nerve block and related neural and cardiovascular toxicities will be warranted.
In conclusion, these preclinical data showed that rimantadine, as well as lidocaine produces local anesthesia against cutaneous nociceptive stimuli, and are more potent than 2-adamantanamine. Rimantadine is equal in potency to lidocaine. Rimantadine and 2-adamantanamine have a longer duration of action at producing infiltration
anesthesia compared with lidocaine.
ACKNOWLEDGEMENTS
The authors gratefully acknowledge the financial support provided for this study by the National Science Council (NSC 100-2314-B-039 -017 -MY3) of Taiwan.
REFERENCES
1. Garrino M.G., Henquin J.C. Adamantane derivatives: a new class of insulin
secretagogues. Br. J. Pharmacol. (1987) 90 583-591.
2. Merello M., Nouzeilles M.I., Cammarota A., Leiguarda R. Effect of memantine (NMDA antagonist) on Parkinson's disease: a double-blind
crossover randomized study. Clin. Neuropharmacol. (1999) 22 273-276.
3. Evidente V.G., Adler C.H., Caviness J.N., Gwinn-Hardy K. A pilot study on the motor effects of rimantadine in Parkinson's disease. Clin. Neuropharmacol.
(1999) 22 30-32.
4. Chen Y.W., Chu C.C., Chen Y.C., Wang J.J., Hung C.H. The local anesthetic effect of memantine on infiltrative cutaneous analgesia in the rat. Anesthesia
and analgesia (2011) 113 191-195.
5. Dolin R., Reichman R.C., Madore H.P., Maynard R., Linton P.N., Webber- Jones J. A controlled trial of amantadine and rimantadine in the prophylaxis of influenza A infection. N. Engl. J. Med. (1982) 307 580-584.
6. Wingfield W.L., Pollack D., Grunert R.R. Therapeutic efficacy of amantadine HCl and rimantadine HCl in naturally occurring influenza A2 respiratory
illness in man. N. Engl. J. Med. (1969) 281 579-584.
7. Alves Galvao M.G., Rocha Crispino Santos M.A., Alves da Cunha A.J.
Amantadine and rimantadine for influenza A in children and the elderly.
Cochrane Database Syst. Rev. (2012) 1 CD002745.
8. Jefferson T., Demicheli V., Di Pietrantonj C., Rivetti D. Amantadine and rimantadine for influenza A in adults. Cochrane Database Syst. Rev. (2006)
CD001169.
9. McLure H.A., Rubin A.P. Review of local anaesthetic agents. Minerva
Anestesiol (2005) 71 59-74.
10. Chen Y.W., Huang K.L., Liu S.Y., Tzeng J.I., Chu K.S., Lin M.T., Wang J.J.
Intrathecal tri-cyclic antidepressants produce spinal anesthesia. Pain (2004)
112 106-112.
11. Chen Y.W., Wang J.J., Liu T.Y., Chen Y.C., Hung C.H. Systemic
dextromethorphan and dextrorphan are less toxic in rats than bupivacaine at
equianesthetic doses. Can. J. Anaesth. (2011) 58 55-61.
12. Hung C.H., Liu K.S., Shao D.Z., Cheng K.I., Chen Y.C., Chen Y.W. The systemic toxicity of equipotent proxymetacaine, oxybuprocaine, and bupivacaine during continuous intravenous infusion in rats. Anesth. Analg.
(2010) 110 238-242.
13. Chen Y.W., Chu C.C., Chen Y.C., Hung C.H., Hsueh M.I., Wang J.J.
Clonidine as adjuvant for oxybuprocaine, bupivacaine or dextrorphan has a significant peripheral action in intensifying and prolonging analgesia in response to local dorsal cutaneous noxious pinprick in rats. Neurosci. Lett.
(2011) 496 186-190.
14. Chen Y.W., Chu K.S., Lin C.N., Tzeng J.I., Chu C.C., Lin M.T., Wang J.J.
Dextromethorphan or dextrorphan have a local anesthetic effect on infiltrative
cutaneous analgesia in rats. Anesth. Analg. (2007) 104 1251-1255.
15. Chen Y.W., Liu K.S., Wang J.J., Chou W., Hung C.H. Isobolographic analysis of epinephrine with bupivacaine, dextromethorphan, 3-methoxymorphinan, or dextrorphan on infiltrative anesthesia in rats: dose-response studies. Reg.
Anesth. Pain Med. (2008) 33 115-121.
16. Chen Y.W., Chu C.C., Chen Y.C., Wang J.J., Hung C.H., Shao D.Z.
Nisoxetine produces local but not systemic analgesia against cutaneous
nociceptive stimuli in the rat. Eur. J. Pharmacol. (2012) 675 22-25.
17. Hung C.H., Chu C.C., Chen Y.C., Chen Y.W., Li Z.Y., Wang J.J. Spinal anesthesia with diphenhydramine and pheniramine in rats. Eur. J. Pharmacol.
(2011) 673 20-24.
18. Leung Y.M., Wu B.T., Chen Y.C., Hung C.H., Chen Y.W. Diphenidol
inhibited sodium currents and produced spinal anesthesia. Neuropharmacology
(2010) 58 1147-1152.
19. Carbonell A.M., Harold K.L., Mahmutovic A.J., Hassan R., Matthews B.D., Kercher K.W., Sing R.F., Heniford BT. Local injection for the treatment of suture site pain after laparoscopic ventral hernia repair. The American surgeon
(2003) 69 688-691.
20. Suraseranivongse S., Chowvanayotin S., Pirayavaraporn S., Kongsayreepong S., Gunnaleka P., Kraiprasit K., Petcharatana S., Montapaneewat T. Effect of bupivacaine with epinephrine wound instillation for pain relief after pediatric inguinal herniorrhaphy and hydrocelectomy. Regional anesthesia and pain
medicine (2003) 28 24-28.
21. Chen Y.W., Chu C.C., Chu K.S., Shieh J.P., Chien C.C., Wang J.J., Kao C.H.
Phenothiazine-type antipsychotics elicit cutaneous analgesia in rats. Acta
Anaesthesiol. Taiwan (2010) 48 3-7.
22. Muth-Selbach U., Hermanns H., Stegmann J.U., Kollosche K., Freynhagen R., Bauer I., Lipfert P. Antinociceptive effects of systemic lidocaine: involvement
of the spinal glycinergic system. Eur. J. Pharmacol. (2009) 613 68-73.
23. Brau M.E., Dreimann M., Olschewski A., Vogel W., Hempelmann G. Effect of drugs used for neuropathic pain management on tetrodotoxin-resistant Na(+) currents in rat sensory neurons. Anesthesiology (2001) 94 137-144.
Table 1. The percentage of maximal possible effect (%MPE), duration, and area under curves (AUCs) of drugs as infiltrative cutaneous anesthesia in rats.
%MPE Complete Block Time
(min)
Time to Full Recovery
(min) AUCs (%min)
Rimantadine 100 ± 0 15 ± 2 66 ± 5a 3599 ± 267
Lidocaine 100 ± 0 19 ± 2 49 ± 5 3318 ± 365
The %MPE, duration of action, AUCs for rimantadine and lidocaine (meanS.E.M.) at the same dose of 53.3 μmol/kg (n = 8 rats for each dose of each drug) were obtained from Figure 2. Of note, all of the rats displayed complete blockade (100%MPE) of any function tested. The symbol
(a) indicates P = 0.042 when rimantadine compared with lidocaine using two-sided Student's t with unequal variances.
Table 2. The 25% effective doses (ED25s), ED50s, and ED75s of drugs for infiltrative cutaneous anesthesia in rats.
Drug ED25 ( 95% CI ) ED50 ( 95% CI ) ED75 ( 95% CI )
Rimantadine 15.0 (13.8 – 16.9)ab 23.8 (21.1 – 26.8)ab 37.5 (36.0 – 39.6)ab
Lidocaine 19.4 (17.8 – 22.3) 26.4 (22.7 – 30.6) 35.8 (33.4 – 38.1)
2-Adamantanamine 42.8 (39.8 – 44.7)a 64.6 (55.0 – 75.9)a 97.6 (94.7 – 102.1)a
The ED50s of drugs (μmol/kg) were obtained from Figure 2. CI = confidence interval. Symbols (a,b) indicated P<0.01 when compared with lidocaine and 2-adamantanamine, respectively, using a one-way ANOVA and pairwise Tukey’s HSD test for paired comparisons.
CH
3H
3C
NH COCH
2N
C
2H
5C
2H
5NH
2H
3C NH
22-Adamantanamine
Rimantadine
Lidocaine
Fig. 1.
Time (min)
0 15 30 45 60 75 90 105 120 135 150
%PE (possible effect)
0 20 40 60 80
100 53 molkg
33 mol/kg 23 molkg 13 mol/kg Saline
Rimantadine
2-Adamantanamine
Lidocaine
0 15 30 45 60 75 90 105 120 135 150
PE %(possible effect%)
0 20 40 60 80
100 160 mol/kg
120 mol/kg 80 mol/kg 40 mol/kg 20 mol/kg
0 15 30 45 60 75 90 105 120 135 150
PE %(possible effect%)
0 20 40 60 80
100 53 mol/kg
40 mol/kg 20 mol/kg 10 mol/kg 5 mol/kg
Fig. 2.
ED ( effective dose )
25 50 75
F u ll R ec ov er y T im e ( m in )
0 10 20 30 40 50 60
2-Adamantanamine (AT) Rimantadine (RT)
Lidocaine (LD)
AT = RT > LD
Fig. 3.
FIGURE LEGENDS
Fig. 1. The chemical structures of 2-adamantanamine, rimantadine, and lidocaine.
Fig. 2. Time courses (4 or 5 doses in each drug) of cutaneous anesthesia with 2- adamantanamine, rimantadine, and lidocaine in rats. Saline (vehicle) group was used as control. Values are expressed as meanS.E.M. For each group of the time course
study, n=8 rats.
Fig. 3. Full recovery time (duration) of 2-adamantanamine, rimantadine, and lidocaine effect as infiltrative cutaneous anesthesia at doses of ED25, ED50, and ED75 (n = 8 at each testing point). Data are mean±S.E.M. The difference in duration was evaluated via using 2-way ANOVA followed by pairwise Tukey's HSD test.