1
Chapter 17:
Electrochemistry Electrochemistry
The study of the interchange of The study of the interchange of chemical and electrical energy chemical and electrical energy
2
Review of Terms
oxidation-reduction (redox) reaction:
involves a transfer of electrons from the reducing agent to the oxidizing agent.
• oxidation (氧化): loss of electrons
• reduction (還原): gain of electrons
3
Half-Reactions
The overall reaction is split into two half- reactions, one involving oxidation and one reduction.
8H
++ MnO
4?+ 5Fe
2+→ Mn
2++ 5Fe
3++ 4H
2O
Reduction: 8H
++ MnO
4?+ 5e
?→ Mn
2++ 4H
2O Oxidation: 5Fe
2+→ 5Fe
3++ 5e
?4
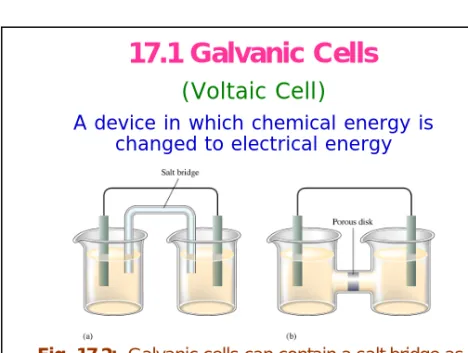
17.1 Galvanic Cells
(Voltaic Cell)
A device in which chemical energy is changed to electrical energy
Fig. 17.2: Galvanic cells can contain a salt bridge as in (a) or a porous-disk connection as in (b).
5
Fig. 17.3: An electrochemical process involves electron transfer at the interface between the electrode and the solution. (a) The species in the solution acting as the reducing agent supplies electrons to the anode. (b) The species in the solution acting as the oxidizing agent receives electrons from the cathode.
• Oxidation occurs at the Anode.
• Reduction occurs at the Cathode.
6
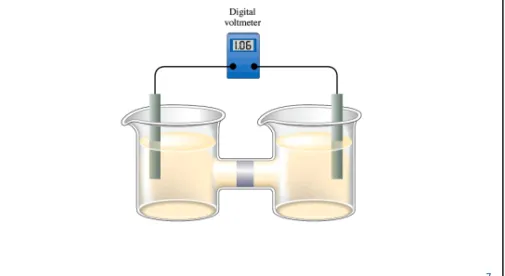
Cell Potential
Cell Potential
orElectromotive Force (emf):
The “pull” or driving force on the electrons.
單位: V (1 V = 1 J/C) 測量工具:
Ø
Voltmeter: 有微小電流,造成能量損耗故所測得之 V < E
cellØ
Potentiometer: 加一反向電壓,直到電流為零故無能量損耗,測得 V = E
cell7
Fig. 17.4: Digital voltmeters draw only a negligible current and are convenient to use.
8
以SHE 為參考電極可定出其他半反應之電位,例如:
•Cu2++ 2e− → Cu E°= 0.34 V vs. SHE
•SO42−+ 4H+ + 2e− → H2SO3+ H2O E°= 0.20 V vs. SHE
17.2 Standard Reduction Potentials
標準還原電位
The E
°values corresponding to reduction half-reactions with all solutes at 1M and all gases at 1 atm.
標準氫電極 (
standard hydrogen electrode, SHE)
2H++ 2e− → H2 E° = 0 VWhere [H+] = 1 M, PH2 = 1 atm
9
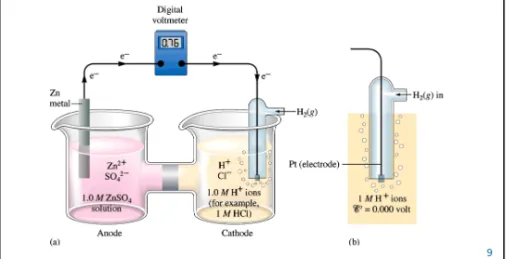
Fig. 17.5: (a) A galvanic cell involving the reactions Zn → Zn2++ 2e–(at the anode) and 2H+ + 2e-→H2(at the cathode) has a potential of 0.76 V. (b) The standard hydrogen electrode where H2(g) at 1 atm is passed over a platinum electrode in contact with 1 M H+ ions. This electrode process (assuming ideal behavior) is arbitrarily assigned a value of exactly zero volts.
10
11
Fig. 17.6: A galvanic cell involving the half-reactions Zn→Zn2++ 2e–(anode) and Cu2++ 2e–→Cu
(cathode), with Eºcell= 1.10 V.
0 Cu Cu 0
Zn Zn 0
cell
= E
→ 2++ E
2+→E
1.10 V = 0.76 V + 0.34 V
12
A
當結合 2 half-reactions 得到平衡之氧化還原 反應式時,有兩點注意事項:
1. The sign of the potential for the reversed half-reaction also must be reversed.
2. The value of E
ois not changed when a half-reaction is multiplied by an integer.
Sample Exercise 17.1
13
Line notation:
to describe electrochemical cells Anode 寫在左邊、Cathode 寫在右邊
例如: a galvanic cell based on
2 Al3+(aq)+ 3 Mg(s )→2 Al(s )+ 3 Mg2+(aq)
Line notation 為: Mg(s)|Mg2+(aq)||Al3+(aq)|Al(s)
2MnO4-(aq)+6H+(aq)+5ClO3-(aq)→2Mn2+(aq)+3H2O(l)+5ClO4-(aq) Line notation 為:
Pt(s)|ClO3-(aq),ClO4-(aq),H+(aq)||MnO4-(aq),Mn2+(aq),H+(aq)|Pt(s)
14
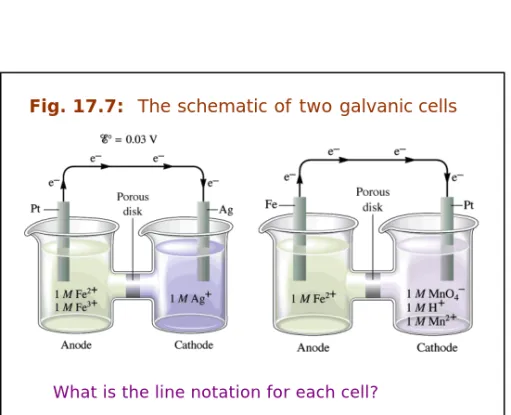
Fig. 17.7: The schematic of two galvanic cells
What is the line notation for each cell?
Read textbook!
15
emf potential difference V work J ch e C
= ( ) = ( )
arg ( ) 17.3 Cell Potential, Electrical Work,
and Free Energy
q E = − w
max max
max
max
qE or w qE
w = = −
−
∵ w 是以系統而言,系統對外 作功,w < 0,故加 “ – ” 以得 正值之 E。
In any real spontaneous process, some energy is always wasted – the actual work realized is always less than the calculated maximum.
16
∆ G = − nFE
∆ G ° = − nFE °
n = number of moles of electrons
F = Faraday = 96,485 coulombs per mole of electrons
E > 0 ⇒ ∆G < 0 --- spontaneous
Sample Exercise 17.3 & 17.4
17
Michael Faraday lecturing at the Royal Institution before Prince Albert and others (1855). The faraday was named in honor of Michael Faraday (1791-1867), an English-man who may have been the greatest experimental scientist of the nineteenth century. Among his many achievements were the invention of the electric motor and generator and the development of the principles of electrolysis.
18
17.4 Dependence of Cell Potential on Concentration
例如:
Cu(s )+ 2Ce4 +(aq)→Cu2 +(aq)+ 2Ce3 +(aq), Eo= 1.36 V 如果將 [Ce4 +] ↑(即 [Ce4+] > 1 M) ⇒ Ecell ↑
而若將 [Cu2 +] or [Ce3 +] ↑ ⇒Ecell ↓
可根據 Le Châtelier’s principle 判斷
Sample Exercise 17.5
19
Concentration Cell
濃度電池: a cell in which both compartments have the same components but at different
concentrations.例如:
自然會傾向使[Ag+] 相同的方 向進行反應。
一般濃度電池的 cell potential 都不大。
20
Sample Exercise 17.6
Determine the direction of electron flow and
designate the anode and cathode for the cell
represented below.
21
The Nernst Equation
With Nernst equation, we can calculate the potential of a cell in which some or all of the components are not in their standard states.
) ln(Q RT G G = ∆
o+
∆
) ln(Q RT nFE nFE = −
o+
−
o
o
nFE
G and nFE
G = − ∆ = −
∆ Q
) 303 log(
. or 2
)
ln( Q
nF E RT
E nF Q
E RT
E =
o− =
o−
) 0591 log(
. 0
C, 25
at Q
E n E =
o−
°
Sample Exercise 17.7
22
Calculation of Equilibrium Constants for Redox Reactions
Nernst equation 所計算的 E 為電池放電前之 maximum potential,一旦開始放電,濃度改變,
E 亦隨之改變 (
↓) 直到達到平衡。
At equilibrium, Q = K and E
cell= 0
RT K nFE
RT K nFE nF K
E RT
nF Q E RT E
o o
o
o
303 . ) 2 log(
, )
ln(
) ln(
) ln(
: equation Nernst
=
=
⇒
=
−
=
0591 . ) 0 log(
C, 25 at
nEo
K =
°
Sample Exercise 17.8
23
Ion-selective electrodes
∵ Cell potential is sensitive to concentrations of the reactants & products
∴ 可藉測量 E 來定量特定離子濃度,例如 pH meter。
pH meter 由下列三部份組成:
(1) a standard electrode of known potential
(2) a special glass electrode that changes potential depending on the conc.
of H+in the solution where it is dipped (3) a potentiometer
24
A battery is a galvanic cell or, more commonly, a group of galvanic cells connected in series.
17.5 Batteries
http://www.nsc.gov.tw/dept/acro/version01/battery/basictheory.htm 25
電池設計與原理
電池為一將化學能轉換成電能的裝置,因具有可攜帶、多 種組合、高能量密度以及無排放噪音與廢氣的優點,所以在許 多的領域受到普遍的應用。
構成電池的四個主要部分為
• 電極 (electrode):依其所發生的氧化或還原反應又有陽 極(負極; anode) 與陰極 (正極; cathode)之分。
• 電解液 (electrolyte)
• 隔離膜 (separation film): 為隔離電極的裝置,以避免 兩極上的活性物質直接接觸而造成電池內部的短路。
• 罐體 (shell):作為電池的外殼,主要用以保護內部結構,
所以需要有良好的機械結構、耐熱耐震動耐腐蝕等性能。
常見的罐體造型有圓筒型、方型、鈕扣型等。
26
電池的種類
電池可透過電池本身的充放電特性與工作性質大致分類如下:
♣
一次電池 (primary cell)
僅能被使用一次的電池,無法透過充電的方式再補充已被 轉化掉的化學能,故稱為一次電池。此類電池常見的有乾 電池、水銀電池與鹼性電池等。
一次電池的應用最早也最為廣泛,市面上販售的不可充電 電池幾乎皆屬此類,如鈕扣型水銀電池、1號、2號以及 3號電池等等。
27
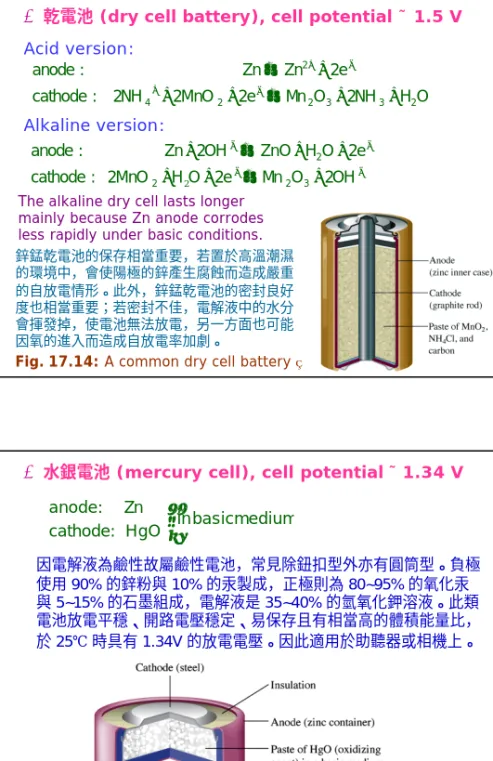
Ø乾電池(dry cell battery), cell potential ˜ 1.5 V Acid version:
Alkaline version:
O H 2NH O Mn 2e 2MnO 2NH
: cathode
2e Zn Zn :
anode
2 3 3 2 2
4
2
+ +
→ + +
+
→
+ −
− +
−
−
−
−
+
→ + +
+ +
→ +
2OH O Mn 2e O H 2MnO : cathode
2e O H ZnO 2OH
Zn :
anode
3 2 2
2 2
The alkaline dry cell lasts longer mainly because Zn anode corrodes less rapidly under basic conditions.
Fig. 17.14: A common dry cell battery⇒ 鋅錳乾電池的保存相當重要,若置於高溫潮濕 的環境中,會使陽極的鋅產生腐蝕而造成嚴重 的自放電情形。此外,鋅錳乾電池的密封良好 度也相當重要;若密封不佳,電解液中的水分 會揮發掉,使電池無法放電,另一方面也可能 因氧的進入而造成自放電率加劇。
28
Ø水銀電池(mercury cell), cell potential ˜1.34 V medium
basic HgO in
: cathode
Zn :
anode
因電解液為鹼性故屬鹼性電池,常見除鈕扣型外亦有圓筒型。負極 使用 90% 的鋅粉與 10% 的汞製成,正極則為 80~95% 的氧化汞 與 5~15% 的石墨組成,電解液是 35~40% 的氫氧化鉀溶液。此類 電池放電平穩、開路電壓穩定、易保存且有相當高的體積能量比,
於 25℃ 時具有 1.34V 的放電電壓。因此適用於助聽器或相機上。
Fig. 17.15: A mercury battery of the type used in calculators.
29
♣
二次電池 (secondary battery)
二次電池所指的就是可以被重複使用的電池。透過充電的 過程,可以使得電池內的活性物質再度的回復到原來的狀 態,因而能再度的提供電力。這類的電池有鉛蓄電池 (lead storage battery)、鎳鎘電池
(nickel cadmium battery)、
鎳氫電池 (nickel hydrogen battery)、二次鋰電池 (secondary lithium battery) 以及鋰離子電池 (lithium ion battery) 和高分子鋰電池 (polymer lithium battery) 等。
30
Ø鉛蓄電池(lead storage battery)
O 2H 2PbSO 2HSO
2H PbO Pb : rxn net
– – – – – – – – – – – – – – – – – – – – – – – – – – – – – – – – –
O 2H PbSO 2e
3H HSO PbO : cathode
2e H PbSO HSO
Pb :
anode
2 4 4
2
2 4 4
2
4 4
+
→ +
+ +
+
→ + + +
+ +
→ +
+ −
−
− +
−
− +
Fig. 17.13: One of the six cells in a 12-V lead storage battery. The anode consists of a lead grid filled with spongy lead, and the cathode is a lead grid filled with lead dioxide. The cell also contains 38% (by mass) sulfuric acid.
鉛蓄電池在 25℃ 時能提供 ~2V 的電壓。具有電動勢 大、操作溫度廣、結構簡單、技術成熟與價格低廉等 的優勢,再加上良好的循環壽命,使得此種電池的產 量與產值在電池產出中具有相當重要的地位。其應用 以汽機車領域為主,或用於UPS、無線電機、緊急照 明設備、通信電機以及工業用電機設備等處。此外,
也使用於電廠中,作為緊急的電力來源之用。
31
Ø鎳鎘電池(nickel-cadmium (Ni-Cd) battery)
−
−
−
−
+
→ + +
+
→ +
2OH Ni(OH) 2e
O 2H NiO : cathode
2e Cd(OH) 2OH
Cd :
anode
2 2
2
2
鎳鎘電池電壓值為1.2V,體積能量密度約 130~200 Wh/L,重量能量 密度則在40~50 Wh/kg 間。因發展已久,成本較低;再加上循環壽命 長達2000~4000 次,及大電流放電的特性、適用溫度範圍廣、自放電 率小等優點,故佔有率頗高。不過受到 ”記憶效應”的影響,效能會隨充 放電次數增加而下降。鎳鎘電池的市場佔有率在數年前相當高,但受到 環保意識抬頭的影響,有鎘污染疑慮的鎳鎘電池佔有率已逐年下降。
Ø鎳氫電池
鎳氫電池與鎳鎘電池相似,但鎳氫電池的陰極活性物質則是儲氫合金。
所能提供的電壓約為1.2 V,具有不錯的能量密度 (50 ~ 60 Wh/kg or 250 ~ 300 Wh/L),及良好的循環壽命。因不含鎘,不會像鎳鎘電 池有鎘污染的問題。但鎳氫電池在高溫下效能較差,且在一般使用上有 自放電率高及記憶效應的問題。
32
Ø鋰離子電池(lithium ion battery)
鋰離子電池以鋰鈷氧化物、鋰錳氧化物、鋰鎳氧化物等作為陽極活性 材料,在陰極材料的部分則為碳材料,電解質也有許多種可能 LiPF6, LiClO4, LiBF4等,使用有機電解液。電壓可達到3.6V,而能量密度為 250~300 Wh/L(或90~110 Wh/kg)。該電池具有相當高的能量密度及 工作電壓,再加上長循環壽命與較無記憶效應的特性,使得鋰離子二 次電池佔有率日益上升。但其成本過高,過充、放電時有安全顧慮而 需保護迴路等問題仍是需要克服的缺點。
6C + LiCoO2 →LixC6+ Li1-xCoO2 net rxn:
6C + xLi+ + xe-→LixC6 cathode:
LiCoO2→Li1-xCoO2+ xLi++ xe- anode:
33
Ø高分子鋰離子電池(polymer lithium ion battery) 高分子鋰離子電池以高分子電解質取代液態鋰離子電池所使用的有機 電解液,以避免後者容易揮發燃燒與造成漏液的現象,使高分子鋰電 池有更佳的安全性。此種高分子電解質,一方面可作為傳導離子的媒 介、一方面又可作為隔離膜用,再加上與鋰金屬的反應性低,故在安 全性方面受到的質疑較小,也因此受到廣泛研究與開發。 分類上,
高分子鋰離子電池因所使用的高分子電解質有不同而有完全固態的固 態高分子電解質與加入可塑劑的膠態高分子電解質兩類。
anode: Li (in polymer) →Li+(in polymer) + e– cathode: Li+(in CoO2) + e–+ CoO2→Li CoO2
the most promising new reusable battery
34
♣
燃料電池 ( fuel cell )
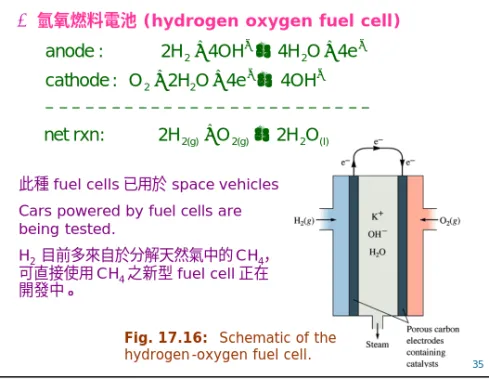
與前述兩者有相當大的不同,又被稱為連續電池。特色是陰 陽兩極並無活性物質的存在,而是透過外部的系統提供,所 以只要持續地提供活性物質,電池就可以持續的放電。在陽 極部分,真正進行氧化反應的是空氣或是氧氣;而陰極部分 則是以氫或者是煤氣等為主。此類電池如氫氧燃料電池 (hydrogen oxygen fuel cell) 等。
此類電池尚在發展中,且受限於其較大的體積,主要用在發 電機組上或最為備用能源。近來由於技術的提昇,有逐漸小 型化的趨勢,並運用於電動車輛等領域。
♣
其他
太陽能電池(solar cells)、熱起電力電池等
35 (l)
2 2(g) 2(g)
2 2
2 2
O 2H O
2H : rxn net
4OH 4e
O 2H O : cathode
4e O 4H 4OH 2H :
anode
→ +
→ + +
+
→ +
−
−
−
−
– – – – – – – – – – – – – – – – – – – – – – – – –
Ø氫氧燃料電池(hydrogen oxygen fuel cell)
Fig. 17.16: Schematic of the hydrogen-oxygen fuel cell.
此種fuel cells 已用於 space vehicles Cars powered by fuel cells are being tested.
H2目前多來自於分解天然氣中的CH4, 可直接使用CH4 之新型fuel cell 正在 開發中。
36
A gathering of several cars powered by fuel cells (Los Angeles Memorial Coliseum).
37
17.6 Corrosion
• Corrosion involves oxidation of the metal.
• ~1/5 年產之鋼鐵是用以 replace rusted metal.
• 由Table 17.1 可看出除了Au外,常用為 structural or decorative 用之金屬的 standard reduction potentials 均比O2小(less positive).
• Some metals, such as copper, gold, silver and platinum, are relatively difficult to oxidize. These are often called noble metals.
Au3++ 3e–→Au Eo = 1.50 V O2+ 4H++ 4e–→2H2O Eo = 1.23 V
⇒故Au 在空氣中不會氧化
38
Corrosion of Iron
Fe2O3的水合(hydration) 程度會影響鐵銹的顏色 鐵鏽形成的 3 要件:鐵、氧氣、水
酸與鹽分均會加速 鐵的生鏽
§ Fe(s) → Fe2+(aq) + 2 e−
§ Fe2+(aq) → Fe3+(aq) + e−
Ÿ O2(g) + 2 H2O(l) + 4 e−→ 4 OH−(aq) 2 Fe3+(aq) + 6 OH−(aq)→ Fe2O3⋅3H2O
39
¶改變鐵的“體質” – 添加鉻 (Cr) & 鎳 (Ni),即為不鏽鋼
¶覆蓋“金鐘罩”
- 鍍金、銀等貴重金屬
- 鍍鋁以於表面形成緻密氧化鋁層
- 犧牲打 (galvanizing): 鍍活性較高之金屬,如鋅或錫 - 塗佈無機鹽類,上釉
- 塗佈油漆
Prevention of Corrosion
40
¶“cathodic protection” – a method most often employed to protect steel in buried fuel tanks and pipelines
⇒ 以 active metal (如 Mg) 以導線連接至欲保護之 tank or pipeline,因為 Mg 比 Fe 為更好之 reducing agent,可防 止 Fe 被氧化,但 Mg anode 須定期更換;ship’s hull 亦以 類似法防鏽 (用 Ti)。
Fig. 17.18: Cathodic protection of an underground pipe.
41
加 NaHCO3 的目的:
- to help remove any aluminum oxide coating that forms - to make the cleaning solution more conductive
銀器變黑了怎麼辦?
Al foil + NaHCO3
O2(g) + 4Ag(s) + 2H2S(g) → 2Ag2S(s) + 2H2O(l)
2Al(s) + 3Ag2S(s) + 6H2O (l) → 6Ag + 2Al3+(aq) + 6OH-(aq) + 3H2S(g) 生活小撇步
42
17.7 Electrolysis
• An electrolytic cell uses electrical energy to produce a chemical change that would otherwise not occur spontaneously.
• 生活中的重要例子: 電池充電、製鋁、chrome plating 等
Fig. 17.19: A standard galvanic cell vs. a standard electrolytic cell.
43
4
How much chemical change occurs with the flow of a given current for a specified time?
1. Q = I ×t 1A = 1C/s 2. Q/F = moles of e
–3. 由 e
–轉移數 ⇒ 求得 moles of species 4. 求出 mass of species
Stoichiometry of electrolysis
Sample Exercise 17.9
44
Electrolysis of water
anode: 2H2O →O2+ 4H++ 4e- –Eo= –1.23 V cathode: 4H2O + 4e-→2H2+ 4OH– Eo= –0.83 V net rxn: 2H2O →2H2+ O2 Eocell= –2.06 V 於 pure water [H+] = [OH–] = 10–7M,上述反應之 E = -1.23 V 而實際上,若以 Pt 為電極接 6V 電池,放入純水中,觀察不到 任何反應 (∵ ions 太少)。
加入少量電解質(salts)則可 加快反應進行。
45
Electrolysis of mixture of ions
Suppose 某一溶液含 Cu2+、Ag+、Zn2+。若外加電壓逐漸 turn up,metals being plated out onto the cathode 順序為何?
Ag++ e-→Ag Eo= 0.80 V Cu2++ 2e-→Cu Eo= 0.34 V Zn2++ 2e-→Zn Eo= –0.76 V
reduction of Ag+最容易發生,即氧化力: Ag+>Cu2+ > Zn2+, 此亦為金屬於 cathode 析出之順序。
以 Eo預測電解產物時須注意 “overvoltage”問題 例如: electrolysis of NaCl(aq ), 於 anode 產生 Cl2而非 O2
2Cl–→Cl2+ 2e- –Eo= –1.36 V 2H2O →O2+ 4H++ 4e- –Eo= –1.23 V 因為氧化水須要施加比預期高許多之電壓,此即稱
“overvoltage”,過電壓之成因複雜。 Sample Exercise 17.10
46
17.8 Commercial Electrolytic Processes
ØProduction of aluminum ØElectrorefining of metals ØMetal plating
ØElectrolysis of sodium chloride Ø … … … … …..
47
因為鋁的活性太高,在自然界中和各種不同元素(尤其是氧) 緊密地結合在一起,很難提煉出來。鋁的生產,初期是使 用化學法,利用活性高之鈉或鎂金屬將鋁還原出來;法國 於1855年採用化學法開始工業生產,是世界最早生產鋁的 國家。
以化學法煉鋁的成本極高,在西元1886年之前,鋁的價值 甚至超過銀和金! 直到西元1886年,年輕的美國歐柏林大 學學生Charles M. Hall和法國化學家Paul L. T. Héroult 於同年發現以電解法來提煉鋁的廉價方法,大量地生產純 鋁,而使鋁的價格「一落千丈」。
Production of aluminum
48
Fig. 17.21: Charles Martin Hall (1863-1914)
49
Fig. 17.22: A schematic diagram of an electrolytic cell for producing aluminum by the Hall-Heroult process.
鋁礦砂(bauxite): 主成分為 Al2O3 Al2O3: m.p. = 2050°C
Al2O3+ cryolite (Na3AlF6) ⇒ m.p. = ~1000°C
Overall cell reaction: 2Al2O3+ 3C →4Al + 3CO2
50
Electrorefining of metals
Purification of metals is an important application of electrolysis. 例如: 銅的精煉純化
Anode: impure Cu Cu→Cu2++ 2e– Cathode:thin sheet of ultrapure Cu Cu2++ 2e–→Cu Electrolyte: CuSO4(aq) 99.95% pure
於anode 上,除 Cu 被氧化外,其他如 Zn →Zn2++ 2e–、 Fe→Fe2++ 2e– 等亦會發生,而noble metal impurities 在 所施電壓下則不會被氧化,沉澱在底下形成sludge,為煉銅之重 要副加產品。
51
Fig. 17.23: Ultrapure copper sheets that serve as the cathodes are lowered between slabs of impure copper that serve as the anodes into a tank containing an aqueous solution of copper sulfate (CuSO4).
52
Metal plating
Anode: plating metal (欲鍍上之金屬) Cathode:object to be plated (被鍍物) Electrolyte: 含欲鍍金屬之ions
例如: “tin cans” – steel cans with a thin Sn coating、
chrome -plated steel bumper for cars 等
Fig. 17.24: (a) A silver-plated teapot. (b) Schematic of the electroplating of a spoon.
53
Electrolysis of sodium chloride
Fig. 17.25: The Downs cell for the electrolysis of molten sodium chloride.
電解熔融之NaCl NaCl: m.p. = 800°C
NaCl + CaCl2⇒m.p. = ~600°C
anode:
2Cl–→Cl2+ 2e– cathode:
Na++ e–→Na
Na must be stored in an inert solvent, such as mineral oil, to prevent its oxidation.
54
電解NaCl 水溶液 (brine):
為工業上製備Cl2& NaOH 之重要方法
Cathode:
þ2H2O + 2e-→H2+ 2OH– Eo= –0.83 V Na++ e–→Na Eo= –2.71 V Anode:
þ2Cl–→Cl2+ 2e- –Eo= –1.36 V
2H2O →O2+ 4H++ 4e- –Eo= –1.23 V (high overvoltage)
如此之電解槽中含有混合之NaOH and NaCl
55
Fig. 17.26: The mercury cell for production of chlorine and sodium hydroxide.
chlor-alkali process: 使用汞作為cathode,因 H2 對於汞電極 有很高之overvoltage,cathode 上將會還原出鈉: Na++ e–→Na。
鈉即與汞形成鈉汞齊液態合金,再於另一槽中與 H2O 反應生成 NaOH。
2Na + 2H2O → 2Na++ 2OH–+ H2 因環保考量,此法已逐漸 被淘汰,改用薄膜隔離 the anode and cathode compartments in brine electrolysis cells ,此 membrane 只可使陽離子 通過,不讓陰離子通過,
故不會有NaOH 被 NaCl 污染的問題。
2 NaCl(aq) + 2 H2O(l) 2 NaOH(aq) + Helectrolysis 2(g) + Cl2(g)56