計畫編號: NSC89-2314-B002-189
計畫名稱:
環境致癌物代謝酵素之基因多形性表現與食道癌關係之研究
The Study of Association between Esophageal Cancer and the Genetic Polymorphism in Carcinogen-metabolic Enzyme
執行期限:88 年 8 月 1 日至 89 年 7 月 31 日 主持人:李章銘 共同主持人:李元麒 執行機構及單位名稱: 台大醫學院外科 email: ntuhlee@yahoo.com 一.中英文摘要: 關鍵詞:食道癌,基因多型性 環境致癌物代謝酵素之基因多型性與環境毒物暴露的交互作用之下,可決定個體致 癌的傾向。本研究乃是在探討第一(CYA1A1,CYP2E1)與第二(GSTM1, GSTT1)型,毒 物代謝酵素的基因多型性,與台灣食道癌致癌危險性的關係。我們收集了 105 位食 道鱗狀上皮細胞癌病患與 266 健康對照組 CYA1A1 與 CYP2E1 以 PCR-RFLP. GSTM1 , GSTT1 以 PCR 測定其基因型。結果:雖然在所有的病患與對照者,其基因型型分佈 沒有顯著的差異。但是在抽煙者中,GSTT1 缺乏與 CYP1A1 變異型有較高的致癌傾向 (GSTT1 缺乏 OR=1.17 5.08, CYP1A1 變異,OR=0.99 2.94)。結論:本實驗顯示, GSTT1 與 CYP1A1 基因多型性可與抽煙等環境因素交互作用,而影響個體食道癌的傾 向。
The interaction between the environmental toxins and the polymorphic xenobiotic metabolizing enzymes can determine the individual susceptibility to chemical carcinogenesis.
Objective: In this study, we investigated the association of the genetic polymorphisms of
phase I (CYP1A1, CYP2E1) and phase II (GSTM1 and GSTT1) xenobiotic metabolizing
enzymes with the risk of esophageal cancer in Taiwan. Method: We recruited 105 cases of esophageal squamous cell carcinoma and 266 persons of healthy controls during 1996-1999 in Taiwan. The genotypes of the genobiotic metabolizing enzymes were determined by PCR (for CYP1A1 and CYP2E1) or PCR-RFLP (for GSTM1 and GSTM1). Results: There was no significant difference in distribution of the genotypes of CYP1A1, CYP2E1, GSTM1 or GSTT1
between the patients and controls. However, multivariate analysis in the cigarette smokers showed that GSTT1 absent genotype significantly increased the risk of esophageal cancer,
with an OR (95% CI) of 2.44 (1.17-5.08). Cigarette smokers with variant allele of CYP1A1
also had a marginal increase of risk to develop esophageal cancer as compared to the wild
CYP1A1 genotype (OR = 1.70; 95% CI= 0.99-2.94). In contrast to the results found in
cigarette smokers, the GSTT1 absent genotype was less frequent in patients of esophageal
squamous cell carcinoma with an OR of 0.29 (95% CI=0.11-0.73). Conclusion: Our results revealed that the GSTT1 and CYP 1A1 genetic polymorphism might interact with cigarette smoke to modulate the risk of esophageal cancer in Taiwan.
二.計畫緣由與目的:
The individual cancer susceptibility can be determined by the interaction of environmental and genetic factors. Many environmental factors have been identified to associate with the risk of esophageal cancer (1). In most of the western countries, cigarette smoking and alcohol drinking are the most well known factors, which exert a dose dependent and synergistic effect on the risk of esophageal cancer (2). The dietary factors as malnutrition or consumption of pickled vegetable, which is contaminated with nitrosamine, are also considered important for the risk of esophageal cancer in the Chinese population (3). After exposure to chemical carcinogens, a biological detoxification system would be initiated to eliminate the toxins. The xenobiotic metabolizing enzymes are responsible for most of the detoxification processes. These enzymes can be classified into two major groups, phases I and II enzymes. Phase I enzymes are mainly composed of families of cytochrome P450 (CYP) which can initially oxidize the carcinogens. The phase II enzymes include glutathione s-transferase (GST), NADPH: quinone oxidoreductase (NQO, DT-diaphroase) and N-acetyltransferase (NAT) etc, which can reduce the electrophilic intermediates and facilitate their excretion. Many of these intermediates produced in the processing of these enzymes are carcinogenic in character. Therefore variation in the enzymatic activities may result in different accumulation of active carcinogens and substantially influence the carcinogenic susceptibility for individuals.
CYP1A1 and CYP2E1 are important members of the cytochrome P450 family. CYP1A1
encodes for an inducible enzyme that catalyzes the bioactivation of polycyclic aromatic hydrocarbons (PAH), the most important cigarette-related pollutant. For example, the benzo(a)pyrene, one of the PAH families, can be transformed by CYP1A1 into the
electrophilic product, diol-epoxide, which would facilitate the DNA adduct formation (4). Genetic variants of CYP1A1 were detected with the mutation in the 3’ non-coding region
(MspI polymorphism) and in exon 7 of heme binding region with substitution of isoleucine to
valine (Ile-Val polymorphism). CYP2E1 is the major ethanol-oxidizing enzyme of
nonalcohol dehydrogenase, which catalyzes the conversion of ethanol to acetaldehyde (5). Its substrates include ethanol, N-nitrosamine, benzene, styrene, butadiene and urethane (6). RFLP using Rsa I and Dra I restriction enzymes have been used to detect genetic variants in introns 5 and 6 of CYP2E1 respectively (7). The biological consequences produced by the variant genotypes of CYP1A1 and CYP2E1 are still unknown. However, the genetic
polymorphism of CYP2E1 has been found to influence the metabolism of benzene (8); and a
difference in aryl hydrocarbon hydroxyalase (AHH) activity has been detected between the genetic variants of CYP1A1 MspI polymorphism (9).
GST belongs to one of the phase II enzymes, which is responsible for conjugating the electrophilic intermediates, produced by the phase I enzymes, to reduced glutathione (10). It
is composed of at least five classes in its family designated, α, µ, π, σ and θ. GST is broadly expressed in mammalian organs including gastrointestinal tract (48). The GSTT1 and GSTM1
encode for the enzymes µ and θ of GST respectively, and are responsible for the metabolism
of different substrates. For example, 1,3-butadiene can be effectively metabolized by GSTT1
but is a poor substrate of GSTM1 (12); while the metabolism of another reactive intermediate
of butadine, monoepoxybutane, is for the most part influenced by GSTM1 (13). The deletion
of GSTT1 and GSTM1 genes has been identified among the normal population, and leads to
absence of the enzymatic activities. Under exposure to trans-stilbene oxide, the cell with
GSTM1 absent genotype was found more susceptible to DNA damage of sister chromatid
exchange (SCE) (14). Similarly, SCE was increased in the GSTT1 absent genotype when
exposed to 1,3-butadiene, haloalkanes or haloalkenes existing in the cigarette-related pollutants (15).
The genetic polymorphisms of these phase I and II enzymes were found to associate with a higher risk of various aerodigestive cancers, i.e. CYP 1A1 or CYP 2E1 with lung cancer
(16-17); GSTT1 and GSTM1 with head and neck, laryngeal or oral cancer (18-20). The Val/Val CYP1A1 genotype combined with GSTM1 null genotype was found to associate with a higher
risk of esophageal cancer in heavy smokers (21). A study from the high-risk area of China also revealed that the wild CYP2E1 and non-null GSTM1 genotypes could increase the risk of
esophageal squamous cell carcinoma (29). Although most of the inhabitants in Taiwan belong to the ethnic Chinese population, the effect of genetic polymorphism of xenobiotic metabolizing enzymes on cancer susceptibility might be different under different environmental exposure. In this study, we investigated the association of the genetic polymorphism of GSTT1, GSTM1, CYP1A1, and CYP2E1 with the risk of esophageal cancer
in Taiwan.
三.結果與討論
Results
Totally, 105 patients and 266 controls were recruited in this study. The data of the
subjects with incomplete epidemiological records or inadequate amount of extracted DNA for genotyping were discarded in the analysis. The distribution of GSTM1, GSTT1, CYP1A1,
and CYP2E1 genotypes and the prevalence of cigarette smoking, alcohol drinking and areca
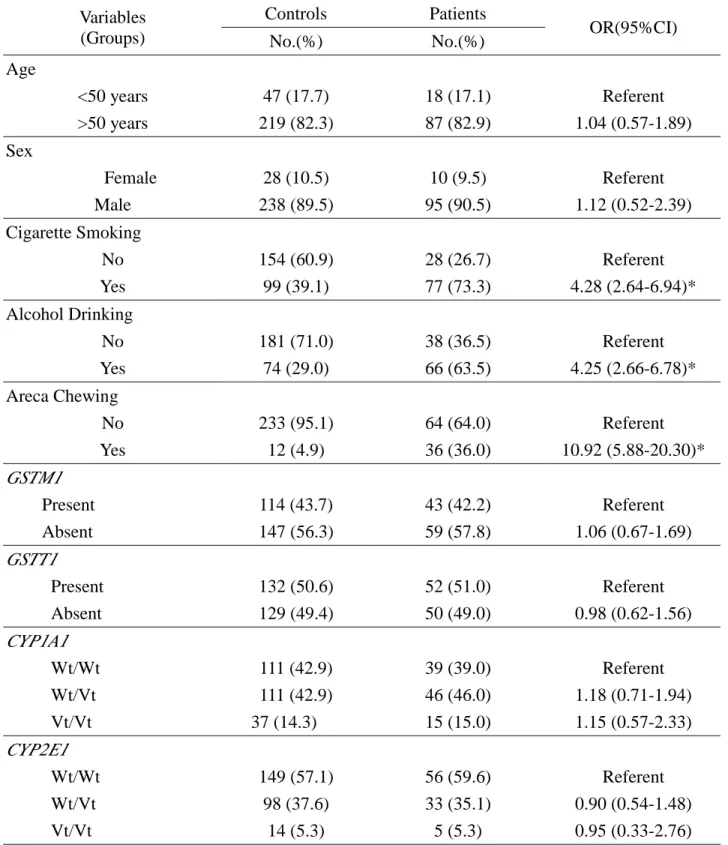
chewing were listed in Table 1. Habits of cigarette smoking, alcohol drinking and areca chewing were significant factors to influence the risk of esophageal cancer (p<0.001 for each factor respectively). The distributions of age and gender were not significantly different between patients and controls. In addition, there was no significant difference in frequency of genotypes GSTM1, GSTT1, CYP1A1, or CYP2E1 between the two groups. However,
when subjects were dichotomized with the habit of cigarette smoking, the patients of esophageal cancer in cigarette smokers had a higher frequency of GSTT1 absent genotype
the frequency of GSTT1 absent genotype was significantly lower in patients of esophageal
cancer (OR=0.30, 95% CI: 0.12-0.72) than in the controls (Table 2). As for the genotypes of
GSTM1, CYP1A1 or CYP2E1, there was no significant different distribution between patients
and controls either in cigarette smokers or non-smokers (Table 2).
Because of the similarity in enzymatic activities between GSTM1 and GSTT1, interaction
may exists between these two enzymes and therefore modify the risk of esophageal cancer. Table 3 shows the combined effect of GSTT1 and GSTM1, dichotomized by cigarette smokes.
We found no evident interaction between GSTM1 and GSTT1 in cigarette-smokers.
However, in the non-cigarette smoker, the combined GSTT1 and GSTM1 absent genotype
further reduced the risk of cancer with an OR of 0.08 (95% CI: 0.01-0.48), compared to those with simultaneous presence of GSTT1 and GSTM1. The effect by GSTM1 alone is not
evident, as the OR (95% CI) of GSTT1 present /GSTM1 absent genotype being 1.06
(0.38-2.94)(Table 3). The interaction of GSTs and CYPs was not remarkable in that the trends of
cancer risk were not significantly changed by the combination of GSTs and CYPs (data not
shown).
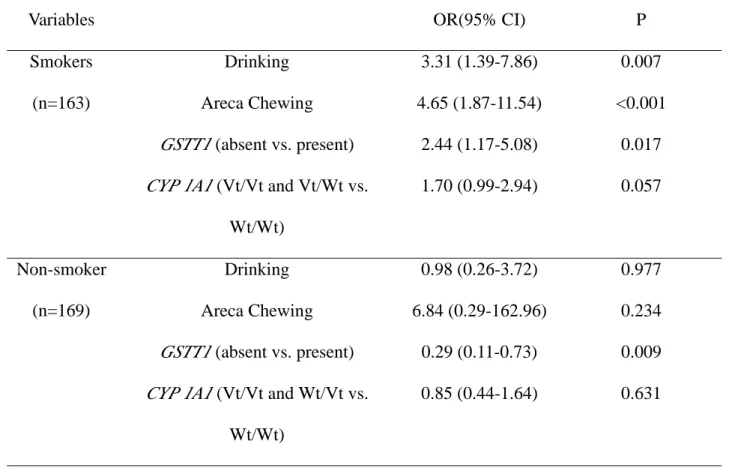
Since the cancer risk determined by these polymorphic xenobiotic enzymes was found different in cigarette and non-cigarette smokers, we also dichotomize the subjects with cigarette smoke in the analysis of multiple logistic regression. As shown in table 4, alcohol drinking, areca chewing and GSTT1 present genotype were found significant for the
risk of esophageal cancer with ORs (95% CI) of 3.31 (1.39-7.86), 4.65 (1.87-11.54), and 2.44 (1.17-5.08) respectively. Smokers with CYP1A1 MspI heterozygote variant genotype had a
1.76-fold risk (95% CI=0.80-3.86, p=0.16) to develop esophageal cancer as compared to those with wild genotype (data not shown in the table). Similarly, smokers with homozygous variant genotype had a 2.83-fold risk (95% CI=0.88-9.14, p=0.08) to develop esophageal cancer (data not shown in the table). The significance was even strengthened, when we treated the genotypes of CYP1A1 MspI polymorphism as a continuous variable
using the wild genotype as baseline (OR=1.70, 95%CI=0.99-2.94; p=0.057 for homozygous and heterozygous variant genotypes vs. wild genotype) (Table 4). In the non-smokers, the
GSTT1 present genotype had a significant protective effect with an OR of 0.29 (95% CI:
0.11-0.73, p<0.01). The effects of alcohol drinking, areca chewing and CYP1A1 were not
significant in this group.
Discussion
Although the environmental toxins are eliminated through metabolism by the xenobiotic metabolizing enzymes, the processes of which may also produce a variety of reactive intermediates that are genotoxic in nature. Whether the carcinogens are detoxified or activated in the process may determine the polymorphic xenobiotic metabolizing enzyme to be “favorite” or “unfavorite” toward the individual cancer susceptibility. Our results demonstrated that the GSTT1 absent genotype had a higher risk to develop esophageal cancer
has a protective effect for the cancer susceptibility. Both of these effects were significant under logistic regression, adjusting with the other significant environmental factors. These findings require cautious interpretation and further investigation.
As a member of GST family, GSTT1 shares a common property of the family in
conjugating reduced glutathione to electrophilic intermediates, produced by oxidation of the phase I enzymes. There are also some functional variation between GSTT1 and other
members of the GST family. For example, the main substrates of GSTT1 are epoxybutane,
ethylene oxide, halomethane, and methyl bromide, while bezo (a) pyrene, styrene-7,8-oxide and trans-stilbene oxide can be effectively metabolized by GSTM1 (25). The common
substrate of GST, 1-chloro-2, 4-dinitrobenzene is poorly metabolized by GSTT1 (12). It is
also found that chemopreventive agents of benzyl isothiocyanate, coumarin or ethoxyquin can induce the GSTT1 activity, which is distinct from the mechanisms that regulate levels of other GSTs (26). The cigarette-related carcinogens, 1,3-butadiene, various haloalkanes or
haloalkenes, can be effectively detoxified by GSTT1, and absence of GSTT1 would increase
chromosome aberration or sister chromatid exchange (SCEs) for individuals (15). On the other hand, GSTT1 can also activate some environmental toxins and thus increase their
genotoxicity. Landi et al (27) found that the presence of GSTT1 could increase the
mutagenecity created by brominated trihalomethanes (THMs), the carcinogenic disinfection by-products frequently found in chlorinated drinking water. Two members in the dihaloalkanes family, dichloromethane (DCM) and dibromomethane (DBM), which are widely applied by the consumers and industry, were found exclusively activated by GSTT1
(26). In the carcinogenic test, DCM can induce a more evident genotoxicity in mouse than in rat or hamster, which was thought due to a higher constitutive GSTT1 expression in the live
and lung of mouse (28). In agreement with the previous in vitro observations, our results
implied that GSTT1 might detoxify some cigarette-related carcinogens of the esophagus in
that, for the cigarette smokers, the risk of esophageal cancer was increased by the absence of
GSTT1 (OR: 2.44; 95%CI: 1.17-5.08). However, GSTT1 might also activate the
environmental toxins encountered by the non-smokers so as to lower the risk of esophageal cancer by the absence of GSTT1 in non-smokers (OR: 0.29; 95%CI: 0.11-0.73). The
protective effect of GSTT1 absent genotype for the non-smokers was further enhanced when it was combined with GSTM1 absent genotype (OR: 0.08; 95%CI: 0.01-0.48 using GSTM1
present/GSTT1 present as referent).
There are more than 4000 compounds produced by cigarette smoking, including polycyclic aromatic hydrocarbons (PAH), heterocyclic hydrocarbons, N-nitrosamines, aromatic amines, aldehydes, volatile carcinogens, inorganic compounds, and radioactive elements etc (34). Exposure to these carcinogens can increase the risk of DNA damage, as evidenced by the non-linear dose-response correlation between smoking and PAH-DNA adducts in lymphocytes (35). The level of PAH adduct in lymphocytes seemed to be
modified by genetic (GSTM1 absent genotype) or nutritional (β-carotene and -α-tocopherol) factors in the heavy smokers, according to the study of Mooney et al (36). Even in the same individual, variation in absorption, transportation, and accumulation of active carcinogens of cigarette in various organs can produce an organ-specific tumorigenicity by the constituents. For example, the metabolite of cigarette smoke, 2-naphthylamine can be concentrated in urine and increase the susceptibility to bladder cancer (37).
In our study, the effects of alcohol drinking, cigarette smoking and areca nut chewing are significantly associated with esophageal cancer, which was consistent with the previous studies (3,33,50). However, we didn’t evaluate the dietary effects, which was shown important to influence the risk of esophageal cancer in the Chinese population (2, 46). In Hong Kong, a study for never-smoker and never-drinker revealed that the risk of esophageal cancer could be increased by the consumption of prickled vegetables (31), which is rich in compounds of NOC as nitrite, nitrate, N-nitrosodimethylamine, and N-nitrosodiethylamine (32). Extracts of pickled vegetables, collected from the high-risk area of Linxian in China, could produce a dose-dependent mutagenecity in Salmonella typhimurium. It is note worthy that the effect required to be fortified by a microsomal activation system from the rat liver (30). It is also of interest to furhter investigate whether these dietary factors can interact with genetic polymorphism of the xenobiotic metabolizing enzymes to influence the risk of esophageal cancer.
The GSTT1 absent genotype was found to increase the risk of smoking related head and neck
cancer (19), pharyngeal and oral cancer (38). It was also associated with risk of bladder cancer in general population (39) or in the non-cigarette smokers (40). GSTM1 was found to
be more consistently associated with the risk of lung cancer. A meta-analysis of 12 case-control studies by McWilliams et al (41) has demonstrated a 1.4-fold risk to develop lung cancer in the GSTM1 absent genotype. Associations of GSTM1 with bladder, laryngeal, or
head and neck cancer have also been reported but with conflicting results (25). The effect
GSTT1, GSTM1 and other xenobiotic metabolizing enzymes can synergy with each other to
increase the cancer susceptibility of lung (42). The impact of genetic polymorphism can be also modified by a variety of environmental factors. For example, a more evident effect of
GSTM1 absent genotype on lung cancer risk was observed in the individuals who smoked less
than 35 pack-year (43). Insufficient intake of vitamin C and other nutrients can aggravate the susceptibility to lung cancer in the GSTM1 absent genotype (44). As for the esophageal
cancer, a study in the high-risk area of China showed that the wild CYP2E1 and non-null GSTM1 genotypes were over presented in patients of esophageal squamous cell carcinoma
(29). These two genotypes had an addictive effect to increase the risk of esophageal cancer. In Japan, the GSTM1 null genotype combined with Val/Val genotype of Ile/Val CYP1A1
polymorphism was found to be associated with a higher risk of esophageal cancer in heavy smokers (21). Another report by Morita et al did not find the similar association for
Previous in vitro study has also shown that the variant allele of CYP1A1 MspI
polymorphism can preferentially increase the aryl hydrocarbon hydroxylase (AHH) inducibility by 3-methylcholanthrene (9). The important product of the PAH family in the cigarette pollutants, benzo(a)pyrene, can be transformed by CYP1A1 into the electrophilic
product, diol-epoxide and facilitate the DNA adduct formation (4). Foundry workers with the CYP1A1 MspI variant genotypes were found to have an increased level of DNA adducts in
leukocytes (45). Consistent with these observations, we found that cigarette smokers with
CYP1A1 MspI variant allele had a higher susceptibility to esophageal cancer. Multivariate
analysis revealed that the risk of esophageal cancer was increased by the CYP1A1 MspI
variant allele with an OR of 1.76 for the heterozygous variant genotype and 2.83 for the homozygous variant. Treating the CYP1A1 MspI genotypes as continuous variables and
using the wild genotype as baseline, a borderline significance was observed for the genotypes containing the variant allele (OR=1.70, 95%CI=0.99-2.94, p=0.057 for homozygous and heterozygous variant genotypes vs. wild genotype). This effect is not seen in CYP2E1
genotypes, nevertheless.
In our control, the genotype distribution of CYP1A1 MspI, CYP2E1, GSTT1 and GSTM1 is
similar to the previous reports in normal healthy from Taiwan (18, 23). The distribution of
CYP 1A1 MspI genotypes in our study is also similar to that of the Japanese population but
had a lower percentage of wild genotype than the ethnic Caucasian (42.9 % vs. 70-85 %) (47). The percentage of GSTM1 absent genotype is similar between the Asian and Caucasian
population, which is around 50%. While the deletion of GSTT1 was found more frequent in
the Asian population including the ethic Chinese (around 50%) than the Caucasian (11-18%) or African population (24-38%) (25).
In conclusion, our result demonstrated that the risk of esophageal cancer could be
modulated by the interaction of gene-gene and gene-environment interaction. The genotypes with CYP1A1 MspI variant allele and absence of GSTT1 could increase the risk of esophageal
cancer, when the individuals were exposed to the toxins from cigarette smoke, while the absence of GSTT1 might be “protective” for the non-smokers, which exerted a synergistic
effect with the absence of GSTM1.
四.計畫成果自評:
本研究提供食道癌的癌化過程的解釋模式,這些資料顯示,食道癌乃是環境因子與遺傳 因子交互作用的產物,由此有助於食道癌高危險群的發現,而期於早期發現及早期治 療,但是本研究因個案數不多,因此尚需更多的類似研究,以取得更確定的結論。
五.參考文獻:
1. Schottenfeld D. Epidemiology of the esophageal cancer. Semin Oncol.,11: 92-100, 1984. 2. Yu M. C., Garabrant, D. H., Peter, J. M., and Mack, T. M. Tobacco, alcohol, diet,
occupation, and carcinoma of the esophagus. Cancer Res., 48: 3843-3848, 1988.
3. Yang C. S. Research on esophageal cancer in China: a review. Cancer Res., 4: 2633-2644, 1980.
4. Dahl A. R., and Lewis, J. L. Respiratory tract uptake of inhalants and metabolism of xenobiotics. Ann. Rev. Pharm. Toxicol., 33, 383-407, 1993.
5. Gonzalez F. J. The molecular biology of cytochrome P450s. Pharm. Rev., 40: 243-288, 1988.
6. Guengerich F. P., and Shimada, T. Oxidation of toxic and carcinogenic chemicals by human cytochrome P-450 enzymes. Chem. Res. Toxicol., 4: 391-407, 1991.
7. Uematsu F., Kikuchi, H., Ohmachi, T., Sagami, I., Motomiya, M., Kamataki, T., Komori, M., and Watanabe, M. Two common RFLPs of the human CYP2E gene. Nucleic Acids Res., 19: 2803, 1991.
8. Nedelcheva V., Gut, I., Soucek, P., Tichavska, B., Tynkova, L., Mraz, J., Guengerich, F. P., and Ingelman-Sundberg, M. Metabolism of benzene in human liver microsomes: individual variations in relation to CYP2E1 expression. Arch. Toxicol., 73: 33-40, 1999.
9. Kiyohara C., Hirohata, T., and Inutsuka, S. The relationship between arylhydrocarbon hydroxilase and polymorphisms of the CYP1A1 gene. Jpn. J. Cancer, 87:18-24, 1996. 10. Mannervik B., and Danielson, U. H. Glutathione transferase: structure and catalytic activity. CRC. Crit. Rev. Biochem., 23: 283-337, 1988.
12. Wiencke J. K., Pemble, S., Ketterre, B., and Kelsey, K. T. Gene deletion of glutathione S-transferase:correlation with induced genetic damage and potential role in endogenous
matagenesis. Cancer Epidemiol., Biomark. Prev., 4: 253-259, 1995.
13. Strange R. C., and Fryer, A. A. The glutathione S-transferases: influence of polymorphism on cancer susceptibility. IARC Sci. Publ., 148: 231-249, 1999.
14. Wiencke J. K., Kelsey, K. T., Lamela, R. A., and Toscano, W. A. Jr Human glutathione S-transferase deficiency as a marker of susceptibility to epoxide-induced cytogenetic damage. Cancer Res., 50: 1585-1590, 1990.
15. Hengstler J. G., Arand, M., Herrero, M. E., and Oesch, F. Polymorphisms of N-acetyltransferases, glutathione S-transferases, microsomal epoxide hydrolase and
sulfotransferases: influence on cancer susceptibility. Recent Results Cancer Res., 154: 47-85, 1998.
16. Kawajiri K., Nakachi, K., Imai, K, Yoshii, A., Shinoda, N., and Watanabe, J.
Identification of genetically high risk individuals to lung cancer by DNA polymorphisms of the cytochrome P450 1A1 gene. FEBS Lett., 263: 131-133, 1990.
17. Persson I., Jahansson, I., Bergling, H., Dahl, M. L., Seidegard, J., Rylander, R., Rannung A., Högberg, J., and Ingelman-Sundberg, M. Genetic polymorphism of cytochrome P450 2E1
in a Swedish population. Relationship to incidence of lung cancer. FEBS Lett., 319: 207-211, 1993.
18. Hung H. C., Chuang, J., Chien, Y., C., Chern, H., D., Chiang, C. P., Kuo,Y. S., Hildesheim A., and Chen, C. J. Genetic polymorphisms of CYP2E1, GSTM1 and GSTT1; environmental factors and risk of oral cancer. Cancer Epidemiol., Biomark. Prev., 6: 901-905, 1997.
19. Cheng L., Sturgis, E. M., Eicher, S. A., Char, D., Spitz, M. R., and Wei, Q. Glutathione-S-transferase polymorphisms and risk of squamous-cell carcinoma of the head and neck. Int. J. Cancer, 84: 220-224, 1999.
20. Jourenkova N., Reinikainen, M., Bouchardy, C., Dayer, P., Benhamou, S., and Hirvonen, A. Larynx cancer risk in relation to glutathione S-transferase M1 and T1 genotypes and tobacco smoking. Cancer Epidemiol., Biomark. Prev., 7: 19-23,1998.
21. Nimura Y., Yokoyama, S., Fujimori, M., Aoki, T., Adachi, W., Nasu, T., He, M., Ping, Y, M., and Lida, F. Genotyping of the CYP1A1 and GSTM1 genes in esophageal carcinoma patients with special reference to smoking. Cancer, 80: 852-857, 1997.
22. Morita S., Yano, M., Tsujinaka, T., Ogawa, A., Taniguchi, M., Kaneko, K., Shiozaki, H., Doki, Y., Inoue, M., and Monden, M. CYP1A1, CYP2E1 and GSTM1 polymorphisms are not associated with susceptibility to squamous-cell carcinoma of the esophagus. Int. J. Cancer, 71:192-195, 1997.
23. Yu M. W., Chiu, Y. H., Yang, S. Y., Santella, R. M., Chern, H. D., Liaw, Y. F., and Chen, C. J. Cytochrome P450 1A1 genetic polymorphisms and risk of hepatocellular carcinoma among chronic hepatitis B carriers. Br. J. Cancer, 80: 598-603, 1999.
24. Pemble S., Schroeder, K. R., Spencer, S. R., Meyer, D. J., Hallier, E., Bolt, H. M., Ketterer, B., and Taylor, J. B. Human glutathione S-transferase theta (GSTT1): cDNA cloning and the characterization of a genetic polymorphism. Biochem. J., 300: 271-276, 1994.
25. Rebbeck T. R. Molecular epidemiology of the human glutathione s-transferase genotypes GSTM1 and GSTT1 in cancer susceptibility. Cancer Epidemiol. Biomarkers Prev., 6: 733-743, 1997.
26. Sherratt P. J., Manson, M. M., Thomson, A. M., Hissink, E. A., Neal, G. E., van Bladeren, P. J., Green,T., and Hayes, J. D. Increased bioactivation of dihaloalkanes in rat liver due to induction of class theta glutathione S-transferase T1-1. Biochem. J., 335: 619-630, 1998.
27. Landi S., Hanley, N. M., Warren, S. H., Pegram, R. A., and DeMarini, D. M. Induction of genetic damage in human lymphocytes and mutations in Salmonella by trihalomethanes: role of red blood cells and GSTT1-1 polymorphism. Mutagenesis, 14: 479-82, 1999.
28. Green T. Methylene chloride induced mouse liver and lung tumours: an overview of the role of mechanistic studies in human safety assessment. Hum. Exp. Toxicol., 16: 3-13, 1997. 29. Tan W., Song, N., Wang, G. Q., Liu, Q., Tang, H. J., Kadlubar, F. F., and Lin, D. X. Impact of genetic polymorphisms in cytochrome P450 2E1 and glutathione S-transferases M1, T1, and P1 on susceptibility to esophageal cancer among high-risk individuals in China. Cancer
Epidemiol., Biomark. Prev., 9: 551-556, 2000.
30. Lu S. H., Camus, A. M., Tomatis, L., and Bartsch, H. Mutagenicity of extracts of pickled vegetables collected in Linhsien County, a high-incidence area for esophageal cancer in Northern China. J. Natl. Cancer Inst., 66: 33-36, 1981.
31. Cheng K. K., Day, N. E., Duffy, S. W., Lam, T. H., Fok, M., and Wong, J. Pickled vegetables in the aetiology of oesophageal cancer in Hong Kong Chinese. Lancet, 339: 1314-1318, 1992.
32. Lu S. H., and Lin, P. Recent research on the etiology of esophageal cancer in China. Z. Gastroenterol., 20: 361-367, 1982.
33. Castellsague X., Munoz, N., De, Stefani, E., Victora, C. G., Castelletto, R., Rolon, P. A., and Quintana, M. J. Independent and joint effects of tobacco smoking and alcohol drinking on the risk of esophageal cancer in men and women. Int. J. Cancer, 82: 657-664, 1999.
34. Newcomb P. A., and Carbone, P. P. The health consequences of smoking. Cancer. Med. Clin. North. Am., 76: 305-331, 1992.
35. van Schooten F. J., Godschalk, R. W., Breedijk, A., Maas, L. M., Kriek, E., Sakai, H., Wigbout, G., Baas, P., Van't Veer, L., and Van Zandwijk, N.
32P-postlabelling of aromatic DNA adducts in white blood cells and alveolar macrophages of smokers: saturation at high exposures. Mut. Res., 378: 65-75, 1997.
36. Mooney L. A., Bell, D. A., Santella, R. M., Van Bennekum, A. M., Ottman, R., Paik, M., Blaner, W. S., Lucier, G. W., Covey, L., Young, T, L., Cooper, T. B., Glassman, A. H., and Perera, F. P. Contribution of genetic and nutritional factors to DNA damage in heavy smokers. Carcinogenesis, 18: 503-509, 1997.
37. Piolatto G., Negri, E., La Vecchia, C., Pira, E., Decarli, A., and Peto, J. Bladder cancer mortality of workers exposed to aromatic amines: an updated analysis. Br. J. Cancer, 63: 457-459, 1991.
38. Jourenkova-Mironova N., Voho, A., Bouchardy, C., Wikman, H., Dayer, P., Benhamou, S., and Hirvonen, A. Glutathione S-transferase GSTM1, GSTM3, GSTP1 and GSTT1 genotypes and the risk of smoking-related oral and pharyngeal cancers. Int. J. Cancer, 81: 44-48, 1999. 39. Abdel-Rahman S. Z., Anwar, W. A., Abdel-Aal, W. E., Mostafa, H. M., and Au, W. W. GSTM1 and GSTT1 genes are potential risk modifiers for bladder cancer. Cancer Det. Prev., 22:129-138, 1998.
40. Brockmoller J., Cascorbi, I., Kerb, R., and Roots, I. Combined analysis of inherited polymorphisms in arylamine N-acetyltransferase 2, glutathione S-transferases M1 and T1, microsomal epoxide hydrolase, and cytochrome P450 enzymes as modulators of bladder cancer risk. Cancer Res., 56: 3915-3925, 1996.
41. McWilliams J. E., Sanderson, B. J., Harris, E. L., Richert-Boe, K. E., and Henner, W. D. Glutathione S-transferase M1 (GSTM1) deficiency and lung cancer risk. Cancer Epidemiol Biomarker. Prev., 4: 589-594, 1995.
42. el-Zein R., Zwischenberger, J. B., Wood, T. G., Abdel-Rahman, S. Z., Brekelbaum, C., and Au, W. W. Combined genetic polymorphism and risk for development of lung cancer. Mut. Res., 381:189-200, 1997.
43. Alexandrie A. K., Ingelman Sundberg, M., Seidegar, J., Tornling, G., and Rannug, A. In: Proceedings of the International ISSX Workshop on Glutathione S-transferses. London, Taylor and Francis.
44. Garcia-Closas M., Kelsey, K. T., Wiencke, J. K., and Christini, D. C. Nutrient intake as a modifier of the association between lung cancer and glutathione S-transferase μ deletion. Proc. Am. Assoc. Cancer Res., 36: 281, 1995.
45 Hemminki K., Dickey, C., Karlsson, S., Bell, D., Hsu, Y, Tsai, W. Y., Mooney, L. A., Savela, K., and Perera, F. P. Aromatic DNA adducts in foundry workers in relation to exposure, life style and CYP1A1 and glutathione transferase M1 genotype. Carcinogenesis, 18: 345-350, 1997.
46. Gao Y. T., McLaughlin, J. K., Gridley, G., Blot, W. J., Ji, B. T., Dai, Q., and Fraumeni, J. F. Jr. Risk factors for esophageal cancer in Shanghai, China. II. Role of diet and nutrients. Int. J. Cancer, 58: 197-202, 1994.
47. Kawajiri K. Chapter 15. CYP1A1. IARC Sci. Publ. 148,159-1 72, 1999.
48. Tsuchida S., and Sato, K. Glutathione transferase and cancer. Crit. Rev. Biochem. Mol. Biol., 27: 337-384, 1992.
49. Ko Y. C., Huang, Y. L., Lee, C. H., Chen, M. J., Lin,LM., and Tsai, C. C. Betel quid chewing, cigarette smoking and alcohol consumption related to oral cancer in Taiwan. J. Oral Pathol. Med., 24: 450-453, 1995.
50. Sankaranarayanan R., Duffy, S. W., Padmakumary, G., Nair, S. M., Day, N. E., and Padmanabhan, T.K. Risk factors for cancer of the oesophagus in Kerala, India. Int. J. Cancer, 49: 485-489, 1991.
Table 1 Odds Ratio (OR) with 95% Confidence Interval (CI) for GSTM1, GSTT1, and
CYP1A1 polymorphisms in esophageal cancer
Controls Patients
Variables
(Groups) No.(%) No.(%) OR(95%CI)
Age <50 years 47 (17.7) 18 (17.1) Referent >50 years 219 (82.3) 87 (82.9) 1.04 (0.57-1.89) Sex Female 28 (10.5) 10 (9.5) Referent Male 238 (89.5) 95 (90.5) 1.12 (0.52-2.39) Cigarette Smoking No 154 (60.9) 28 (26.7) Referent Yes 99 (39.1) 77 (73.3) 4.28 (2.64-6.94)* Alcohol Drinking No 181 (71.0) 38 (36.5) Referent Yes 74 (29.0) 66 (63.5) 4.25 (2.66-6.78)* Areca Chewing No 233 (95.1) 64 (64.0) Referent Yes 12 (4.9) 36 (36.0) 10.92 (5.88-20.30)* GSTM1 Present 114 (43.7) 43 (42.2) Referent Absent 147 (56.3) 59 (57.8) 1.06 (0.67-1.69) GSTT1 Present 132 (50.6) 52 (51.0) Referent Absent 129 (49.4) 50 (49.0) 0.98 (0.62-1.56) CYP1A1 Wt/Wt 111 (42.9) 39 (39.0) Referent Wt/Vt 111 (42.9) 46 (46.0) 1.18 (0.71-1.94) Vt/Vt 37 (14.3) 15 (15.0) 1.15 (0.57-2.33) CYP2E1 Wt/Wt 149 (57.1) 56 (59.6) Referent Wt/Vt 98 (37.6) 33 (35.1) 0.90 (0.54-1.48) Vt/Vt 14 (5.3) 5 (5.3) 0.95 (0.33-2.76)
Table2 Joint Effects of GSTM1, GSTT1, or CYP1A1 with Cigarette Smoking in Esophageal
Cancer
Variables Controls (%) Patients (%) ORs (95% CI)
GSTM1 Present 41 (41.8) 29 (38.7) Referent Absent 57 (58.2) 46 (61.3) 1.14 (0.61-2.11) GSTT1 Present 55 (56.1) 32 (42.7) Referent Absent 43 (43.9) 43 (57.3) 1.72 (0.94-3.15)* CYP1A1 Wt/Wt 43 (43.4) 28 (37.8) Referent Wt/Vt 45 (45.5) 33 (44.6) 1.13 (0.56-2.17) Vt/Vt 11 (11.1) 13 (17.6) 1.82 (0.71-4.61) CYP2E1 Wt/Wt 56 (57.7) 47 (66.2) Referent Wt/Vt 37 (38.1) 20 (28.2) 0.64 (0.33-1.26) Smokers Vt/Vt 4 (4.1) 4 (5.6) 1.19 (0.28-5.02) GSTM1 Present 67 (44.1) 14 (51.8) Referent Absent 85 (55.9) 13(48.2) 0.72 (0.32-1.66) GSTT1 Present 70 (46.1) 20 (74.1) Referent Absent 82 (53.9) 7 (25.9) 0.30 (0.12-0.72)** CYP1A1 Wt/Wt 65 (43.6) 11 (42.3) Referent Wt/Vt 62 (41.6) 13 (50.0) 1.24 (0.52-2.97) Vt/Vt 22 (14.8) 2 (7.7) 0.54 (0.11-2.62) CYP2E1 Wt/Wt 88 (57.5) 9 (39.1) Referent Wt/Vt 55 (36.0) 13 (56.5) 2.31 (0.93-5.77) Non-smokers Vt/Vt 10 (6.5) 1 (4.3) 0.98 (0.11-8.54)
Table3 Combined Effects of GSTT1 and GSTM1 on Esophageal Cancer
GSTM1 Present Absent
Variables
GSTT1 Present Absent Present Absent
Patients (%) 11 (14.7) 18 (18.4) 21 (28.0) 25 (33.3) Controls (%) 23 (23.5) 18 (18.4) 32 (32.7) 25 (25.5) ORs (95%CI) Referent 2.09 (0.79-5.53) Referent 1.52 (0.70-3.34) Smokers
ORs (95%CI) Referent 2.09 (0.79-5.53) 1.37 (0.55-3.41) 2.09 (0.85-5.18)
Patients (%) 8 (29.6) 6 (22.2) 12 (44.4) 1 (3.7)
Controls (%) 29 (19.0) 38 (25.0) 41 (27.0) 44 (29.0) ORs (95%CI) Referent 0.57 (0.18-1.83) Referent 0.08 (0.01-0.42)*
Non-smokers
ORs (95%CI) Referent 0.57 (0.18-1.83) 1.06 (0.38-2.94) 0.08 (0.01-0.48)*
Table 4 Multivariate analysis of risk factors for esophageal cancer in cigarette and
non-cigarette smokers
Variables OR(95% CI) P
Drinking 3.31 (1.39-7.86) 0.007
Areca Chewing 4.65 (1.87-11.54) <0.001
GSTT1 (absent vs. present) 2.44 (1.17-5.08) 0.017 Smokers
(n=163)
CYP 1A1 (Vt/Vt and Vt/Wt vs.
Wt/Wt) 1.70 (0.99-2.94) 0.057 Drinking 0.98 (0.26-3.72) 0.977 Areca Chewing 6.84 (0.29-162.96) 0.234 GSTT1 (absent vs. present) 0.29 (0.11-0.73) 0.009 Non-smoker (n=169)
CYP 1A1 (Vt/Vt and Wt/Vt vs.
Wt/Wt)
0.85 (0.44-1.64) 0.631