Optimizing Glycemic Control of Diabetes Mellitus in Older Adults – A Tailored Approach
Yuh-Min Song
1,21Division of Endocrinology/Metabolism, Department of Internal Medicine, Taichung Tzu Chi Hospital, Buddhist Tzu Chi Medical Foundation;
2School of Medicine, Tzu Chi University, Hualien, Taiwan
Abstract
The prevalence as well as the incidence of diabetes mellitus has been increasing worldwide. In an aging society, this disorder in older adults contributes to these increases. Older people are more vulnerable than younger people to developing excessive fat deposition and reduction in skeletal muscle because of a seden- tary lifestyle, lower energy expenditure, and physical alterations due to aging, which can lead to the develop- ment of insulin resistance. The capacity of pancreatic beta cells to regenerate and differentiate is reduced in older people, which predisposes them to insulin deficiency. These two pathophysiological alterations underlie the development of glucose intolerance. With significantly longer life spans thanks to the advances in health care, it is imperative to attain optimal glycemic control in this specific population to prevent diabetes-related chronic complications. In addition to life style modifications such as dietary control and exercise for obese patients and those who could benefit from moderate weight loss, antidiabetic agents are frequently required to achieve prespecified treatment goals. Delivery of these medications in an efficient and safe manner must be tailored to individual requirements to maintain an intricate balance between reasonable glycemic control and hypoglycemia. Older adults with diabetes are vulnerable to hypoglycemia due to a long history of the disease and frailty from aging. As long as factors that impact the pharmacokinetics and pharmacodynamics of these agents are considered, such as renal function and adherence to polypharmacy, oral agents are more welcomed by older people because of convenience of administration and proved clinical efficacy. When oral agents fail, insulin therapy may be unavoidable when trying to pursue an optimal glycemic target. (J Intern Med Taiwan 2019; 30: 132-149)
Key Words: Diabetes mellitus, Glycemic control, Older adult
Reprint requests and correspondence:Dr. Yuh-Min Song
Address:Division of Endocrinology/Metabolism, Department of Internal Medicine, Taichung Tzu Chi Hospital, Buddhist Tzu Chi Medical Foundation, No.
88, Sec. 1, Fengxing Rd., Tanzi Dist., Taichung City 427, Taiwan
The scope of diabetes mellitus in the older adults
As people age, they may have more chronic diseases than their younger counterparts. The inci- dence of diabetes is increasing with the increase in the geriatric population1,2. Taiwan’s Ministry of the
Interior reported that Taiwan has officially entered the stage of an “aged society” as Taiwanese people over 65 years old accounted for 14.05% of the coun- try’s total population at the end of March, 2018. In 2017, the International Diabetes Federation esti- mated that 122.8 million people worldwide between 65 and 99 years old had diabetes, with a prevalence
of 18.8%3. The increasing prevalence of diabetes in older adults is not only due to deteriorating pancre- atic beta cell function and insulin sensitivity from increased adiposity and reduced skeletal muscle mass in the process of aging, but also the occur- rence of this disease at an earlier age than previ- ously. Geriatric adults have a longer life expectancy thanks to improved health care policies and quality nowadays. Physicians must provide optimal man- agement of this disease in the expanding geriatric population4,5.
Pathophysiology of type 2 diabetes mellitus in older adults
The pathophysiology of type 2 diabetes mel- litus (T2DM) in older adults is not much differ- ent from that in their younger counterparts, but the severity might be exaggerated through the combined effects of deteriorating pancreatic beta cell function and the insulin effect as people age. Many studies have reported that the turnover, regeneration, and proliferation of pancreatic beta cells are impaired in the process of aging6-17. The replication refrac- tory period and the time between cell divisions (G0 stage of the cell cycle) appear to lengthen with age18. In a study using the frequently sampled intravenous glucose tolerance test in normal-weight study sub- jects with comparable baseline glucose levels and fat mass, older men had a 50% loss of beta cell func- tion compared with the younger group19.
Impaired insulin function (insulin resistance) at various tissue levels in the elderly could be exag- gerated by decreased physical activity and abnormal adipose tissue deposition with a simultaneous loss of skeletal muscle mass. This is called sarcopenic obesity, a relatively newly-defined clinical entity gaining widespread attention as having significant impact in geriatric healthcare. The chronological age per se may have no independent influence on the development of insulin resistance when adipos- ity is considered in the analysis. Visceral adiposity,
a notable major factor causing insulin insensitivity, together with reduced beta cell function, results in the development of glucose intolerance20-26.
The life style of older adults also contributes to the development of decreased insulin sensitiv- ity. Reduced energy expenditure as they become more sedentary, and lack of access to proper exer- cise facilities may result in excessive accumula- tion of adipose tissue, especially visceral fat, with a concomitant reduction in lean muscle mass, mainly in the skeletal musculature which is the major site of glucose disposal27-31. A study investigating the relationship between skeletal muscle mass (using dual-energy X-ray absorptiometry) and various components of metabolic syndrome in different age groups in Korea, found that, sarcopenia was an early predictor for the development of diabetes and meta- bolic syndrome, particularly in the elderly, even in the absence of obesity32. Although a sedentary life in older adults may increase body adiposity, studies showed that increased physical activity significantly improved insulin sensitivity, decreased the inci- dence of diabetes, and improved glycemic control in the elderly with diabetes33,34. Exercise benefits insulin sensitivity at the molecular level. Mitochon- drial function in terms of adenosine triphosphate (ATP) production is reduced in the older popula- tion compared with their younger counterparts, and exercise reverses the age-related decline in oxida- tive capacity and ATP production. These findings support the evidence of enhanced insulin sensitivity after exercise training35.
The goal of glycemic control in older adults with diabetes
although many older adults with diabetes are robust enough to lead an independent life, a signifi- cant number are frail generally and have comorbidi- ties such as impaired vision, reduced muscle mass, reduced bone density, and diabetic neuropathy (sen- sory, motor or autonomic), which may independently
or in clusters lead to inability to maintain homeo- stasis, especially in the presence of acute stress.
When there is accompanying impaired renal func- tion or an impaired counter-regulatory response to hypoglycemia after a long history of diabetes, the risk of hypoglycemia-associated complications increases36-42. Although some investigators found that a higher glycated hemoglobin (HBA1c) level was associated with walking difficulty, low physical performance, increased incident frailty, and lower extremity mobility limitations in women between 70 and 79 years old43, there is controversy concerning the impact of hyperglycemia on the general perfor- mance of older adults. In another study, higher blood glucose was associated with increased incident frail- ty in nondiabetic elderly people. However, in elderly people with diabetes, a U-shaped phenomenon was noted, with blood glucose levels higher than 180 mg/
dL and lower than 160 mg/dL both linked to higher incident frailty. The causality underlying this phe- nomenon requires more research44. In a recent study using the Clinical Frailty Scale in elderly diabetes patients in Japan, more severe frailty was associat- ed with advancing age, low levels of HbA1c, serum
albumin, total cholesterol, and high-density lipopro- tein cholesterol, and low systolic blood pressure, as well as low body weight, suggesting a role of reverse metabolism owing to malnutrition as a cause of frail- ty45. With increasing recognition that hypoglycemia is a major drawback in intensive glycemic control, many academic societies have modified targets to less stringent levels tailored to the general perfor- mance of this special group of patients. The current guidelines of the academic society of Taiwan for gly- cemic control in older adults with diabetes are no exception46. Older adults who are functionally and cognitively intact and have a significant life expec- tancy should receive diabetes care using goals devel- oped for younger adults. The goals may be relaxed for older adults not meeting the above criteria (Table 1)46,47. But, hyperglycemia leading to symptoms or risk of acute hyperglycemic complications should be avoided in all patients48-53.
Starting with non-Pharmacological management of diabetes in older adults
When diabetes is diagnosed or already exists
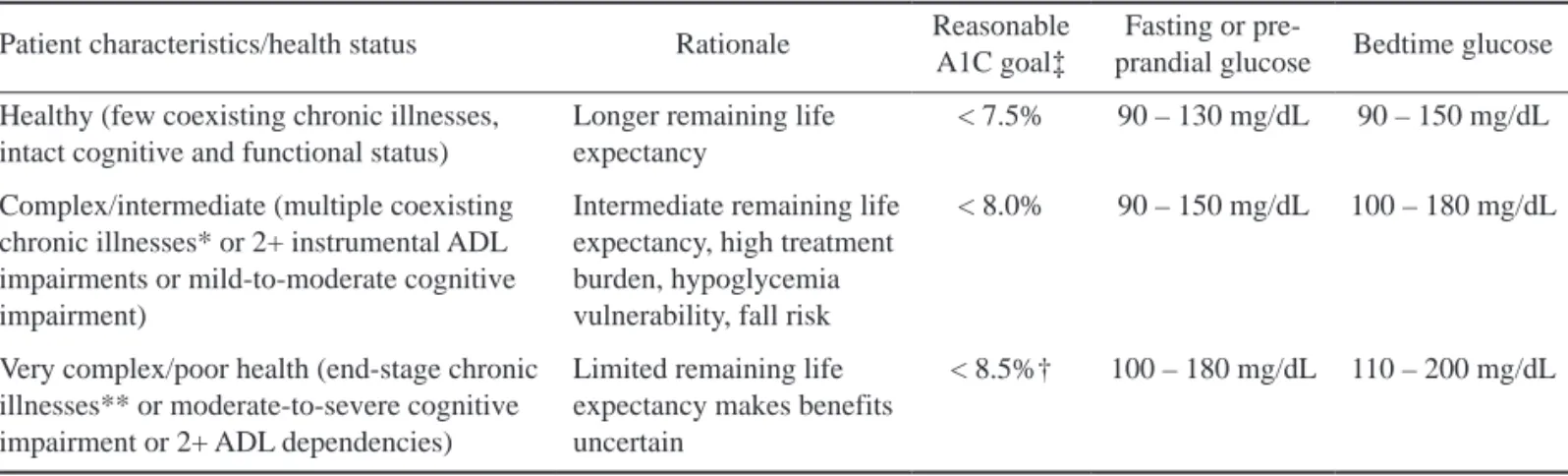
Table 1. A framework for treatment goals for glycemia in older adults with diabetes (adapted from 46, 47) Patient characteristics/health status Rationale Reasonable
A1C goal‡
Fasting or pre-
prandial glucose Bedtime glucose Healthy (few coexisting chronic illnesses,
intact cognitive and functional status)
Longer remaining life expectancy
< 7.5% 90 – 130 mg/dL 90 – 150 mg/dL
Complex/intermediate (multiple coexisting chronic illnesses* or 2+ instrumental ADL impairments or mild-to-moderate cognitive impairment)
Intermediate remaining life expectancy, high treatment burden, hypoglycemia vulnerability, fall risk
< 8.0% 90 – 150 mg/dL 100 – 180 mg/dL
Very complex/poor health (end-stage chronic illnesses** or moderate-to-severe cognitive impairment or 2+ ADL dependencies)
Limited remaining life expectancy makes benefits uncertain
< 8.5%† 100 – 180 mg/dL 110 – 200 mg/dL
ADL, activities of daily living. ‡A lower A1C goal may be set if achievable without recurrent or severe hypoglycemia or undue treat- ment burden. *Coexisting chronic illnesses are conditions serious enough to require medications or lifestyle management and may include arthritis, cancer, congestive heart failure, depression, emphysema, falls, hypertension, incontinence, stage 3 or worse chronic kidney disease, myocardial infarction, and stroke. The term“multiple” means at least 3, but many patients may have 5 or more. **The presence of a single end-stage chronic illness, such as stage 3 - 4 congestive heart failure or oxygen-dependent lung disease, chronic kidney disease requiring dialysis, or uncontrolled metastatic cancer, may cause significant symptoms or impairment of functional status and significantly reduce life expectancy. †A1C of 8.5% equates to an estimated average glucose of ~200 mg/dL. Looser A1C targets above 8.5% are not recommended as they may expose patients to more frequent high glucose values and acute risks from gly- cosuria, dehydration, hyperglycemic hyperosmolar syndrome, and poor wound healing.
in an older adult, the impact of geriatric syndrome on the management of diabetes has to be considered when planning treatment. This syndrome has mul- tiple facets manifesting as functional disabilities in vision and hearing, falls, depression, cognitive impairment, and malnutrition. These disabilities can not only can lead to frailty with loss of inde- pendence in daily living and a low quality of life, but may also become major obstacles in the treat- ment and care of patients with diabetes. A thorough medical history is needed to identify any coexist- ing diabetes-related complications or comorbidities.
Even in newly diagnosed patients, the establish- ment of diabetes almost always has gone through a long journey during which certain cardiovascu- lar risk factors could have already developed and which could raise the risk of cardiovascular events if unnoticed and untreated54-57. Efficient and holis- tic care management requires a well-organized team of physicians, nurse practitioners, nurses, diabetes educators, dietitians, pharmacists, social workers, and mental health professionals. The involvement of both the patient and family in an active treat- ment strategy is also highly recommended. Self- monitoring of blood glucose, for example, can alert the patient and caregivers to glycemic excursions or swings, and can provide physicians with useful information needed to adjust treatment58-61.
As a rule, lifestyle modification including regular physical exercise with mild-to-moderate loss of body weight in obese patients should be com- menced before or at the same time as pharmacolog- ical therapy. Nutritional counseling and exercise training resulting in a loss of body weight in can- didate patients has been found to not only improve insulin sensitivity but also beta cell function. Both aerobic exercise and whole-body resistance training are feasible options in older adults to increase skel- etal muscle mass and decrease fat deposition, with improvement in insulin sensitivity and better gly- cemic control33,62-70. There is some concern about
body weight loss in the elderly71. Researchers have found that when intentional weight loss is achieved by a combination of planned caloric calculation and regular aerobic exercise such as resistance training, the loss of skeletal muscle mass is minimal and is accompanied by increases in physical function and bone density72-74.
Flexibility training and balance training are recommended 2-3 times/week for older adults with diabetes. The American Diabetes Association rec- ommends yoga and tai chi, based on individual pref- erence, to increase flexibility, muscular strength, and balance47. The exercises should be designed to avoid harm to the feet and joints of geriatric people, who are more vulnerable to injury than younger people. Walking barefoot on a pebble route is abso- lutely contraindicated for older patients since the pro- prioceptive or pressure sensation may be impaired, especially in those with a long history of diabetes with a high risk of diabetic neuropathy59,75,76.
Pharmacological management of diabetes in older adults
Oral antidiabetic agents (OADs) are still the most commonly prescribed agents in diabetic patients regardless of age. Unless specific clinical conditions such as acute illness or a catabolism from severe hyperglycemia require the use of insulin therapy, oral agents warrant appraisal in older adults because of their convenience in administration and proved efficacy in glycemic control, with concern about safety issues77-80. The characteristics of cur- rently approved medications for T2DM are summa- rized in Table 2.
Biguanides
Biguanides have long been the first line of OADs considered due to their efficacy in lowering glucose and safety profile. Contraindications include significantly impaired renal or liver function, heart failure, or previous difficulty with these medica-
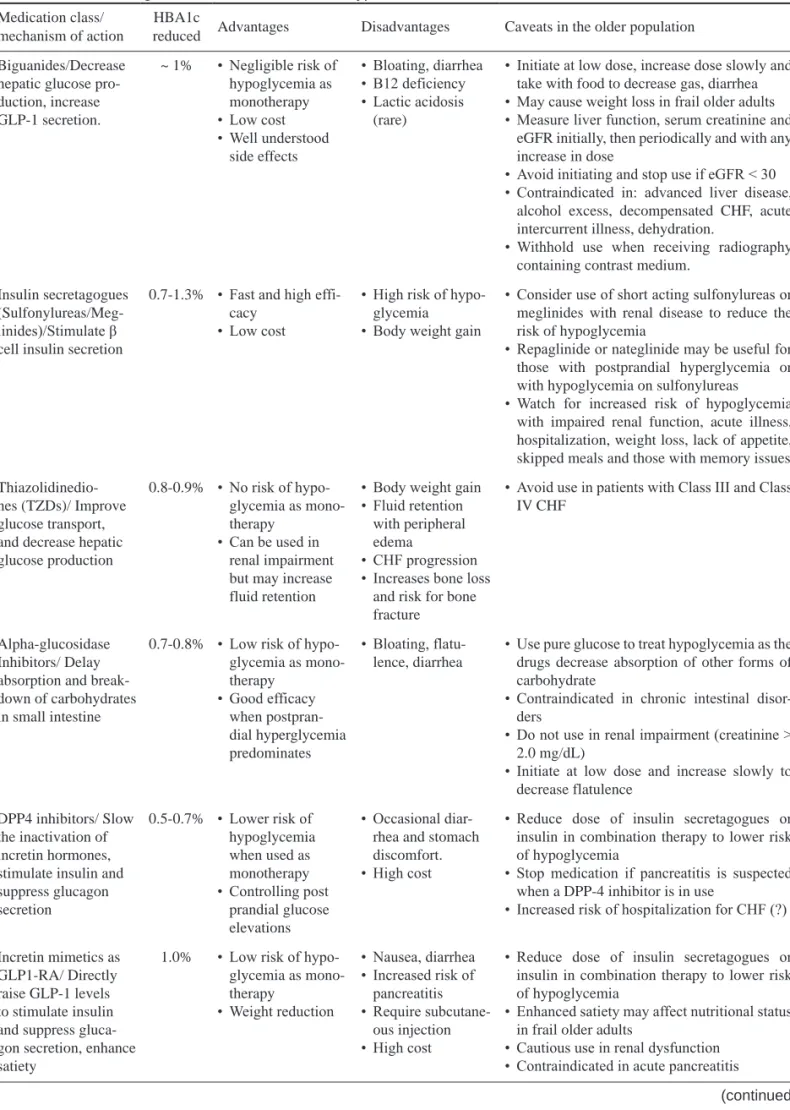
Table 2. Antidiabetic agents used in older adults with type 2 diabetes Medication class/
mechanism of action
HBA1c
reduced Advantages Disadvantages Caveats in the older population Biguanides/Decrease
hepatic glucose pro- duction, increase GLP-1 secretion.
~ 1% • Negligible risk of hypoglycemia as monotherapy
• Low cost
• Well understood side effects
• Bloating, diarrhea
• B12 deficiency
• Lactic acidosis (rare)
• Initiate at low dose, increase dose slowly and take with food to decrease gas, diarrhea
• May cause weight loss in frail older adults
• Measure liver function, serum creatinine and eGFR initially, then periodically and with any increase in dose
• Avoid initiating and stop use if eGFR < 30
• Contraindicated in: advanced liver disease, alcohol excess, decompensated CHF, acute intercurrent illness, dehydration.
• Withhold use when receiving radiography containing contrast medium.
Insulin secretagogues (Sulfonylureas/Meg- linides)/Stimulate β cell insulin secretion
0.7-1.3% • Fast and high effi- cacy
• Low cost
• High risk of hypo- glycemia
• Body weight gain
• Consider use of short acting sulfonylureas or meglinides with renal disease to reduce the risk of hypoglycemia
• Repaglinide or nateglinide may be useful for those with postprandial hyperglycemia or with hypoglycemia on sulfonylureas
• Watch for increased risk of hypoglycemia with impaired renal function, acute illness, hospitalization, weight loss, lack of appetite, skipped meals and those with memory issues Thiazolidinedio-
nes (TZDs)/ Improve glucose transport, and decrease hepatic glucose production
0.8-0.9% • No risk of hypo- glycemia as mono- therapy
• Can be used in renal impairment but may increase fluid retention
• Body weight gain
• Fluid retention with peripheral edema
• CHF progression
• Increases bone loss and risk for bone fracture
• Avoid use in patients with Class III and Class IV CHF
Alpha-glucosidase Inhibitors/ Delay absorption and break- down of carbohydrates in small intestine
0.7-0.8% • Low risk of hypo- glycemia as mono- therapy
• Good efficacy when postpran- dial hyperglycemia predominates
• Bloating, flatu- lence, diarrhea
• Use pure glucose to treat hypoglycemia as the drugs decrease absorption of other forms of carbohydrate
• Contraindicated in chronic intestinal disor- ders
• Do not use in renal impairment (creatinine >
2.0 mg/dL)
• Initiate at low dose and increase slowly to decrease flatulence
DPP4 inhibitors/ Slow the inactivation of incretin hormones, stimulate insulin and suppress glucagon secretion
0.5-0.7% • Lower risk of hypoglycemia when used as monotherapy
• Controlling post prandial glucose elevations
• Occasional diar- rhea and stomach discomfort.
• High cost
• Reduce dose of insulin secretagogues or insulin in combination therapy to lower risk of hypoglycemia
• Stop medication if pancreatitis is suspected when a DPP-4 inhibitor is in use
• Increased risk of hospitalization for CHF (?)
Incretin mimetics as GLP1-RA/ Directly raise GLP-1 levels to stimulate insulin and suppress gluca- gon secretion, enhance satiety
1.0% • Low risk of hypo- glycemia as mono- therapy
• Weight reduction
• Nausea, diarrhea
• Increased risk of pancreatitis
• Require subcutane- ous injection
• High cost
• Reduce dose of insulin secretagogues or insulin in combination therapy to lower risk of hypoglycemia
• Enhanced satiety may affect nutritional status in frail older adults
• Cautious use in renal dysfunction
• Contraindicated in acute pancreatitis
(continued)
diovascular disease derived from a meta-analysis of randomized trials reported uncertainty regard- ing its benefit in reducing cardiovascular risks when used as a first line OAD84. Furthermore, with the advent of novel antidiabetic agents, the role of met- formin as a first line oral antidiabetic agent has been challenged in the past several years, as consistent findings of significant heart protection from newer agents have been reported in long-term, prospective, randomized control trials. The use of the sodium- glucose co-transporter subtype 2 inhibitor (SGLT2i) empagliflozine was found to result in profound reduction in all-cause mortality and cardiovascu- lar mortality, as well as hospitalization from heart failure in the EMPA-REG trial in diabetic patients with high risks of cardiovascular events85. The administration of the glucagon-like peptide-1 recep- tor agonist (GLP-1RA) liraglutide was also found to significantly reduce the cardiovascular risk com- tions in patients, mainly from gastrointestinal irri-
tation. The United Kingdom Prospective Diabetes Study found that patients taking metformin had sig- nificantly lower rates of myocardial infarction and related mortality than patients taking sulfonylureas (SUs) or insulin therapy81. A survey from a health- care database in Canada investigated mortality in new users of oral antidiabetic agents over 5 years.
The mortality rates in patients on metformin mono- therapy (13.8%) or in combination with SUs (13.6%) were significantly lower than those on SU mono- therapy (24.7%)82. A retrospective study in China analyzed 3400 pairs of diabetic patients who were started on metformin and lifestyle modification or life style modification alone. Over 5 years, those on metformin therapy had significant risk reductions in all-cause mortality by 29.5% and cardiovascular events by 30-35% (except heart failure)83. However, a review article on the impact of metformin on car-
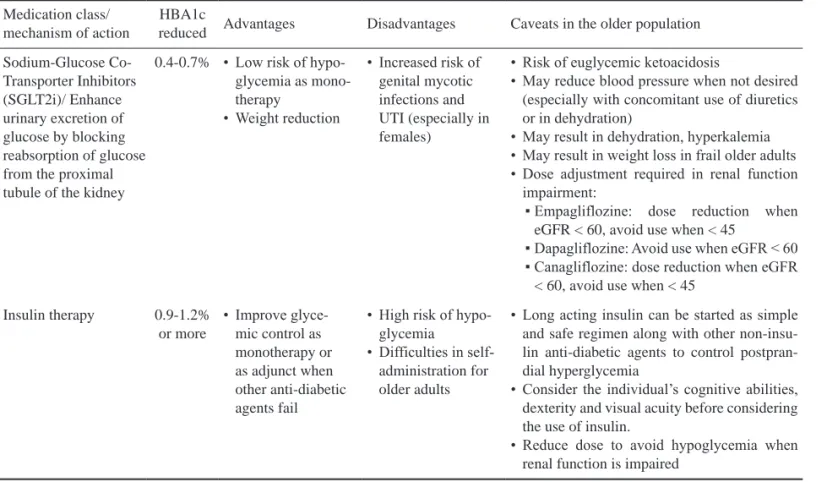
Medication class/
mechanism of action
HBA1c
reduced Advantages Disadvantages Caveats in the older population Sodium-Glucose Co-
Transporter Inhibitors (SGLT2i)/ Enhance urinary excretion of glucose by blocking reabsorption of glucose from the proximal tubule of the kidney
0.4-0.7% • Low risk of hypo- glycemia as mono- therapy
• Weight reduction
• Increased risk of genital mycotic infections and UTI (especially in females)
• Risk of euglycemic ketoacidosis
• May reduce blood pressure when not desired (especially with concomitant use of diuretics or in dehydration)
• May result in dehydration, hyperkalemia
• May result in weight loss in frail older adults
• Dose adjustment required in renal function impairment:
▪ Empagliflozine: dose reduction when eGFR < 60, avoid use when < 45
▪ Dapagliflozine: Avoid use when eGFR < 60
▪ Canagliflozine: dose reduction when eGFR
< 60, avoid use when < 45 Insulin therapy 0.9-1.2%
or more
• Improve glyce- mic control as monotherapy or as adjunct when other anti-diabetic agents fail
• High risk of hypo- glycemia
• Difficulties in self- administration for older adults
• Long acting insulin can be started as simple and safe regimen along with other non-insu- lin anti-diabetic agents to control postpran- dial hyperglycemia
• Consider the individual’s cognitive abilities, dexterity and visual acuity before considering the use of insulin.
• Reduce dose to avoid hypoglycemia when renal function is impaired
Abbreviations: CHF: congestive heart failure.
Table 2. Antidiabetic agents used in older adults with type 2 diabetes (continued)
pared with a placebo86. Various academic societies have endorsed the use of these newer antidiabetic agents as second line medications added to metfor- min in diabetic patients who are at risk of or already have cardiovascular disorders (e.g. heart failure, existing coronary heart disease, or recent acute cor- onary syndrome), because of solid evidence of car- dioprotection compared with other agents47,52,87,88.
The dosage of metformin must be adjusted according to renal function as estimated by the glo- merular filtration rate (GFR) (eGFR, mL/min/1.73 m2) derived from serum creatinine levels. In a study in 451 diabetic patients, a dosage-response curve was noted with a range between 500 mg and 2000 mg daily. A further titration up to 2500 mg daily did not result in further significant benefit in gly- cemic control89. A recent study in Japanese diabetic patients 21 to 84 years old (mean age of 64) with a mean eGFR of 78.3 ± 19.5 mL/min/1.73 m2 sug- gested that the efficacy of metformin is dose-related and a daily dose of 1500 mg had a significant glu- cose-lowering effect. A further titration up to 2250 mg daily, the maximum dose used in that study pro- tocol, had a trend toward further improvement in the glycemic profile. Dosing frequencies of two and three times per day in patients taking 1500 mg/day resulted in similar efficacy. Most side effects were in the gastrointestinal system90. Inconsistencies in the recommended highest dosages in different studies could be caused by individual responsive- ness to metformin when given as a monotherapy.
Diabetes treatment is rarely limited to the use of a single agent and combination therapy is almost always used because of the multiple pathophysiolog- ical processes underlying diabetes development and progression. As with other OADs, the selection of the proper dosage and frequency and timing of met- formin administration largely depends on the char- acteristics of co-administered agents91.
One risk of metformin is buildup of lactic acid with poor renal function. The United States
Food and Drug Administration (USFDA) advises that metformin not be used when the eGFR is < 30 mL/min/1.73 m2 92. Although the eGFR declines as people age, age per se may not be a factor contrib- uting to the accumulation of lactic acid with met- formin use. Renal function is the dominant clinical factor of concern when metformin is considered, regardless of age93.
Sulfonylureas and metiglinides as insulin secretagogues
The second most commonly prescribed OADs in most regions and countries are the sulfolnylureas (SUs). As the earliest one among the various class- es of OADs in the drug development history, SUs have always been one of the most-widely prescribed till nowadays, although there have been voices and noises arising several years ago debating on its role in the management of diabetes out of the following observations and concerns: 1. possible links between its use and increased risk of cardiovascular as well as all-cause mortalities, and 2. the debut of other new antidiabetic agents that have lower risk in causing severe hypoglycemia with an appreciable antidiabet- ic efficacy at the same time, as well as significant benefits in cardio- and renal protection94-100. Never- theless, when used judiciously, SUs are high efficacy antidiabetic agents which can bring glycemic control to a prespecified target in a shorter time compared with other oral therapies101,102. Among longer dura- tion SUs, gliclazide is less likely to cause hypogly- cemia than glyburide and glimepiride103.
Meglitinides, a class of non-sulfonylurea insu- lin secretagogues, effectively lower the postprandial glucose level to achieve smooth glycemic excursion after a meal. They carry a lower risk of hypoglyce- mia due to their fast-on and fast-off pharmacokinet- ics in insulin stimulation, an advantage especially beneficial for older adults. With a rapid onset of action after administration before or with a meal, meglitinides are flexible and feasible for patients
who have irregular meals. For a given dose of repa- glinide, inactive metabolites are mainly excreted via the bile (∼90%). Only 8% is excreted in the urine and less than 2% of the parent drug is recovered in the feces104-107. In diabetic patients with chronic kidney disease of stage 2-3, repaglinide has the same phar- macokinetic characteristics as seen in diabetics with normal renal function and thus carries a lower risk of hypoglycemia risk than long-acting SUs108.
Thiazolidinediones as insulin sensi- tizers
Thiazolidinediones (TZD) improve insulin sensitivity by acting as ligands for the activation of the nuclear peroxisome proliferator-activated receptor gamma in adipocytes. In an earlier study on the mechanisms underlying the insulin-sensi- tizing effect of pioglitazone, it was demonstrated that a shift in fat distribution from visceral to sub- cutaneous areas is associated with improvements in hepatic and peripheral tissue sensitivity to insulin109. In a more recent study using 18F-fluorodeoxyglu- cose-positron emission tomography and computed tomography, pioglitazone was found to significantly decrease the visceral fat volume and its metabolic activity in patients with impaired glucose tolerance or T2DM110. As visceral fat accumulation has been linked to a higher risk of cardiovascular diseases, the redistribution of visceral fat to subcutaneous sites with the use of TZD has been proposed to be pro- tective for the cardiovascular system111. There are debates over the pros and cons of the role of dif- ferent TZDs in heart protection. These agents are contraindicated in patients with evident heart fail- ure or with ischemic heart disease who are vulner- able to the development of heart failure from fluid accumulation, a common side effect of TZDs112-117. The prudent use of TZDs has been proved effective for glycemic control, along with an improved lipid profile such as elevation of high-density lipoprotein cholesterol and lowering of triglycerides, theoreti-
cally beneficial to the cardiovascular system in dia- betes patients with insulin resistance and metabolic syndrome118.
Alpha-glucosidase inhibitors
Alpha-glucosidase inhibitors lower plasma glucose by inhibiting the breakdown of complex car- bohydrate at the small intestinal level with reduced absorption of simple sugar into the blood stream. In a study carried out in elderly patients with diabe- tes, acarbose was found to effectively lower HBA1c levels with improvement in both the fasting and incremental postprandial glucose values. Although the pharmacology of these agents does not involve insulin stimulation, with lowering of plasma glucose and the consequent amelioration of glucotoxicity, insulin sensitivity as assessed by the homeosta- sis model of assessment or insulin clamp method improved in clinical studies119-122. The side effects are mostly gastrointestinal with bloating, flatulence or abdominal distension from gas formation in the large intestine, symptoms that are tolerable for most patients, including the geriatric population120.
Incretin therapy
Incretins, the peptides secreted from the gas- trointestinal tract in response to various nutrients ingested into the alimentary tract, carbohydrates in particular, have been applied to clinical use in the past decade with success. Among the various incretins, glucagon-like peptide-1 (GLP-1) is the most widely investigated and appreciated in gly- cemic control strategies. When secreted from the L-cells located at the distal end of the ileum, GLP-1 is rapidly degraded by the dipeptidyl peptidase-4 (DPP4) from nearby intestinal epithelial cells within a few minutes so only 25% of the secreted amount reaches the portal circulation. A further 40-50% is destroyed in the liver and thus only 10-15% of the originally secreted amount enters the systemic circu- lation and may reach the pancreas to exert an insuli-
notropic effect (incretin effect). When the activity of DPP4 is inhibited by its antagonist (DPP4 inhibitor = DPP4i), the amount of GLP-1 that reaches the portal or peripheral circulation is enhanced with a con- sequent increase in the amount of insulin secreted from pancreatic beta cells. GLP-1 not only stimulates secretion of insulin but also suppresses glucagon secretion from the neighboring alpha cells. Through this synergistic effect, the circulatory glucose level is effectively lowered when plasma glucose is driven into insulin-sensitive peripheral tissues (muscle, liver, and adipose tissue) and when endogenous glucose production is reduced (gluconeogenesis from muscle and glycolysis from liver) due to the suppressed glucagon effect123,124. Beta cell dysfunc- tion plays a more dominant role in the pathophysi- ology underlying T2DM than adiposity and insulin resistance in Asian patients compared with the Cau- casian population, and incretin-based therapy has been proposed to have a more prominent role in the management of diabetes in East Asian people125,126. The low risk of hypoglycemia when used as mono- therapy or when added to metformin merits its use in older adults with diabetes. With the exception of linagliptin, which is eliminated through the hepatic pathway, the dosages of DPP4i must be adjusted to renal function127,128. DPP4i can be used as mono- therapy or in combination with any other oral anti- diabetic agents or insulin therapy, but physicians should be cautious when prescribing concomitant SUs as the risk of hypoglycemia is significantly raised. The dose of SUs should be reduced or halved in this regimen129. The low risk of hypoglycemia in incretin therapy is derived from the lowering of the intracellular ATP/adenosine diphosphate (ATP/
ADP) ratio during the glycolysis pathway in the beta cells when the ambient plasma blood glucose and hence the intra-cellular glucose level is accordingly low. Physiologically, a low ATP/ADP ratio would lead to the opening of the ATP-sensitive potas- sium (kATP) channels located on the cell membrane
with consequent suppression of insulin secretion from beta cells, preventing a further lowering of blood glucose. However, when SUs are in use, these insulin secretagogues stimulate insulin secretion via high affinity with the sulfonylurea receptor-1 on the cell membrane even when the plasma glucose level is low, a coupling leading to the stimulation of insulin secretion through closing of the nearby kATP channels. Hypoglycemia ensues because of unchecked insulin secretion in the presence of SUs.
At the same time, the high insulin levels incurred by SU action may suppress glucagon secretion, with loss of the protective role of this counter-regulatory hormone against hypoglycemia130. In a meta-analy- sis on the concomitant use of DPP4-i and SUs com- pared with SUs alone, the hypoglycemia risk was increased by 50% in the combination group with one excess case in every 17 cases thus treated131. In a Swedish nationwide study on the impact of SUs combined with metformin versus DPP4i combined with metformin, investigators found significantly higher risks of developing hypoglycemia, fatal and non-fatal cardiovascular diseases, and all-cause mortality in the SU-combination group. Univari- ate analyses showed increasing age and frailty were both risk factors, among others, in developing hypo- glycemia and other parameters of interest132. The SU that carried the highest risk of hypoglycemia was glibenclamide. It has a long half-life and the metabolites still exert a secretagogue effect, espe- cially when the plasma concentration builds up in patients with impaired renal function133. A higher risk of hypoglycemia was also noted when insulin as second line therapy was added to metformin, com- pared with adding DPP4i134. Thus, strong evidence shows that DPP4i carry a significantly lower risk of hypoglycemia when used alone or in combina- tion with other antidiabetic agents, excluding SUs and insulin therapy, in the elderly diabetic popula- tion. This group is prone to hypoglycemia caused by inadequate nutrition, frailty from other comorbidi-
ties, or overtreatment medically135.
GLP-1RAs administered by injection have gained a significant role in the management of dia- betes since their debut in 2005. They not only have proved efficacious in lowering glucose, but also offer significant cardiovascular protection, with evidence obtained from randomized, placebo-controlled car- diovascular outcome trials136. In the LEADER trial, liraglutide significantly reduced the rate of the first occurrence of death from cardiovascular causes, nonfatal myocardial infarction, or nonfatal stroke compared with a placebo in type 2 diabetes patients 50 years old or older with a history of coronary heart disease, cerebrovascular disease, peripheral vascu- lar disease, or chronic kidney disease, and those 60 years old or older with at least one cardiovascular (CV) risk factor137. A meta-analysis of completed and ongoing clinical trials concluded that the CV benefits of the GLP-1RA regimens showed a class effect138.
These novel findings on the benefits of anti- diabetic agents, together with those addressed in the next section on sodium-glucose co-transporter subtype 2 (SGLT2) inhibitors, have gradually lead to a paradigm shift in the management of diabe- tes beyond glycemic control as the primary and only goal toward cardiovascular and renal protec- tion.139-141.
Sodium-glucose co-transporter inhibitors
SGLT2 inhibitors (SGLT2i) are novel OADs that lower glucose by promoting excessive glycos- uria via inhibition of tubular reabsorption of fil- tered plasma glucose through the glomerulus. This working mechanism does not involve insulin secre- tion but may cause physiologically adaptive pro- cesses after glucose is drained from the body. This reduced plasma glucose concentration after exces- sive glycosuria is followed by reduced insulin secre- tion as well as enhanced glucagon secretion with a
net increase in endogenous glucose production via gluconeogenesis, and formation of ketone bodies from metabolism of free fatty acids derived from the effect of glucagon on fat metabolism. An advan- tage when initiating these antidiabetic agents is sig- nificant reduction of body weight. This is welcomed by overweight and obese diabetic patients who may also obtain better glycemic control from reduction of fatty tissue, especially visceral fat, associated with improvement in insulin sensitivity142-144.
SGLT2i can be used as monotherapy or in com- bination with any other antidiabetic agents, inclu- sive of insulin therapy145. In addition to the expected glucose lowering effect, the Empagliflozin, Cardio- vascular Outcomes, and Mortality in Type 2 Dia- betes trial, one of the first large scale randomized, placebo-controlled clinical trials of its kind, found that empagliflozine could significantly reduce the risk of heart failure and related mortality146. The multiple nonglycemic effects of this new group of antidiabetic agents have been explored in an attempt to explain the mechanisms underlying these unex- pected cardiovascular benefits. Plausible multifac- eted experimental findings have been proposed, which include reductions in body weight, adipose tissue (visceral adiposity predominantly), and blood pressure (diuretic hypothesis), improvement in arte- rial stiffness, and less hyperinsulinemia147. An increase in ketone body formation which spares the failing heart from excessive oxygen consumption during synthesis of ATP, proposed as the “theory of thrifty substrate”, has also been theorized as the cause of the significant cardiovascular protec- tion.148. The cardioprotective benefits of SGLT2i have been generally endorsed by clinical research outcomes and meta-analyses from multiple relevant studies149,150.
SGLT2i lower plasma glucose by eliminating glucose into the urine, and adequate renal function is critical for the clinical effect. When renal func- tion is impaired, the amount of glucose filtered
through the glomerulus is also reduced, followed by weakened glycemic control. Generally, these agents are contraindicated when the eGFR is lower than 30 ml/min/1.73 m2 because of insignificant low- ering of glucose and possible dehydration instead.
The USFDA advises that physicians evaluate risk factors for kidney injury before starting these agents (including reduced blood volume, chronic kidney insufficiency, heart failure, and concomitant med- ications such as diuretics, angiotensin-converting enzyme inhibitors, angiotensin II receptor blockers, and non-steroidal anti-inflammatory drugs), follow up renal function periodically. The drugs should be discontinued promptly whenever there is evidence of acute kidney injury151.
The significant glucosuric effect with the use of these agents may cause local irritation of the urethral mucosa or even urinary tract or genital infection. Risk factors for development of fungal genital infection include female gender and a previ- ous history of fungal genital infections152. Clinical studies have shown that most infections are mycotic and antibiotics are effective as standard therapy. The antidiabetic regimen rarely needs to be stopped153. Hypovolemia can occur due to the diuretic effect.
Patients taking diuretics for any other clinical reason should be observed to avoid impaired renal hemody- namics, hypotension, or orthostatic hypotension154. The use of SGLT2i in older adults has gener- ally been deemed safe when the risks of hypogly- cemia, volume depletion-related events, genital/
urinary tract infection, polyuria, and increment in ketone body formation are considered before and during follow-up and when used judiciously155-157.
Insulin therapy
If patients can accept the use of injections, and there are no contraindications to its use, insulin is generally considered to have the best treatment out- comes in terms of glycemic control for patients of all ages, as long as the dosage and frequency of admin-
istration are well designed and tailored to individual requirements. Insulin injections can be adminis- tered as initial therapy, especially in patients with extremely high plasma glucose levels (e.g. when HBA1c is > 9%) and other signs of a catabolic state from extreme hyperglycemia, or as add-on or com- bination therapy whenever more than one OAD does not give reasonable glycemic control. Insulin therapy consists of multiple modalities of admin- istration, including basal, basal plus (one basal + one or two short-acting insulin injections adminis- tered before the larger meal(s) in a day), basal-bolus (one basal + short-acting insulin injection admin- istered before each main meal), or mixed insulin with various formulae (e.g. 30/70, 25/75, or 50/50 as the short-acting/intermediate-acting ratio in the mixture). The regimen is tailored to each patient’s clinical characteristics including the required fre- quency of administration, the injection devices available, dexterity of the individuals in handling injections, and family support for those who are dependent in daily activities. Conclusions from randomized, controlled clinical trials show better glycemic control with more frequent and complex insulin regimens compared with simpler regi- mens158-160. However, complex regimens may not be feasible for elderly people with physical or mental dysfunction. Therefore, for reasons of convenience and better adherence to therapy, long-acting insulin is usually started first with the aim of obtaining an acceptable fasting glucose level and a narrowing of the postprandial glucose excursion, when other factors such as dietary control and exercise inten- sity are maintained. Chien et al.161 conducted a pro- spective study of the effects and complications of starting a basal insulin regimen in elderly (72.5 ± 5.3 year-old) and younger (52.6 ± 8.1 year-old) dia- betic patients who had failed OAD therapy. After 24 weeks of treatment with insulin glargine, there were similar reductions in fasting blood glucose and HBA1c in both groups (81.3 ± 79.9 mg/dL vs 93.0 ±
82.5 mg/dL; 1.18 ± 1.76% % vs 1.49 ± 2.12% in the elderly and the younger groups, respectively, with p > 0.5 for intergroup comparison in both param- eters). The incidence of hypoglycemia was low in both groups without statistical significance (9.4% vs 15.0%, elderly vs young, p = 0.4733 for intergroup comparison). This study indicates that basal insulin therapy (starting with 0.24 ± 0.11 U/kg in the elderly group) with gradual titration of dose according to careful clinical evaluation is safe in older adults with diabetes who do not achieve adequate glycemic control with two or more OADs. When the goal of fasting glucose has been reached but there is inade- quate glycemic control as evidenced by high HBA1c or postprandial glucose profiles, a switch to a more complex regimen may be needed to avoid the annoy- ing symptoms of chronic hyperglycemia, such as body weight loss, thirst, polyuria, nocturia, postural hypotension due to dehydration, and a decreased immune response. A study of Japanese diabetic patients who failed SU therapy found similar reduc- tions of HBA1c from similar beginning levels (~
9%) of -14.7~ -17.8% in patients with complex basal- bolus regimens and simpler pre-mixed regimens.
Despite the absence of statistical significance, the absolute HBA1c levels reached were numerically lower in the basal-bolus group (6.9(6.2~7.3)% than the pre-mixed group (7.4(6.9~8.7)%)162.
Conclusions
As the number of older adults with diabetes increases, healthcare professionals face more chal- lenges in its management in this group of patients.
A multi-disciplinary team is needed to deliver profi- cient and competent care using judicious prescription and evaluation of the advantages and disadvantages of various antidiabetic regimens. The goal is not only to achieve adequate glycemic control but also to prevent and minimize diabetes-related complica- tions and comorbidities to lengthen the healthy life span of elderly patients living with diabetes.
References
1. Boeckxstaens P, Peersman W, Goubin G, et al. A practice- based analysis of combinations of diseases in patients aged 65 or older in primary care. BMC Family Practice 2014; 15:
159-64.
2. Jacob L, Breuer J, Kostev K. Prevalence of chronic diseases among older patients in German general practices. GMS German Medical Science 2016;14: Doc03. doi:10.3205/
000230.
3. 3. Global picture. In: Karuranga S, da Rocha Fernandes J, Huang Y, et al. editors. International Diabetes Federation.
IDF atlas. 8th ed. Brussels, Belgium: International Diabetes Federation 2017; 40-65.
4. Boyle JP, Thompson TJ, Gregg EW, et al. Projection of the year 2050 burden of diabetes in the US adult population:
dynamic modeling of incidence, mortality, and prediabetes prevalence. Population Health Metrics 2010; 8: 29-40.
5. Waldeyer R, Brinks R, Rathmann W, et al. Projection of the burden of Type 2 diabetes mellitus in Germany: a demo- graphic modelling approach to estimate the direct medical excess costs from 2010 to 2040. Diabet Med 2013; 30:
999-1008.
6. Butler AE, Janson J, Bonner-Weir S, et al. Beta-cell deficit and increased beta-cell apoptosis in humans with type 2 dia- betes. Diabetes 2003; 52: 102-10.
7. Menge BA, Tannapfel A, Belyaev O, et al. Partial pancreatec- tomy in adult humans does not provoke beta-cell regenera- tion. Diabetes 2008; 57: 142-9.
8. Meier JJ, Butler AE, Saisho Y, et al. Beta-cell replication is the primary mechanism subserving the postnatal expansion of beta-cell mass in humans. Diabetes 2008; 57: 1584-94.
9. Tschen SI, Dhawan S, Gurlo T, et al. Age-dependent decline in beta-cell proliferation restricts the capacity of beta-cell regen- eration in mice. Diabetes 2009; 58: 1312-20.
10. Rankin MM, Kushner JA. Adaptive beta-cell proliferation is severely restricted with advanced age. Diabetes 2009; 58:
1365-72.
11. Reers C, Erbel S, Esposito I, et al. Impaired islet turnover in human donor pancreata with aging. Eur J Endocrinol 2009;
160: 185-91.
12. Perl S, Kushner JA, Buchholz BA, et al. Significant human beta-cell turnover is limited to the first three decades of life as determined by in vivo thymidine analog incorporation and radiocarbon dating. J Clin Endocrinol Metab 2010; 95: E234- E239.
13. Gunasekaran U, Gannon M. Type 2 Diabetes and the Aging Pancreatic Beta Cell. Aging 2011; 3: 565-75.
14. Gregg BE, Moore PC, Demozay D, et al. Formation of a human β-cell population within pancreatic islets is set early in life. J Clin Endocrinol Metab 2012; 97: 3197-206.
15. Saisho Y, Butler AE, Manesso E, et al. β-Cell mass and turno- ver in humans: effects of obesity and aging. Diabetes Care 2013; 36: 111-7.
16. Kushner JA. The role of aging upon β cell turnover. J Clin Invest 2013; 123: 990 -5.
17. Lee PG, Halter JB. The Pathophysiology of Hyperglycemia in Older Adults: Clinical Considerations. Diabetes Care 2017;
40: 444-52.
18. Salpeter SJ, Klein AM, Huangfu D, et al. Glucose and aging control the quiescence period that follows pancreatic beta cell replication. Development 2010; 137: 3205-13.
19. Chen M, Bergman RN, Pacini G, et al. Pathogenesis of age- related glucose intolerance in man: insulin resistance and decreased beta-cell function. J Clin Endocrinol Metab 1985;
60: 13-20.
20. Coon PJ, Rogus EM, Drinkwater D, et al. Role of body fat dis- tribution in the decline in insulin sensitivity and glucose toler- ance with age. J Clin Endocrinol Metab 1992; 75: 1125-32.
21. Racette SB, Evans EM, Weiss EP, et al. Abdominal Adipos- ity Is a Stronger Predictor of Insulin Resistance than Fitness Among 50 - 95 Year Olds. Diabetes Care 2006; 29: 673-8.
22. Karakelides H, Irving BA, Short KR, et al. Age, obesity, and sex effects on insulin sensitivity and skeletal muscle mito- chondrial function. Diabetes 2010; 59: 89-97.
23. Srikanthan P, Hevener AL, Karlamangla AS. Sarcopenia Exacerbates Obesity-Associated Insulin Resistance and Dys- glycemia: Findings from the National Health and Nutrition Examination Survey III. PLoS ONE 2010; 5: e10805.
24. Lee SW, Youm Y, Lee WJ, et al. Appendicular Skeletal Muscle Mass and Insulin Resistance in an Elderly Korean Population:
The Korean Social Life, Health and Aging Project-Health Examination Cohort. Diabetes Metab J 2015; 39: 37-45.
25. Choi KM. Sarcopenia and sarcopenic obesity. Korean J Intern Med 2016; 31: 1054-60.
26. Sinclair AJ, Abdelhafiz AH, Rodríguez-Mañas L. Frailty and sarcopenia - newly emerging and high impact complications of diabetes. Journal of Diabetes and Its Complications 2017;
31: 1465-73.
27. Willey KA, Singh FMA. Battling Insulin Resistance in Elderly Obese People with Type 2 Diabetes - Bring on the heavy weights. Diabetes Care 2003; 26: 1580-8.
28. Kanaya AM, Harris T, Goodpaster BH, et al. Health, Aging, and Body Composition (ABC) Study. Adipocytokines attenu- ate the association between visceral adiposity and diabetes in older adults. Diabetes Care 2004; 27: 1375-80.
29. Petersen KF, Shulman GI. Etiology of insulin resistance. Am J Med 2006; 119: S10-S16.
30. Amati F, Dubé JJ, Coen PM, et al. Physical Inactivity and Obesity Underlie the Insulin Resistance of Aging. Diabetes Care 2009; 32: 1547-9.
31. Bradley D, Hsueh W. Type 2 Diabetes in the Elderly: Chal- lenges in a Unique Patient Population. J Geriatr Med Gerontol 2016; 2: 014. 10.23937/2469-5858/1510014
32. Moon SS. Low skeletal muscle mass is associated with insulin resistance, diabetes, and metabolic syndrome in the Korean population: The Korea National Health and Nutrition Exam- ination Survey (KNHANES) 2009-2010. Endocrine Journal 2014; 61: 61-70.
33. Kahn SE, Larson VG, Beard JC, et al. Effect of exercise on insulin action, glucose tolerance, and insulin secretion in aging. Am J Physiol 1990; 258: E937-E943.
34. Flack KD, Davy KP, Hulver MW, et al. Aging, Resistance
Training, and Diabetes Prevention. Journal of Aging Research 2011; Article ID 127315.
35. Ghosh S, Lertwattanarak R, Lefort N, et al. Reduction in reac- tive oxygen species production by mitochondria from elderly subjects with normal and impaired glucose tolerance. Diabe- tes 2011; 60: 2051- 60.
36. Chen LK, Chen YM, Lin MH, et al. Care of elderly patients with diabetes mellitus: A focus on frailty. Ageing Research Reviews 2010; 9: S18-S22.
37. Halter JB, Musi N, McFarland Horne F, et al. Diabetes and cardiovascular disease in older adults: current status and future directions. Diabetes 2014; 63: 2578-89.
38. Sinclair AJ, Rodriguez-Mañas L. Diabetes and Frailty: Two Converging Conditions? Can J Diabetes 2016; 40: 77-83.
39. Perkisas S, Vandewoude M. Where frailty meets diabetes.
Diabetes Metab Res Rev 2016; 32 Suppl 1: 261-7.
40. Sinclair AJ, Abdelhafiz AH, Rodríguez-Mañas L. Frailty and sarcopenia - newly emerging and high impact complications of diabetes. Journal of Diabetes and Its Complications 2017;
31: 1465-73.
41. Chhetri JK, Zheng Z, Xu X, et al. The prevalence and inci- dence of frailty in Pre-diabetic and diabetic community dwelling older population: results from Beijing longitudinal study of aging II (BLSA-II). BMC Geriatrics 2017; 17: 47-54.
42. Yanase T, Yanagita I, Muta K, et al. Frailty in elderly diabetes patients. Endocrine Journal 2018; 65: 1-11.
43. Kalyani RR, Tian J, Xue QL, et al. Hyperglycemia is Asso- ciated with the Incidence of Frailty and Lower Extremity Mobility Limitations in Older Women. J Am Geriatr Soc 2012; 60: 1701-7.
44. Zaslavsky O, Walker RL, Crane PK, et al. Glucose Levels and Risk of Frailty. Gerontol A Biol Sci Med Sci 2016; 71:
1223-9.
45. Yanagita I, Fujihara Y, Eda T, et al. Low glycated hemoglobin level is associated with severity of frailty in Japanese elderly diabetes patients. J Diabetes Investig 2018; 9: 419-25.
46. DAROC Clinical Practice Guidelines for Diabetes Care - 2018, Taiwan, Diabetes Association of the R.O.C., 2018.
47. American Diabetes Association. 11. Older adults: Standards of Medical Care in Diabetesd2018. Diabetes Care 2018; 41:
S119-S125.
48. Kirkman MS, Briscoe VJ, Clark N, et al. Diabetes in Older Adults: Consensus Report. J Am Geriatr Soc 2012; 60: 2342- 56.
49. Pilotto A, Noale M, Maggi S, et al. Hypoglycemia Is Inde- pendently Associated with Multidimensional Impairment in Elderly Diabetic Patients. BioMed Research International 2014; Article ID 906103.
50. Milanesi A, Weinreb JE. Diabetes in the Elderly. [Updated 2017 Mar 24]. In: De Groot LJ, Chrousos G, Dungan K, et al. editors. Endotext [Internet]. South Dartmouth (MA):
MDText.com, Inc.; 2000.
51. Kumar R, Wassif WS. Management of Type 2 Diabetes in Frail and Elderly Patients. Curre Res Diabetes & Obes J 2018;
7: 555705.
52. Meneilly GS, Knip A, Miller DB, et al. 2018 Clinical Practice Guidelines. Diabetes in Older People - Diabetes Canada Clin-
ical Practice Guidelines Expert Committee. Can J Diabetes 2018; 42: S283-S295.
53. Strain WD, Hope SV, Green A, et al. Type 2 diabetes mel- litus in older people: a brief statement of key principles of modern day management including the assessment of frailty.
A national collaborative stakeholder initiative. Diabet Med 2018; 35: 838-45.
54. Araki A, Ito H. Diabetes mellitus and geriatric syndromes.
Geriatr Gerontol Int 2009; 9: 105-14.
55. Rodríguez-Pascual C, Rodriguez-Justo S, García-Villar E, et al. Quality of life, characteristics and metabolic control in dia- betic geriatric patients. Maturitas 2011; 69: 343-7.
56. Zekry D, Frangos E, Graf C, et al. Diabetes, Comorbidities, and Increased Long-term Mortality in Older Patients Admit- ted for Geriatric Inpatient Care. Diabetes & Metabolism 2012; 38: 149-55.
57. Corriere M, Rooparinesingh N, Kalyani RR. Epidemiology of Diabetes and Diabetes Complications in the Elderly: An Emerging Public Health Burden. Curr Diab Rep 2013 Dec;
13: 10.1007/s11892-013-0425-5.
58. Moghissi E. Management of Type 2 Diabetes Mellitus in Older Patients: Current and Emerging Treatment Options.
Diabetes Ther 2013; 4: 239-56.
59. Kezerle L, Shalev L, Barski L. Treating the elderly diabetic patient: special considerations. Diabetes Metabolic Syndrome and Obesity: Targets and Therapy 2014; 7: 391-400.
60. Dardano A, Penno G, Del Prato S, et al. Optimal therapy of type 2 diabetes: a controversial challenge. Aging 2014; 6:
187-206.
61. Bigelow A, Freeland B. Type 2 Diabetes Care in the Elderly.
The Journal for Nurse Practitioners 2017; 13: 181-6.
62. Sigal RJ, Kenny GP, Boulé NG, et al. Effects of aerobic train- ing, resistance training, or both on glycemic control in type 2 diabetes: a randomized trial. Ann Intern Med 2007; 147:
357-69.
63. Bloem CJ, Chang AM. Short-Term Exercise Improves β-Cell Function and Insulin Resistance in Older People with Impaired Glucose Tolerance. J Clin Endocrinol Metab 2008;
93: 387-92.
64. Slentz CA, Tanner CJ, Bateman LA, et al. Effects of Exercise Training Intensity on Pancreatic β-Cell Function. Diabetes Care 2009; 32: 1807-11.
65. Solomon TPJ, Haus JM, Kelly KR, et al. Improved Pancreatic β-Cell Function in Type 2 Diabetic Patients After Lifestyle- Induced Weight Loss Is Related to Glucose-Dependent Insu- linotropic Polypeptide. Diabetes Care 2010; 33: 1561-6.
66. Church TS, Blair SN, Cocreham S, et al. Effects of aerobic and resistance training on hemoglobin A1c levels in patients with type 2 diabetes: a randomized controlled trial. JAMA 2010; 304: 2253-62.
67. Hovanec N, Sawant A, Overend TJ, et al. Resistance train- ing and older adults with type 2 diabetes mellitus: strength of the evidence. Journal of Aging Research 2012; Article ID 284635.
68. Mavros Y, Kay S, Anderberg KA, et al. Changes in Insulin Resistance and HbA1c Are Related to Exercise-Mediated Changes in Body Composition in Older Adults with Type 2
Diabetes - Interim outcomes from the GREAT2DO trial. Dia- betes Care 2013; 36: 2372-9.
69. Schellenberg ES, Dryden DM, Vandermeer B, et al. Lifestyle interventions for patients with and at risk for type 2 diabetes:
a systematic review and meta-analysis. Ann Intern Med 2013;
159: 543-51.
70. Simpson KA, Mavros Y, Kay S, et al. Graded Resistance Exer- cise and Type 2 Diabetes in Older adults (The GREAT2DO study): methods and baseline cohort characteristics of a rand- omized controlled trial. Trials 2015; 16: 512-25.
71. Waters DL, Ward AL, Villareal DT. Weight loss in obese adults 65 years and older: a review of the controversy. Exp Gerontol 2013; 48: 1054-61.
72. Nicklas BJ, Chmelo E, Delbono O, et al. Effects of resistance training with and without caloric restriction on physical func- tion and mobility in overweight and obese older adults: a ran- domized controlled trial. Am J Clin Nutr 2015; 101: 991-9.
73. Sardeli AV, Komatsu TR, Mori MA, et al. Resistance Train- ing Prevents Muscle Loss Induced by Caloric Restriction in Obese Elderly Individuals: A Systematic Review and Meta- Analysis. Nutrients 2018; 10: 423.
74. Houston DK, Leng X, Bray GA, et al. Action for Health in Diabetes (Look AHEAD) Movement and Memory Ancillary Study Research Group. A long-term intensive lifestyle inter- vention and physical function: the Look AHEAD Movement and Memory Study. Obesity (Silver Spring) 2015; 23: 77-84.
75. 4. Lifestyle Management: Standards of Medical Care in Dia- betesd-2018. Diabetes Care 2018; 41: S38-S50.
76. Vinik AI, Strotmeyer ES, Nakave AA, et al. Diabetic Neurop- athy in Older Adults. Clin Geriatr Med 2008; 24: 407-39.
77. Stein SA, Lamos EM, Davis SN. A review of the efficacy and safety of oral antidiabetic drugs. Expert Opin Drug Saf 2013;
12: 153-75.
78. Pasquel FJ, Powell W, Peng L, et al. A randomized controlled trial comparing treatment with oral agents and basal insulin in elderly patients with type 2 diabetes in long-term care facilities. BMJ Open Diabetes Research and Care 2015; 3:
e000104.
79. Clemens KK, Shariff S, Liu K, et al. Trends in Antihypergly- cemic Medication Prescriptions and Hypoglycemia in Older Adults: 2002-2013. PLoS ONE 2015; 10: e0137596.
80. Chu WM, Ho HN, Huang KH, et al. The prescribing trend of oral antidiabetic agents for type 2 diabetes in Taiwan - An 8-year population-based study. Medicine 2017; 96:
43(e8257).
81. UK Prospective Diabetes Study (UKPDS) Group. Effect of intensive blood-glucose control with metformin on compli- cations in overweight patients with type 2 diabetes (UKPDS 34). Lancet 1998; 352: 854-65.
82. Johnson JA, Majumdar SR, Simpson SH, et al. Decreased Mortality Associated With the Use of Metformin Compared With Sulfonylurea Monotherapy in Type 2 Diabetes. Diabetes Care 2002; 25: 2244-8.
83. Fung CSC, Wan EYF, Wong CKH, et al. Effect of metformin monotherapy on cardiovascular diseases and mortality: a ret- rospective cohort study on Chinese type 2 diabetes mellitus patients. Cardiovasc Diabetol 2015; 14: 137-50.