210 Mendeleev Commun. 2004
One-pot construction of dihydropyrimidinones in ionic liquids
Sushilkumar S. Bahekar,a Sandip A. Kotharkarb and Devanand B. Shinde*b
a Department of Chemistry, National Cheng Kung University, Tainan-701, Taiwan, R.O.C.
b Department of Chemical Technology, Dr. Babasaheb Ambedkar Marathwada University, Aurangabad (MS) – 431004, India.
Fax: +091 240 401833; e-mail: ssbahekar@rediffmail.com
DOI: 10.1070/MC2004v014n05ABEH001895
The use of the ionic liquid [bmim]Cl·2AlCl3 for the preparation of dihydropyrimidinones is described.
In 1893, the Italian chemist Pietro Biginelli reported that the acid-catalysed one-pot cyclocondensation of ethyl acetoacetate, benzaldehyde and urea gave multifunctionalised dihydro- pyrimidones (DHPMs).1 After nearly 100 years, a resurgence of interest occurred as evidenced by an increase in the number of publications and patents on both the synthesis2–4 and biological activity5–10 of these compounds. Antiviral, antitumor, antibacterial, potent calcium channel blocking and anti- inflammatory activities were ascribed to DHPMs. However, the Biginelli synthesis of DHPMs suffers from relatively low yields of products, in particular, when substituted aromatic aldehydes or thioureas are employed.3,11–14 This has led to the recent dis- closure of several improved synthetic protocols for DHPMs, which involve either modification of the Biginelli synthesis11
or the development of novel but more complex multistep strategies.11–14 In addition, several combinatorial solid-phase approaches have been reported that use microwave condi- tions.15,16
The Biginelli synthesis is an example of the use of less toxic chemicals. Thus, the use of catalysts that contain both Lewis acid and transition metal salts, e.g., BF3–OEt2,17 montmoril- lonite(KSF),18 polyphosphate ester19 and reagents such as InCl3,20 LiBr,21 CeCl3·H2O22 and Mn(OAc)3·2H2O23 gave better yields of DHPMs.
Ionic liquids are environmentally benign alternative solvents for various chemical processes. They have attracted the atten- tion of chemists owing to their unique physical and chemical properties.24,25 Because of their low vapour pressure, ionic
Mendeleev Commun. 2004 211 species do not contribute to volatile organic compound emis-
sion. They have also been referred to as ‘designer solvents’26 since their properties can be altered by the fine tuning of parameters such as the choice of the organic cation, inorganic anion and alkyl chain attached to the organic cation. These structural variations provide an opportunity to devise the most idealised solvent needed for a particular chemical process. Several reactions have been carried out in ionic liquids27 including the Biginelli, Diels–Alder, Wittig and Pechman reactions, the benzoin condensation, catalytic hydrogenation and several enzyme cata- lysed reactions.28
Chloroaluminate ionic liquids have been used in Friedel–
Crafts and other reactions where they play the dual role of both the Lewis acid catalyst and the solvent.27 Thus, we decided to investigate the Biginelli synthesis under these conditions. Here we report a new synthesis of DHPMs in the presence of the Lewis acid [bmim]Cl·2AlCl3 ionic liquid.
The composition of ionic liquids is expressed as the apparent mole fraction of AlCl3, N. Accordingly, they are classified as basic, neutral and acidic liquids when N is 0–0.5, 0.5 and 0.5–0.67, respectively. The reaction of (thio)ureas, aldehydes and α-ketoesters was carried out in liquids with N = 0.33, 0.5 and 0.67, respectively. Positive results were obtained only in case of acidic ionic liquids as expected.
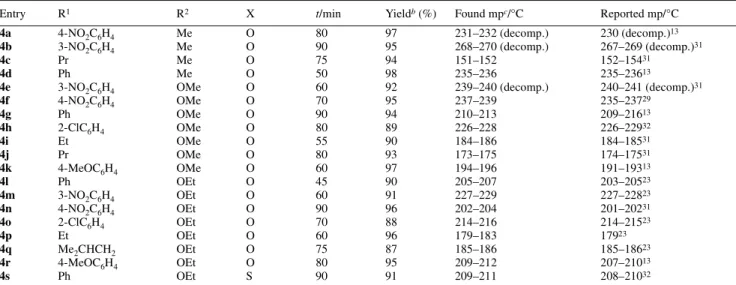
In order to study the effect of substituents on the reactivity of the reactants, a variety of aliphatic and aromaic aldehydes were used. The results are given in Table 1.† In comparison with reported procedures,29 the reaction time for the complete
† The purity of compounds was checked by TLC. The IR spectra were recorded on a JASCO spectrophotometer (Japan) using KBr pellets. The
1H and 13C NMR spectra in CDCl3 were measu red on a FT-NMR spectrophotometer model Ac-300 F (Bruker, Germany) at 300 MHz using TMS as an internal standard. Satisfactory microanalysis data (±0.4% of calculated values) were obtained for all the compounds.
Typical experimental procedure. To a stirred mixture of urea or thio- urea (2.6 mmol), an appropriate α-ketoester (2 mmol) and an aldehyde (2 mmol), the ionic liquid [bmim]Cl·2AlCl3 (11 mmol) was added, and the reaction mixture was stirred for an appropriate time at room tem- perature. The reaction mixture was quenched with cold 6M HCl (15 ml).
The precipitate was filtered off, and the solid was purified by column chromatography (ethyl acetate–hexane) and characterised by IR, 1H and
13C NMR spectroscopy and mass spectrometry.
5-Aceto-4-propyl-6-methyl-3,4-dihydropyrimidin-2(1H)-one 4c: mp 151–
152 °C. 1H NMR ([2H6]DMSO, 300 MHz) d: 8.94 (s, 1H, NH), 7.42 (s, 1H, NH), 4.09 (t, 1H, H-4, J 3.2 Hz), 2.18 (s, 3H, COMe), 2.16 [s, 3H, C(6)–Me], 1.20 (m, 4 H, CH2CH2Me), 0.82 (t, 3H, CH2CH2Me, J 7.0 Hz). 13C NMR, d: 194.5, 153.3, 147.8, 111.1, 50.5, 30.6, 19.3, 17.6, 14.2. IR (KBr, n/cm–1): 3247, 3113, 2956, 1723, 1625. MS (70 eV, EI), m/z: 196 (M+, 1.27%).
Table 1 Dihydropyrimidinones 4a–sa produced according to Scheme 1.
aAll compounds are characterised by IR, 1H and 13C NMR spectroscopy and mass spectrometry. bThe optimised yield is based on the crystalline product obtained. cMelting points are uncorrected.
Entry R1 R2 X t/min Yieldb (%) Found mpc/°C Reported mp/°C
4a 4-NO2C6H4 Me O 80 97 231–232 (decomp.) 230 (decomp.)13
4b 3-NO2C6H4 Me O 90 95 268–270 (decomp.) 267–269 (decomp.)31
4c Pr Me O 75 94 151–152 152–15431
4d Ph Me O 50 98 235–236 235–23613
4e 3-NO2C6H4 OMe O 60 92 239–240 (decomp.) 240–241 (decomp.)31
4f 4-NO2C6H4 OMe O 70 95 237–239 235–23729
4g Ph OMe O 90 94 210–213 209–21613
4h 2-ClC6H4 OMe O 80 89 226–228 226–22932
4i Et OMe O 55 90 184–186 184–18531
4j Pr OMe O 80 93 173–175 174–17531
4k 4-MeOC6H4 OMe O 60 97 194–196 191–19313
4l Ph OEt O 45 90 205–207 203–20523
4m 3-NO2C6H4 OEt O 60 91 227–229 227–22823
4n 4-NO2C6H4 OEt O 90 96 202–204 201–20231
4o 2-ClC6H4 OEt O 70 88 214–216 214–21523
4p Et OEt O 60 96 179–183 17923
4q Me2CHCH2 OEt O 75 87 185–186 185–18623
4r 4-MeOC6H4 OEt O 80 95 209–212 207–21013
4s Ph OEt S 90 91 209–211 208–21032
5-Methoxycarbonyl-4-(3-nitrophenyl)-6-methyl-3,4-dihydropyrimidin- 2(1H)-one 4e: mp 239–240 °C (decomp.). 1H NMR ([2H6]DMSO, 300 MHz) d: 9.31 (s, 1H, NH), 8.09–8.13 (m, 2H, Ar–H), 7.85 (s, 1H, NH), 7.63–7.70 (m, 2H, Ar–H), 5.31 (d, 1H, H-4, J 3.0 Hz), 3.35 (s, 3 H, COOMe, J 3.0 Hz), 2.28 [s, 3H, C(6)–Me]. 13C NMR, d: 165.5, 151.7, 149.6, 147.8, 146.6, 132.8, 130.1, 122.3, 120.8, 98.0, 53.3, 50.8, 17.8.
IR (KBr, n/cm–1): 3358, 3244, 3102, 2957, 1701, 1641. MS (70 eV, EI), m/z: 291 (M+, 5.86%).
5-Methoxycarbonyl-4-(2-chlorophenyl)-6-methyl-3,4-dihydropyrimidin- 2(1H)-one 4h: mp 226–228 °C. 1H NMR ([2H6]DMSO, 300 MHz) d:
9.21 (s, 1H, NH), 7.59 (s, 1H, NH), 7.25–7.40 (m, 4H, Ar–H), 5.62 (d, 1H, H-4, J 2.5 Hz), 3.45 (s, 3H, COOMe), 2.30 [s, 3H, C(6)–Me].
13C NMR, d: 165.4, 151.3, 149.3, 141.4, 131.6, 129.4, 129.0, 128.6, 127.6, 97.6, 51.3, 50.6, 17.6. IR (KBr, n/cm–1): 3367, 3221, 3103, 2948, 1714, 1698. MS (70 eV, EI), m/z: 280 (M+, 5.13%).
5-Methoxycarbonyl-4-ethyl-6-methyl-3,4-dihydropyrimidin-2(1H)-one 4i: mp 184–186 °C. 1H NMR ([2H6]DMSO, 300 MHz) d: 8.96 (s, 1H, NH), 7.30 (s, 1H, NH), 4.01 (m, 1H, H-4), 3.59 (s, 3H, COOMe), 2.16 [s, 3H, C(6)–Me], 1.39 (q, 2H, CH2Me, J 7.5 Hz), 0.77 (t, 3H, CH2Me, J 7.5 Hz). 13C NMR, d: 165.9, 152.7, 148.6, 98.5, 51.3, 50.7, 29.5, 17.7, 8.4. IR (KBr, n/cm–1): 3249, 3118, 2961, 1728, 1708, 1680. MS (70 eV, EI), m/z: 198 (M+, 0.59%).
5-Methoxycarbonyl-4-propyl-6-methyl-3,4-dihydropyrimidin-2(1H)-one 4j: mp 173–175 °C. 1H NMR ([2H6]DMSO, 300 MHz) d: 8.94 (s, 1H, NH), 7.31 (s, 1H, NH), 4.03 (t, 1H, H-4, J 3.2 Hz), 3.59 (s, 3 H, COOMe), 2.15 [s, 3H, C(6)–Me], 1.19–1.40 (m, 4H, CH2CH2Me), 0.82 (t, 3H, CH2CH2Me, J 6.7 Hz). 13C NMR, d: 165.8, 152.6, 148.3, 99.1, 50.6, 49.8, 17.6, 16.9, 13.6. IR (KBr, n/cm–1): 3442, 3252, 3123, 2957, 1726, 1708, 1653. MS (70 eV, EI), m/z: 212 (M+, 0.43%).
5-Ethoxycarbonyl-4-ethyl-6-methyl-3,4-dihydropyrimidin-2(1H)-one 4p:
mp 185–186 °C. 1H NMR ([2H6]DMSO, 300 MHz) d: 8.82 (s, 1H, NH), 7.18 (s, 1H, NH), 4.03–4.09 (m, 3H, H-4 and OCH2Me), 2.16 [s, 3 H, C(6)–Me], 1.41 (m, 2H, CH2Me), 1.17 (t, 3H, OCH2Me, J 6.0 Hz), 0.78 (t, 3H, CH2Me, J 7.5 Hz). 13C NMR, d: 165.3, 152.6, 148.1, 98.7, 58.8, 51.2, 29.4, 17.5, 14.0 and 8.31. IR (KBr, n/cm–1): 3250, 3123, 2962, 1723, 1703, 1675. MS (70 eV, EI), m/z: 212 (M+, 0.43%).
5-Ethoxycarbonyl-4-isobutyl-6-methyl-3,4-dihydropyrimidin-2(1H)-one 4q: mp 185–186 °C. 1H NMR ([2H6]DMSO, 300 MHz) d: 8.86 (s, 1H, NH), 7.32 (s, 1H, NH), 4.01–4.10 (m, 3H, H-4 and OCH2Me), 2.16 [s, 3H, C(6)–Me], 1.69 (m, 1H, CH2CHMe2), 1.35 (m, 1H, CH2CHMe2), 1.17 (t, 3H, OCH2Me, J 7.0 Hz), 1.10 (m, 1H, CH2CHMe2), 0.85 (d, 6 H, CH2CHMe2, J 6.5 Hz). 13C NMR, d: 165.1, 152.6, 147.9, 100.2, 58.8, 48.1, 45.8, 23.5, 22.7, 21.3, 17.4, 14.0. IR (KBr, n/cm–1): 3447, 3244, 3112, 2951, 1701, 1652. MS (70 eV, EI), m/z: 241 (M++ 1, 1.08%).
R2
O O
R1 H
O
H2N NH2 X
N NH R2
O R1
H X
1 2 3
Scheme 1 4
212 Mendeleev Commun. 2004
conversion of starting materials 1, 2 and 3 into products 4 is considerably reduced and the yields of the products are higher.
The method works equally well for urea, thiourea and aliphatic or aromatic aldehydes. Compounds containing electron-with- drawing or electron-releasing groups give the best yield and the highest purity. Furthermore, the ionic system used acted as both a Lewis acid catalyst and a solvent, which is important in reac- tions where stoichiometric amounts are used.
References
1 P. Biginelli, Gazz. Chim. Ital., 1893, 23, 360.
2 B. Schnell, W. Krenn, K. Faber and C. O. Kappe, J. Chem. Soc., Perkin Trans. 1, 2000, 4382.
3 C. O. Kappe, Tetrahedron, 1993, 49, 6937.
4 S. S. Bahekar and D. B. Shinde, Acta Pharm., 2003, 53, 223.
5 C. O. Kappe, Eur. J. Med. Chem., 2000, 35, 1043.
6 M. Modica, M. Santagati, A. Santagati, V. Cutuli, N. Mangano and A. Caruso, Pharmazie, 2000, 55, 500.
7 R. Ku mar, M. Nath and D. L. Tyrrell, J. Med. Chem., 2002, 45, 2032.
8 E. Gossnitzer, G. Feierl and U. Wagner, Eur. J. Pharm. Sci., 2002, 15, 49.
9 K. S. Atwal, G. C. Rovnyak, S. D. Kimball, D. M. Floyd, S. Moreland, B. N. Swanson, J. Z. Gougoutas, J. Schwartz, K. M. Smillie and M. F.
Malley, J. Med. Chem., 1990, 33, 2629.
10 L. Heys, C. G. Moore and P. J. Murphy, Chem. Soc. Rev., 2000, 29, 57.
11 A. D. Patil, N. V. Kumar, W. C. Kokke, M. F. Bean, A. J. Freyer, C. De Brosse, S. Mai, A. Truneh, D. J. Faulkner, B. Carte, A. L. Breen, R. P. Hertzberg, R. K. Johnson, J. W. Westley and B. C. Potts, J. Org.
Chem., 1995, 60, 1182.
12 J. Lu and H. Ma, Synlett., 2000, 63.
13 Y. Ma, C. Qian, L. Wang and M. Yang, J. Org. Chem., 2000, 65, 3864.
14 K. S. Atwal, B. C. O’Reilley, J. Z. Gougoutas and M. F. Malley, Heterocycles, 1987, 26, 1189.
15 P. Lidstrom, J. Tierney, B. Wathey and J. Westman, Tetrahedron, 2001, 57, 9225.
16 M. Kidwai and P. Misra, Synth. Commun., 1999, 29, 3237.
17 E. H. Hu, D. R. Sidler and U. H. Doling, J. Org. Chem., 1998, 63, 3454.
18 F. Bigi, B. Carloni, R. Maggi and G. Sartori, Tetrahedron Lett., 1999, 40, 3465.
19 C. O. Kappe and S. F. Falsone, Synlett., 1998, 718.
20 A. Harja and O. Jano, J. Org. Chem., 2000, 65, 6270.
21 P. B. Prtha, G. Sunil, P. Dipak and S. S. Jagir, Chem. Lett., 2002, 10, 1038.
22 S. D. Bose, F. Liyakat and M. Hari Babu, J. Org. Chem., 2003, 68, 587.
23 K. A. Kumar, M. Kasthuraial, C. S. Reddy and C. D. Reddy, Tetrahedron Lett., 2001, 42, 7873.
24 T. Walton, Chem. Ber., 1999, 99, 2071.
25 P. Wassercheid and W. Keim, Angew. Chem., Int. Ed. Engl., 2000, 39, 3772.
26 M. Free Mantel, Chem. Eng. News, 1998, 76, 32.
27 D. Zhao, M. Wu, Y. Kou and E. Min, Catalysis Today, 2002, 74, 157.
28 J. Peng and Y. Deng, Tetrahedron Lett., 2001, 42, 5917.
29 J. S. Yadav, B. V. S. Reddy, K. B. Reddy, K. S. Raj and A. R. Prasad, J. Chem. Soc., Perkin. Trans. 1, 2001, 1939.
30 J. Lu and Y. Bai, Synthesis, 2002, 466.
31 X. Xu and Y.-G. Wnag, J. Chem. Res. (S), 2003, 377.
32 N. Y. Fu, Y. F. Yuan, Z. Cao, S. N. Wang, J. J. Wang and C. Peppe, Tetrahedron Lett., 2002, 58, 4.
Received: 26th January 2004; Com. 04/2221