Retardation behavior of Sr and Cs in Crushed and Intact Rocks:Two
potential LLW repository Taiwan host rocks
Ming-Chee Wu1,*,Chuan-Pin Lee1, Ching-Yuan Liu2, Tsuey-Lin Tsai3 1*
Department of Earth Sciences, National Cheng Kung University, Tainan,70101, Taiwan, Email :mcwu@mail.ncku.edu.tw
2
Department of Chemical and Materials Engineering, National Central University, Taoyuan, 32001, Taiwan
3
Chemical Division, Institute of Nuclear Energy Research, Taoyuan Longtan 32546, Taiwan
Corresponding author:
Prof. Ming-Chee Wu
Address correspondence to this author at the Department of Earth Science, National Cheng Kung University, Tainan,70101, Taiwan
E-mail:mcwu@mail.ncku.edu.tw
TEL : +886-6-275-4846 FAX:+886-6-275-4846
Abstract
This study investigates sorption and diffusion of Strontium (Sr) and Cesium (Cs) in two potential host rocks (granite from Kinmen Island and basalt from Penghu Island) by using batch and through-diffusion methods in order to establish a reliable safety assessment methodology. These methods were applied to crushed and intact rock samples to investigate the actual geological environment. According to solid-phase analysis, including X-ray diffraction, elemental analysis, auto radiography, and polar microscopy, the sorption component primarily contained iron–magnesium (Fe–Mg) minerals in basalt and granite. Moreover, the distribution coefficient (Kd) of Sr and Cs in various concentrations (~10−2–10−7M) obtained from batch tests indicated a higher sorption capacity in basalt than that in granite because of the 10% Fe–Mg mineral content. The diffusion of Sr and Cs both in crushed granite and basalt reach steady state after 110 days and apparent diffusion coefficient (Da) were 3.29×10-11m2/s (for Sr in crushed granite), 4.17×10-12 m2/s (for Sr in crushed basalt), 2.86×10-11 m2/s (for Cs in crushed granite), 1.82×10-12 m2/s (for Cs in crushed basalt), respectively. However, diffusive result (Da) of Sr and Cs in intact rocks was estimated a lower value than those obtained using crushed rocks. According to the diffusive results in crushed and intact rocks, it showed that major retardation of Sr and Cs depended on the microporous structure of tested media, such as decreases of constrictivity (δ) and increases of tortuosity(τ). In fact, the solid/liquid (S/L) ratio decreased as is the case when switching from batch to column experiments and the sorption effect on minerals became even more negligible in retardation of radionuclide migration.
Keyword: Sorption, diffusion, granite, basalt, distribution coefficients (Kd),
Introduction
Because of the Fukushima Daiichi nuclear crisis in 2011, the Taiwan Government (Atomic Energy Council) and plant operator (Taipower Company, TPC) announced a permit in November, 2011 for the decommissioning of three nuclear plants (six nuclear units) in Taiwan. The oldest operating nuclear units in Taiwan are the two 604-MW General Electric boiling water reactors at the ChinShan plant, which started commercial operation in 1978 and 1979. Moreover, there are three operating nuclear power plants generating 5144 MW in Taiwan, and two Advanced Boiling Water Reactors (ABWR 1350 MW for one unit) under construction at the LungMen plant that would only be allowed to start up after passing strict safety evaluations both by the government and international nuclear safety organizations.
Nuclear power has the advantage of generating huge volumes of electricity while maintaining its waste in a solid form within the plant. The most contentious waste in terms of safety management is the spent fuel that contains approximately 99% of the radioactivity. Achieving a permanent disposal route for spent fuel is a political challenge that is yet to be fully met in any nuclear-powered country.
Since deep geological disposal is currently the preferred way of disposing nuclear waste, the state-run TPC, owner of three nuclear power plants, initiated a long-term spent-fuel disposal program in the 1980s to prepare a geological repository for operation in 2050. To assess the long-term performance of this repository, it is necessary to deal with the problem of high-level radioactive waste (HLW)
considering its long half-life and the associated risk of transporting radionuclides into the environment. Some geological investigations and research and development (R&D) efforts into radionuclide migration have been completed, and the
Cesium (Cs) is a high-yield fission product containing up to 6.5% and 6.2%135Cs and 137
Cs, respectively[2], and selenium (Se) is also a fission product and have a long half-life 2.95×105years. Moreover, Se is a natural trace element that is of
fundamental importance to human health and Cs is released directly in catastrophic nuclear events and it was reported in Fukushima that Cs resulted in a possible contamination of the ocean environment[3]. In addition to its high generation yield, Cs was considered for this study because of the long half-life of137Cs and135Cs (30 and 2.35×106years, respectively), high solubility in water, and chemical properties similar to potassium (K).
The “multi-barrier”concept has been adopted to deal with issues related to nuclear waste repository, such as the use of clays as a buffer/backfill material because of their high sorption capacities. Furthermore, stable and firmly argillaceous formations are suitable as a host rock in the disposal repository. Therefore, granite was initially selected and studied because it is a major host rock on some isolated islands. The sorption and diffusion behavior of Cs on granite, diorite, and mudrock has been studied and reported in literature[4–10].
Batch techniques are frequently used to characterize the sorption of radionuclides in laboratories, and the distribution coefficient (Kd) method is widely used for estimating the retardation of radionuclides. However, there are few systematic sorption studies dealing with sorption in host rocks as compared with those related to a
bentonite-engineered barrier. The Kdvalue obtained by the batch method may be too simple to represent the actual system, and it is found that diffusion methods gives more accurate information relating to radionuclides in deep geology [6, 7]. However, some disagreement between Kdand the retardation factor (Rf) obtained from the two above-mentioned methods due to differences in the solid/liquid (S/L) ratio has been
reported in literature[11–13]. Moreover, the limitation of one-dimensional diffusion tests is that they may only be demonstrated in the diffusion of Cs and Se compacted in crushed granite with a relatively lower or higher length/diameter ratio (L/D < 0.44 or L/D > 1.78)[9-10]. In a repository environment, sorption often occurs on intact rock or fracture because of the hydraulic-geological conditions. It is found that the sorption of Cs on crushed and intact sedimentary rocks was comparable within an order of magnitude[14].
In this study, batch sorption and column through-diffusion experiments in crushed and intact granite and basalt were performed simultaneously. Moreover, the Kdvalues were determined using the two methods and their differences are discussed. The purpose of this study is to determine whether it is possible to compare Kdvalues determined by the batch and static diffusion methods. In fact, such a procedure would be very important in a safety assessment to effectively apply the batch Kdvalues to describe sorption in an actual environment in a potential repository.
Experimental
Rock samples and solid phase analysis
Rock samples were drilled and collected from Kingman and Penghu Islands, located at latitudes and longitudes of 24°N 118°E and 23°N 119°E, respectively. In terms of geology, seismology, meteorology, hydrology, site geometry, and risk in relation to the local population center, both islands are considered to be a potential repository because of the location of the Taiwan Strait approximately 210 km and 50 km away from Taipei, respectively. The Kingman and Penghu Islands, comprising several discrete islands, are approximately 150 km2and 125 km2in area and are composed of 90% granite and 80% basalt, respectively.
In this study, before use, all rock samples were crushed, sieved, and separated into three size groups: ~20–50 mesh (~0.297–0.833 mm), ~50–100 mesh (~0.149–0.297 mm), and ~200–270 mesh (~0.053–0.074 mm). They were then washed three times using de-ionized water (DIW) and dried at 105 ºC for 24 h.
A solid-phase analysis of granite and basalt was performed by powder X-ray diffraction (XRD, D8 Advance, Bruker, Germany) and elemental analysis after sample acid-digestion by inductively coupled plasma–optical emission spectrometry (ICP–OES, JY Ultima-2, France). The major sorption of Cs on both rocks was identified and compared in polar microscopy and autoradiography experiments. The chemical compositions of granite and basalt were listed in Table 1. XRD spectra were compared with those in the International Center for Diffraction Data (JPCDS) database. This showed that major mineral composition of granite (abbreviated as G below) and basalt (abbreviated as B below) included quartz (G & B), biotite (G & B), plagioclase (G & B), feldspar (G & B), goethite (G), and magnetite (B) among other components. The predominant elements of granite and basalt presented as oxides, as
determined by ICP–OES, were SiO2, Al2O3, K2O, Na2O, and Fe2O3.
Polar-microscopy and auto-radiography experiments
After pre-treatment by DIW, the crushed samples were sprayed and glued onto a glass plate and then samples were submerged into the stock solution, containing137Cs with a specific activity of about 200 Bq/mL. After 7 days of immersion, the samples were taken out and rinsed quickly with DIW to remove excess tracer solution on the sample surface. After drying, the samples were covered with thin transparent films, and then contacted with x-ray films. After exposure for two days, the x-ray films were
developed (AGFA, G150, 3-5 min) and fixed (AGFA, G334A.B, 7-10 min). These auto-radiography photos were then compared with the corresponding images from polar-microscopy.
Batch tests
Three centrifuge tubes with different particle sizes (~0.297–0.833, ~0.104–0.173, and ~0.053–0.074 mm)were prepared in various Cs concentrations (~10−2–10−7M) in order to realize the geometric effect in sorption on rocks. Batch tests reported by ASTM were applied[15], and the solid-to-liquid ratio was 1 g:30 mL. A trace amount of the radiotracer137Cs was also added to the liquid with carriers prior to batch tests. After 14 days of shaking, the tubes were centrifuged at 10,380 g (Kokusan H-200, Japan), and the supernatants were radioassayed using an auto-NaI(Tl) gamma detector
(Packard 5002, US) to determine137Cs activity. The pH and Eh values were also measured. The Kdvalue was calculated by the following formula
Kd= C C C0 ‧ V M , (1)
where C is the equilibrium concentration of Cs (cpm/mL), C0is the initial
concentration of Cs in solution (cpm/mL), and V and M are liquid volume (mL) and solid mass (g), respectively.
Through-diffusion tests
In this study, through-diffusion tests were adopted to determine the diffusion coefficient (D) in order to evaluate the transport of radionuclides in compacted media. If D isconstant,Fick’sLaw may be written as
) ( 2 2 2 2 2 2 z C y C x C D t C .
Furthermore, if the compacted layer of crushed granite is assumed to be homogeneous and isotropic, and the diffusion of the radionuclide occurs only in one dimension, the diffusion equation can be written as
2 2 x C D t C a (Da= De/α,α= θ+ ρbKd), (2)
whereα istherock capacity factor. This equation could give a Kdvalue by bulk density (ρb) and porosity (θ). C is the concentration of the liquid. The appropriate boundary conditions and initial conditions are given by
C (0,t) = C0,
C (L,t) = 0,
where C0is the initial Cs concentration at the interface between a tracer-containing reservoir and a slab of rock, and L is the overall length of the rock slab. The
concentration (C) of radionuclide can be expressed as a function of t and x 6 [ 2 0 L t D L A C Q a t )] exp( 2 ) 1 ( 2 2 2 2 2 2 1 L t n D n L a n . (3)
As t→∞,the diffusion process reach equilibrium (steady-state), and the corresponding concentration ratio CR(t) may be rewritten as
] 6 [ ) ( 2 0 L t D VL A C C t CR
t a , (4)where V and A are the volume of the diffusion end and the surface area of the compacted granite powers, respectively. CR(t) is a straight line with a slope m and an intersection tx, in Equation (4). Therefore, a graphical method [16] developed in 1975 by Crank is often employed to determine the apparent diffusion coefficient (Da),
effective diffusion coefficient (De), retardation factor (Rf), and distribution coefficients (Kd) from the values of m, V, L, A, tx, ρb, and θ[7,16,17]
x a t L D 6 2 (5) A L V m De (6) Rf = De/θDa= 1+(ρbKd/θ). (7)
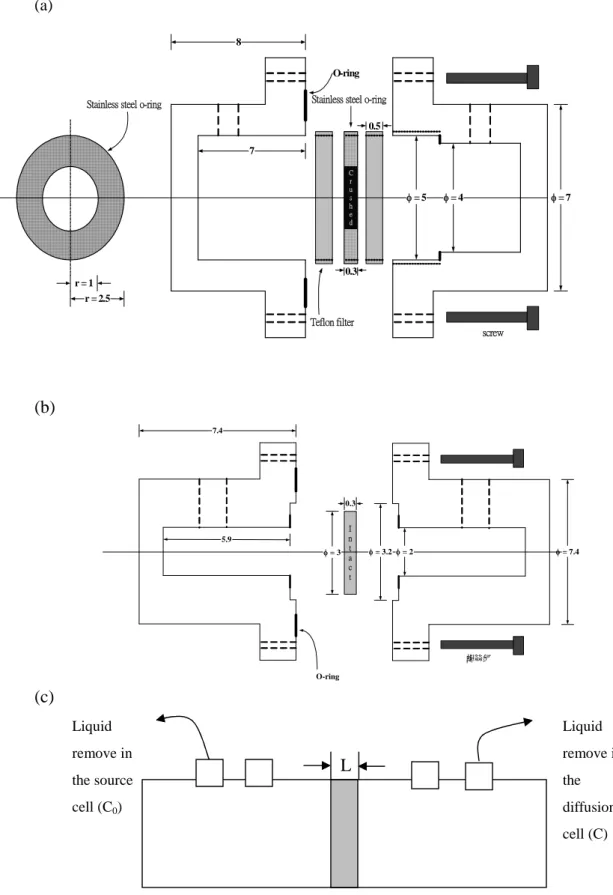
columns (four columns in total) with a bulk density (ρb= 1.8 cm3/g) and a total porosity θof 0.31 and 0.37 in crushed granite and basalt, respectively. In the through-diffusion experiments shown in Fig 1, total volume (V) of samples in both ends (source and diffusion ends) were removed and collected at a constant frequency in order to detect137Cs radioactivity. After removing samples from two diffusion chambers, the same volumes were refilled into two chambers to maintain the initial conditions at boundary (C(0,t) = C0).
Results and Discussion
Sorption on Cs by PA experiments
Fig 2 shows photos of the polar microscopy and autoradiography of thin rocks slices
(< 10 µm) after 7 days. In fact, the thin rock samples were submerged into the stock solution containing137Cs with a specific activity of 200 Bq/mL. Therefore, the
corresponding images of polar microscopy and polar microscopy in Figs 2 and 3 were obtained and compared, respectively. Comparison of the exposure zones shown in
Figs 2 and 3 of the mineral components in basalt and granite responsible for the
sorption of Cs indicates that biotite is a major mineral content.
Distribution coefficients (Kd) from batch tests
According to the previous studies[8-9],Cs sorption on granite belongs to fast-uptake reaction and reaches equilibrium within 4 hours. After 7 days, the pH of Cs on basalt and granite was recorded as approximately 8.63 ± 0.6 and 7.94 ± 1.0, respectively, and showed a Eh within the 150–220 mV range in various Cs concentrations (~10−2–10−7M). Fig 4 displays that Kdin basalt is higher than that in granite at the three sizes (~0.297–0.833, ~0.149–0.297, and ~0.053–0.074 mm). In fact, the specific
surface area (SSA) of crushed basalt and granite by N2-BET increased from 0.61 to 1.96 m2/g as the particle size decreased, and Kdshowed a weaker relationship with SSA than the major mineral components. The comparison among crushed basalt, granite, and mudrock[5–7]in different particle sizes shows that the order variance of SSA in mudrock (~14.76–16.69 m2/g) is greater than that in granite and basalt. Therefore, this demonstrates that the major influence on sorption of Cs in basalt and granite is iron–magnesium(Fe-Mg) minerals, with approximately 14.42% Fe2O3in basalt, such as biotite or magnetite.
Steady-state asymptote
The apparent diffusion coefficient (Da) and effective diffusion coefficient (De) could only be achieved when the through-diffusion experiments reached steady state. In
other words, an important factor, the dimensionless parameter ( 2
L t D td a f ), is
introduced here to check whether the diffusion process reaches equilibrium. Crank (1975)[18]stated that the steady state of diffusion is achieved when tdis higher than 0.45. In this study, the Da, De, and tdvalues of the experimental data shown in Table
2 for basalt and granite are all higher than 0.45, which suggests that the experimental
time is sufficiently long to reach steady-state diffusion. Furthermore, a steady-state asymptote calculated only from the slope and interception was carried out in a lower or higher length/diameter ratio (L/D < 0.44 or L/D > 1.78)in the previous studies
[9-10]. A non-reactive tracer (HTO) was applied to correct and identify the column geometry or retardation factor (Rf) beyond the L/D ratio. In this study, the diffusion length/diameter (L/D) was approximately 0.15 according to the column assembly shown in Fig 1.
The diffusion parameters calculated from Eqs. (5) and (6) are listed in Table 2, summarizing the through-diffusion experiments on compact basalt and granite. In addition, the accumulative concentration curves for basalt and granite obtained by through-diffusion tests of Cs are shown in Fig 5. This shows that the time lag for the crushed and intact rocks to diffuse out is approximately 15 and 20 days, respectively. The through-diffusion tests showed a high R-square value (> 0.9) in each column and exhibited obvious retardation behavior in crushed and intact rocks. Moreover, Table 2 shows the lowest diffusion coefficients (Da = 0.56 × 10−12m2/s and De = 0.39 × 10−12m2/s)in intact basalt than those in other columns. These results indicate that intact basalt has a higher retardation effect than granite. In fact, in addition to the microporous structure (major sorption minerals), it also showed that the major retardation of Cs depended on porosity of compacted media.
Distribution coefficients and Retardation factors
The results in the batch and column tests are shown in Tables 3 and 4. The Kdvalues and retardation factor (Rf) determined with the two methods are comparable within an order of magnitude. In fact, the S/L ratio decreased as is the case when switching from batch to column experiments, and the sorption effect became negligible. More recent studies[19–22]have reported effective diffusivities, i.e., the diffusivity of the radionuclide in the pore fluid, which should in principle be independent of sorption.
Conclusion
To assess the safety of the planned HLW repository, we need to predict the transport rates of radionuclides through these porous materials. In a preliminary geological
survey, laboratory studies are necessary to realize the diffusive transport of important radionuclides, such as137Cs, through potential waste repository hot rocks. In this study, polar microcopy and autoradiography (PA experiments) and batch tests were very useful and straightforward in understanding the sorption behavior of Cs on biotite. Since diffusion is the dominant transport mechanism in the release of Cs through engineered and natural barriers, it is essential to accurately measure the diffusion coefficients (Daand De) for Cs by conducting through-column tests. Granite and basalt were selected and tested in through-diffusion experiments, and both
crushed and intact samples were performed in the work to assess the actual geological environment. The observations indicated that intact basalt has a higher retardation effect than granite, and it may play a more suitable role than granite in the site selection for the HLW repository. In addition to the microporous structure (major sorption minerals), this also showed that the major retardation of Cs depended on the porosity of the compacted media. In fact, the S/L ratio decreased as is the case when switching from batch to column experiments and the sorption effect on minerals became negligible in retardation of radionuclide migration.
Acknowledgments
This study was funded by National Cheng-Kung University, Taiwan, R.O.C., under the project Headquarters of University Advancement.
References
1. Taiwan Power Company (2009) Preliminary Technical Feasibility Study for Final Disposal of Spent Nuclear Fuel - 2009 Progress Report (Summary).
2. Choppin G. R., Liljenzin J.O., Rydberg J. (2002) Radiochemistry and Nuclear Chemistry, Butterworth-Heinemann, U.S.
3. Kawamura H., Kobayashi T., Furuno A., In T., Isihikawa Y., Nakayama T., Shima S., Awaji T., (2011) J. of Nuclear Sci. Tec. 48:1349–1356
4. Hsu C.N., Wei Y.Y., Chuang J.T., Tseng, C.L., Yang J.Y., Ke C.H., Cheng H.P., Teng, S.P. (2002) Radiochim Acta 90:659-664
5. Lee C.P., Lan P.L., Jan Y.L., Wei Y.Y., Teng S.P., Hsu C.N. (2006) Radiochim Acta 94: 679-682
6. Lee CP, Jan Y.L, Lan P.L., Wei Y.Y., Teng S.P., Hsu C.N. (2007) J Radioanal Nucl Chem 274:145-151
7. Lee CP, Tsai SC, Wei YY, Teng SP, Hsu CN (2008) J Radioanal Nucl Chem 275:115-119
8. Lee CP, Kuo YM,Tsai SC, Wei YY, Teng SP, Hsu CN (2008) J Radioanal Nucl Chem 275:343-349
9. Lee CP, Tsai SC, Jan Y.L, Wei YY, Teng SP, Hsu CN (2008) J Radioanal Nucl Chem 275:371-378
10. Tsai TL, Lee CP, Lin TY, Wei HJ, Men LC (2010) J Radioanal Nucl Chem 285:733-739
11. Meier H., Zimmerhachl E., Hecker W., Zeitler G., Menge P. (1988) Radiochimica Acta 44:239.
12. Meier H., Zimmerhachl E., Zeitler G., Menge P., Albrecht W. (1992). Radiochimica Acta 58:341.
13. Eriksen T.E. (1989). Some notes on diffusion of radionuclides through compacted clay. SKB technical report, p 89, Sweden.
15. ASTM (1984) American Society for Testing and Materials. Philadelphia, D 4319-83.
16. Tsai S.C., Ouyang S., Hsu C. N., App. Rad. Isotopes 54 (2001) 209.
17. D. J. Liu, X. H.Fan, J. Yao, B.Wang, J. of Radioanal. Nucl. Chem., 268 (2006) 3 18. J. Crank, The Mathematics of Diffusion, Clarendon Press (1975)
19. Jansson, M., Eriksen, T.E., (2001) A probe for in-situ radionuclide experiments, Technical Report TR-01-14, Svensk Karnbranslehantering AB.
20. Garcia-Gutierrez M., Cormenzana J.L., Missana T., Mingarro M., Molinero J. (2006) J. of Iberian Geology 32 (1):37–53
21. Suzuki S., Haginuma M., Suzuki K. (2007) J. of Nuclear Science and Technology 44:81–89
22. T. Itakura, Airey D.W., Leo C. J., Payne T., McOrist G. D.(2010) J. of
(a)
(b)
(c)
.
Figure 1. Schematic plots of diffusion column: (a) crushed rocks; (b) intact rocks; (c) through-diffusion framework after assembly
Liquid remove in the source cell (C0) Liquid remove in the diffusion cell (C) L r = 2.5 = 7 8 C r u s h e d 0.5 0.3 r = 1 screw Teflon filter
Stainless steel o-ring O-ring Stainless steel o-ring
7 = 5 = 4 I n t a c t = 7.4 5.9 7.4 O-ring 螺絲釘 0.3 = 2 = 3 = 3.2