Ho-Chieh Yu and Kuan-Zong Funga)
Department of Materials Science and Engineering, National Cheng Kung University, Tainan 70101, Taiwan, Republic of China
(Received 13 June 2003; accepted 16 December 2003)
The structural and electrical properties of lanthanum copper oxide were examined as a function of Sr addition. It was observed that the lanthanum oxide and copper oxide formed La2CuO4 with K2NiF4 structure when the powder mixture was heated at
800 °C in ambient pressure. Interestingly, the samples of Sr-doped (15∼25%) lanthanum copper oxides showed single perovskite-based phase after being heated at 800 °C. Without Sr addition, a single-perovskite phase of lanthanum copper oxide was observed only under the oxygen pressure as high as 65 kbar. The stabilization of perovskite structure in lanthanum copper oxide was effectively achieved by the addition of Sr. Based on the titration analysis and pertinent defect reactions, the enhancement of perovskite stability was due to the presence of trivalent copper ions that were created to balance the electrical charge of doping ion (SrLa⬘). With the
increasing concentration of trivalent copper ions (or electron holes equivalently) in Sr-doped samples, lanthanum copper oxide also changed from a semiconductor to metallic conductor. When the Sr dopant exceeded its solubility limit of approximately 25% in the A-site sublattice, the Sr-rich second phases, La2SrCu2O6and Cu2SrO3,
appeared and suppressed the electronic conduction drastically.
I. INTRODUCTION
Lanthanum copper oxides have been considered as po-tential superconducting materials although the supercon-ductivity has not been obtained. Under the ambient pres-sure, the stable compound of lanthanum copper oxides is La2CuO4 with K2NiF4 structure. The K2NiF4 structure
consists of perovskite layers with alternating rock-salt layers. La2CuO4exhibits p-type semiconducting
behav-ior.1,2
It has been reported that Sr-doped La2CuO4 or
La2-xSrxCuO4(x⳱ 0.15∼0.20) showed superconducting
behavior based on the measurement of ideal diamagne-tism at the temperature as high as 40 K.2,3
The addition of Sr also enhances the stability of the tetragonal K2NiF4
structure although the superconducting behavior only oc-curred in the distorted K2NiF4phase. In addition,
lantha-num copper oxides may crystallize in a complete perov-skite structure with a formula of LaCuO3-␦ when
proc-essed at high oxygen pressure.
LaCuO3-␦ was first prepared by Demazeau et al. 4
at 900 °C and 65 kbar. It was indexed as a rhombohedral-ly distorted perovskite. The oxygen stoichiometry of LaCuO3-␦ exists with ␦ ranging from 0 to 0.5 under
various oxygen partial pressures. Accordingly, the crys-tal structure of LaCuO3-␦ also changes from tetragonal,
monoclinic to orthorhombic.5,6
The conduction behavior of LaCuO3-␦oxides changes
from an insulator to metallic conductor as␦ varies from 0.5 to 0. The conductivities of LaCuO3-␦ significantly
improve from 10−6 S/cm to 1000 S/cm as ␦ decreased from 0.5 to 0.0, although the superconducting behavior was not observed.7,8
The nonstoichiometry of LaCuO3-␦
perovskite suggests that oxygen vacancies may be pres-ent in a wide range from 0% to 16.7%. Considering its high conductivity and available oxygen vacancies, LaCuO3-␦ may be used as a catalyst or electrode for
electrochemical reactions. However, in the previous study it was reported that LaCuO2.5of CaMnO2.5
struc-ture was obtained at ambient pressure by low tempera-ture reduction of LaCuO3-␦prepared at high pressure.
9,10
Nonetheless, the synthesis of LaCuO3-␦under high
oxy-gen partial pressure is not suitable for practical applica-tions. An effective and simple way to process LaCuO3-␦
is desired.
In many conducting oxides, the addition of aliova-lent dopants is the key factor in changing the structural and electrical properties.11–14 Several Sr-doped lantha-num copper oxides have been reported to crystallize in an oxygen-deficient perovskite structure. Typically, La1-xSrxCuO2-␦ has a tetragonal cell of a ≈ 2√2 ap and a)
Address all correspondence to this author. e-mail: kzfung@mail.ncku.edu.tw
c≈ ap(apis the lattice parameter of the primitive cubic
perovskite) for 0.15 艋 x 艋 0.25.15,16 Recently, La0.75Sr0.25CuO2.44and La0.80Sr0.20CuO2.47 were found
to crystallize in an oxygen-deficient perovskite structure with the resistivity of 4.8 × 10−4
ohm-cm and 6.8 × 10−4
ohm-cm, respectively.17
Due to its superior electrical property, the perovskite La1-xSrxCuO2.5-␦has been
inves-tigated as a potential cathode material for solid oxide fuel cells.18
For such a high-temperature application, the structural stability and electrical properties of the mate-rial are extremely important. Although it may seem ob-vious that Sr addition affects the crystallization and elec-trical conductivity of lanthanum cuprate perovskite, the role of Sr on the structural and electrical properties of Sr-doped lanthanum copper oxides has not been thor-oughly studied. It is believed that the electronic defects created by Sr addition have a strong influence not only on the structure evolution/phase transformation but also on the electrical conductivity. Therefore, in this study, vari-ous amount of Sr was added into lanthanum copper oxide based on the formula of La1-xSrxCuO2.5-␦. The crystal
structure was characterized as a function of Sr concen-tration using x-ray diffraction. The valence of Cu was determined using iodometric titration. Electrical conduc-tivity of various Sr-doped lanthanum copper oxides was measured using the four-point-probe direct current (dc) technique. Finally, the effects of Sr addition were exam-ined and discussed in light of defect chemistry.
II. EXPERIMENTAL A. Sample preparation
Samples of La1-xSrxCuO2.5-␦powder were synthesized
by conventional ceramic processing. In the preparation of samples, appropriate amounts of La2O3, SrCO3, and CuO
were mixed and ball-milled in ethanol solution for 24 h. After being dried, the powder mixture was then calcined at 800 °C for 24 h in air and sintered at 960 °C in air. B. Characterization
1. XRD analysis
The crystal structure of the samples were examined by x-ray powder diffraction (XRD) at room temperature with Cu K␣ radiation, and the scanning rate was at 2°/min. The lattice parameters were determined by using Si as an internal standard with a scanning rate of 0.2°/min.
2. Scanning electron microscopy and energy-dispersive x-ray spectrometry
The microstructures including grain structure and sur-face morphology of the sintered samples were observed using field-emission scanning electron microscopy
(FE-SEM) (Model S4200, Hitachi), Tokyo, Japan. The surfaces of sintered samples were fine polished using 0.3-m diamond paste. The polished samples were ther-mally etched at 880 °C for 2 h. Subsequently, the etched samples were inserted into the FE-SEM for observation. Energy dispersive x-ray spectrometry (EDS) was per-formed on the surface of samples to semiquantitatively estimate the chemical composition of the phases present. 3. Iodometric titration
Iodometric titration was used to determine the oxida-tion state of Cu ions. After being pulverized and dried at 110 °C for 1 h, the powder was then sieved through a 200-mesh screen. Then, about 0.06 g of calcined powder was weighted and placed in a 100 mL Erlenmeyer flask, filled with flowing N2gas, and then mixed with 1 g of KI
powder. Subsequently, the sample was dissolved in 10 mL of 8 N HCl. For the determination of Cu2+ions, as the second step, KI was added after all Cu3+ions were reduced by HCl. Finally, the solutions of both first and second step were titrated with 0.02 N Na2S2O3to a starch
end point.19
4. Conductivity measurement
Due to the high conductivity of doped lanthanum cop-per oxides, the electrical conductivity was measured us-ing the four-point-probe dc technique at various tempera-tures up to 800 °C. Bar-shaped specimens with a dimen-sion of 5 × 5 × 40 mm (height × width × length) were prepared by die-pressing followed by cold isostatic pressing under the pressure of 200 Mpa. Platinum paste was applied on the cross-section surfaces (5 × 5 mm) connected with Ag wires. Two more Ag wires were con-nected to samples at 3 mm away from both ends and used as the voltage probes. After applying a source of constant current, the conductivity of the measured sample was determined from the voltage drop across the voltage probes, the current applied and the geometric factor. III. RESULTS AND DISCUSSION
A. Structure analysis of La1-xSrxCuO2.5-␦ (0⭐ x ⭐ 0.4)
The raw materials of La2O3, SrCO3, and CuO were
first mixed based on a perovskite formula of La1-xSrxCuO2.5-␦(x varied from 0 to 0.4). The samples of
undoped and Sr-doped lanthanum copper oxide were heated at 960 °C for 20 h in air, and the corresponding x-ray traces are shown in Fig. 1. In the undoped sample consisting of La2O3and CuO with a mole ratio of 1:2, no
reflections representing a perovskite phase were ob-served. As a result, the undoped mixture shows the pres-ence of La2CuO4and excess CuO. La2CuO4exhibits an
orthorhombic K2NiF4 structure 1,20
with lattice param-eters of a⳱ 3.816 Å, b ⳱ 3.782 Å and c = 13.208 Å.
The formation of La2CuO4 consumed equimolar La2O3
and CuO from the original mixture (La2O3:CuO⳱ 1:2).
Thus, excess CuO was observed. This result indicates that La2O3and CuO heated in air could not form a stable
lanthanum copper oxide with a perovskite structure. On the contrary, with the addition of Sr into lanthanum cop-per oxide, the reflection intensities representing La2CuO4
and CuO gradually reduced and a perovskite-based struc-ture formed. For instance, with the addition of 10% Sr into the mixture of La2O3and CuO, a deformed
perov-skite, La1-xSrxCuO2.5-␦ with an orthorhombic structure
was observed in addition to La2CuO4 and CuO. When
the addition of Sr increased to 15%, both La2CuO4and
CuO phases were no longer present. Finally, a single orthorhombic perovskite structure was observed, and the lattice parameters measured were a ⳱ 5.5040 Å,
b ⳱ 10.6011 Å, and c ⳱ 3.8785 Å. When the
con-c e n t r a t i o n o f S r O d o p a n t s i n con-c r e a s e d t o 2 0 % , La0.8Sr0.2CuO2.5-␦ perovskite transformed from
ortho-rhombic to tetragonal phase. This tetragonal phase, La0.8Sr0.2CuO2.5-␦, consists of an oxygen-deficient
perovskite subcell with lattice parameters of a ⳱ 10.8666 Å and c ⳱ 3.8570 Å for La0.8Sr0.2CuO2.5-␦.
Based on the XRD analysis shown in Fig. 2, a single tetragonal phase was observed when x fell in the range between 0.2 and 0.25. However, a small amount of a secondary phase was presented when the concentration of SrO was up to 30%. When the concentration of Sr increased to more than 35%, the secondary phases La2SrCu2O6 (JCPDS:83-1839) and Cu2SrO3 (JCPDS:
39-0250) appeared from La1-xSrxCuO2.5-␦ perovskite
matrix.
Table I summarizes the lattice parameters a, b, c, the unit cell volumes, and ␦ value of the La1-xSrxCuO2.5-␦
phase of the samples. The volume of the tetragonal perovskite unit cell decreased with increasing strontium content when 0.2 艋 x 艋 0.25. Apparently, the crystal structure of La1-xSrxCuO2.5-␦ obtained is highly
depen-dent upon the amount of Sr addition. Furthermore, the Sr addition may also affect the concentration of electronic defects in lanthanum copper oxide. In La1-xSrxCuO2.5-␦,
Cu is the only cation that may vary its valence and affect the crystal structure.21,22Therefore, the dependence of Sr addition on the Cu valence as well as the structure varia-tion was examined using Cu titravaria-tion analysis.
FIG. 1. X-ray pattern for Sr-doped lanthanum copper oxides based on the formula of La1-xSrxCuO2.5-␦where x varied from 0% to 20%
FIG. 2. X-ray pattern for Sr-doped lanthanum copper oxides based on the formula of La1-xSrxCuO2.5-␦where x varied from 25% to 40%.
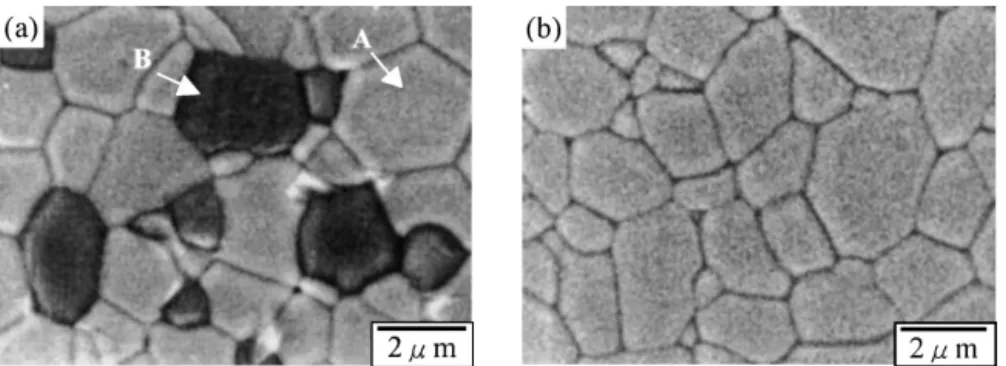
B. SEM microscopy and EDS microanalysis Figure 3 shows the scanning electron microscopy (SEM) micrographs of sintered samples of (a) undoped and (b) 20% Sr-doped lanthanum copper oxides. For un-doped lanthanum copper oxides, two different phases were observed. Based on the EDS analysis, the compo-sition of the matrix phase A is close to La2CuO4. The
average grain size for phase A is 3∼4 m. The dark phase B was found to be CuO. Figure 3(b) shows the micro-graph of 20% Sr-doped lanthanum copper oxides. A single-phase microstructure with the average grain size of 3∼4 m was observed. These results show good agree-ment with the XRD analysis.
C. Titration analysis of Cu
The valence and the concentration of Cu were deter-mined by iodometric titrations. The concentration of Cu3+, expressed as [Cu3+/(Cu2++Cu3+)], was plotted as a function of Sr dopant concentration and is shown in Fig. 4. When the Sr content increased from 10% to 25%, the concentration of Cu3+
also increased linearly from 3% to 18.5%. When the concentration of SrO added in-creased from 30% to 40%, the concentration of Cu3+
, however, decreased from 19% to 8%. From the results of Cu titration, it is clearly shown that the valence change of Cu ions was induced by the addition of Sr ions into lanthanum copper oxides. Such phenomena can be ex-plained by considering the appropriate defect reaction as shown below.
D. Defect reaction for Sr addition
To use a defect equation to illustrate the incorporation of Sr ion into lanthanum copper oxide, a lanthanum-based perovskite needs to be considered first. When the
A cation site is occupied by the trivalent La ion in an ABO3perovskite structure, the B site should be occupied
by a trivalent ion. Thus, in the following discussion, the normal B site was assigned to a trivalent cation. Theo-retically, in lanthanum strontium copper oxide, both di-valent strontium and copper ions need to be considered as the aliovalent dopants of LaBO3 perovskite. Thus,
the following two defect reactions may be used to de-scribe the addition of SrO and CuO into the LaBO3
lat-tice depending upon the defects generated for charge compensation
1⁄
2O2+ 2SrO + 2CuO → 2 SrLa⬘ + 2CuB⬘ + 5OOx
+ VO ••+ 2h•
, (1)
O2+ 2SrO + 2CuO → 2 SrLa⬘ + 2CuB⬘
+ 6OO x+ 4h•
. (2)
In the above defect reactions, Kröger–Vink notation was used. For instance, SrLa⬘ represents the substution of
Sr for La cation sublattice, and CuB⬘ indicates that the
divalent copper ions is located at the trivalent B-cation sublattice. Also, VO
••
represents the oxygen vacancy with two positive charges.
In reaction (1), the negative charges of SrLa⬘ and CuCu⬘
are compensated by oxygen vacancies and electron holes that may associate with Cu2+ions and become Cu3+. In reaction (2), SrLa⬘ and CuCu⬘ are only compensated by
electron holes without the formation of oxygen vacan-cies. In this case, every addition of a Sr ion should create two trivalent copper ions. According to the results of the previous titration analysis, it was found that the defect reaction (1) is the adequate one because [Cu3+] is close to the concentration of Sr ions instead of twice [Sr]. As seen
TABLE I. Crystal lattice of perovskite phase of the samples with different Sr content.
Composition Structure a (Å) b (Å) c (Å) Vcell(Å3) 2.5-␦
La0.85Sr0.15CuO2.5-␦ Orthorhombic 5.504 10.6011 3.8785 226.3044 2.461
La0.8Sr0.2CuO2.5-␦ Tetragonal 10.8666 ⭈⭈⭈ 3.857 455.4461 2.471
La0.775Sr0.225CuO2.5-␦ Tetragonal 10.8668 ⭈⭈⭈ 3.8551 455.2385 2.463
La0.75Sr0.25CuO2.5-␦ Tetragonal 10.8586 ⭈⭈⭈ 3.8568 454.7522 2.461
in the following sections, the presence of Cu3+ signifi-cantly affects both the structural and electrical properties of Sr-doped lanthanum copper oxides.
1. Oxidation state of Cu
As shown in Fig. 1, the crystal structure of Sr-doped lanthanum copper oxide, expressed as La1-xSrxCuO2.5-␦,
varied from the La2CuO4/CuO mixture, orthorhombic, to
the single tetragonal phase. The structure evolution of Sr-doped lanthanum copper oxide may be attributed to the variation of the oxidation state of Cu.
Assuming La2O3 and CuO to crystallize in a
perov-skite structure, two formulas, LaCuO2.5and LaCuO3, are
applicable depending upon the valence of Cu ions being +2 or +3. However, when undoped La2O3 and CuO are
heated in air, the absence of the perovskite phase sug-gests that the presence of divalent Cu ions at the B-sites of the lanthanum copper oxide perovskite lattice would not stably support a perovskite structure because of the repulsion from negatively charged oxygen ions. Instead, La2O3 and CuO formed La2CuO4where the Cu ion
re-mains divalent.
After Sr was added into the mixture of La2O3 and
CuO, La2CuO4 was no longer present and converted to
orthorhombic or tetragonal La1-xSrxCuO2.5-␦ depending
upon the concentration of Sr. As both orthorhombic and tetragonal structures can be viewed as a distorted and enlarged perovskite, the formation of these structures in-dicates that the stability of lanthanum copper oxide perovskite was enhanced by the addition of Sr. From reaction (1), the addition of Sr tends to increase the con-centration of trivalent copper ions which results in the
reduction of oxygen repulsion. Therefore, the stability of the Sr-doped lanthanum copper oxide perovskite was en-hanced by a reduction of oxygen repulsion.
In the 15% Sr-doped lanthanum cuprate, The lattice parameters of the orthorhombic unit cell can be related to the standard perovskite as a ≈ 2√2 ap, b ≈ 2√2 ap, and
c≈ ap, where apis the parameter of a perovskite subcell.
The distortion and superlattice nature of this orthorhom-bic structure is, however, caused by the presence of nu-merous oxygen vacancies in the perovskite lattice. As more Sr was added into lanthanum copper oxide, more trivalent copper ions were obtained. Consequently, the stability of the perovskite was further enhanced. Thus, a more symmetrical tetragonal structure with a ≈ 2√2 ap
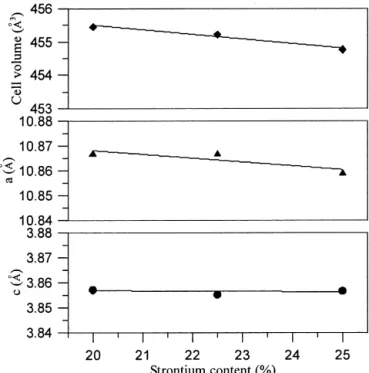
and c≈ ap, was obtained. In Fig. 5, the lattice parameters
decreased with increasing strontium content. On the other hand, the volume of the perovskite subcell also decreases from 455.4461 Å3 to 454.7522 Å3 as the Sr addition increased from x ⳱ 0.2 to 0.25. The smaller lattice also suggests that more Cu3+ ions were created and a higher stability was obtained.
2. Phase separation caused by excess Sr addition As the addition of Sr exceeded more than 30%, from XRD analysis, the phases present were perovskite phase, La2SrCu2O6, and Cu2SrO3. It is known that LaCuO3-␦
with the presence of Cu3+ only exists under very high oxygen partial pressure. In La1-xSrxCuO2.5-␦, although
Cu3+
can be induced by Sr addition, the maximum con-centration of Cu3+
is still limited by the extent of Cu oxidation allowed in air.16
Unless the partial pressure of
FIG. 4. Average valence of Cu ions as a function of Sr concentration (x) in La1-xSrxCuO2.5-␦.
FIG. 5. The lattice parameters (c and a) and cell volume of tetragonal perovskite La1-xSrxCuO2.5-␦versus strontium content.
oxygen can be increased, 25% is the maximum concen-tration of Cu3+allowed in La-based perovskite under the ambient atmosphere. Consequently, phase decomposi-tion or separadecomposi-tion took place when excess Sr ions reacted with La2O3 and/or CuO to form La2SrCu2O6 and
Cu2SrO3respectively.
E. Effect of Sr dissolution on conductivity and conduction mechanism
Considering the possible application of LSCu as an electrode for an intermediate temperature solid oxide fuel cell material, the electrical conductivities of La1-xSrxCuO2.5-␦(x⳱ 0.0∼0.4) samples were measured
from room temperature to 800 °C and plotted as a func-tion of the reciprocal of absolute temperature and are shown in Fig. 6. Without the addition of strontium oxide, the conductivity of the undoped sample with dual phases of La2CuO4 and CuO exhibits semiconducting
behav-ior.23Its conductivity increased from 1.86 × 10−4S/cm to 55 S/cm when the sample was heated from room tem-perature to 800 °C. After Sr was added into lanthanum copper oxide, the conductivity of La1-xSrxCuO2.5-␦(x⳱
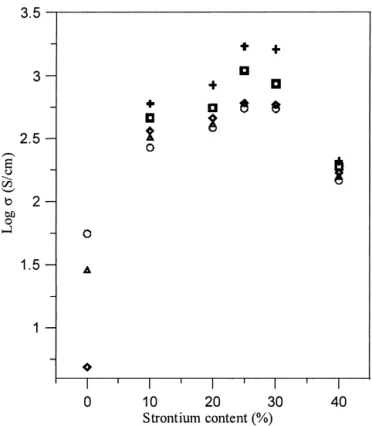
0.1∼0.25) drastically increased to 260∼680 S/cm at 800 °C and showed the metallic conducting behavior. When the conductivity at room temperature, 200 °C, 400 °C, 600 °C, and 800 °C was plotted as a function of Sr concentration (Fig. 7), a maximum conductivity was observed when [x]⳱ 0.25. It is apparent that the con-ductivity of lanthanum copper oxide was also drastically
enhanced by the addition of Sr. in Sr-doped lanthanum copper oxide is fixed by the ad-According to reaction (1), the number of electron holes dition of Sr.24,25Based on the electronic structure model of perovskite oxides suggested by Torrance et al.,25 the unfilled Cu-3d and the filled O-2p bands overlap and form a Cu–O–Cu band. Thus, the transport of charged carriers can occur in the hybrid band and exhibits the behavior of a metallic conductor.26–28
In a metallic con-ductor, the charge carriers have frequent collision with the lattice ions as the concentration of charge carriers is high (fixed by the dopant). As the temperature increases, the lattice vibration rapidly increases. As a result, the mobility of charge carriers will be suppressed at high temperatures. Therefore, a positive slope was obtained when an Arrehenius plot of conductivity was plotted as the reciprocal of absolute temperature as seen in the case of Sr-doped lanthanum copper oxide (Fig. 6). Due to the creation of electronic defects caused by Sr addition, the dissolution of Sr into the lanthanum cuprate perovskite does not only enhance the conductivity but also com-pletely changes the conduction mechanism from semi-conducting to metallic semi-conducting behavior.
When the addition of Sr increased from 25–40%, the conductivity significantly reduced from 2000 to 200 S/cm at 800 °C. As discussed previously, the de-creasing ratio of [Cu3+]/[Cu2+ + Cu3+] was observed
FIG. 6. The electrical conductivities of La1-xSrxCuO2.5-␦plotted as a
function of temperature from 293 K to 1073 K where x⳱ 0, 0.1, 0.2, 0.25, 0.4.
FIG. 7. The conductivity plotted as a function of Sr concentration (when measured at various temperature: +, room temperature; 䊐, 200 °C;◊, 400 °C; ⌬, 600 °C; and 䊊, 800 °C).
because of the formation of La2SrCu2O6 and Cu2SrO3.
Consequently, the concentration of electron holes in 40% Sr-doped sample was significantly reduced and the corresponding electronic conduction was suppressed dramatically.
IV. CONCLUSIONS
An undoped mixture of La2O3 and CuO could not
crystallize in a perovskite structure when heated at 960 °C in air. On the contrary, a perovskite-based struc-ture was obtained when 15%∼25% Sr was added into the mixture. From the titration analysis and appropriate con-sideration of defect reactions, it is concluded that the concentration of trivalent copper ions was enhanced to balance the charge of SrLa⬘ in the Sr-doped samples.
Consequently, the presence of Cu3+was able to stabilize the perovskite structure at the ambient pressure. The ex-istence of Cu3+also changes the conduction behavior of lanthanum copper oxide from a semiconductor to a me-tallic conductor. The conductivity of the 25% Sr-doped sample was significantly enhanced to 2000 S/cm by the increased concentration of electron hole (or Cu3+) com-pared with approximately 2 × 10−4S/cm of the undoped sample at room temperature. However, it is believed that a limited amount of trivalent copper ions was allowed in the perovskite lattice under the ambient pressure. When the addition of Sr ions exceeded this limit (∼25%), the secondary phases La2SrCu2O6 and Cu2SrO3 appeared.
The conductivity was also drastically reduced to 200 S/cm due to the decreased concentration of electron hole (or Cu3+
) when 40% of Sr was added into the mixture.
ACKNOWLEDGMENT
This work was financially supported by the National Science Council of Taiwan, Republic of China (Grant No. NSC 92-2120-M-006-003).
REFERENCES
1. N. Nguyen, J. Choisnet, M. Hervieu, and B. Raveau, J. Solid State Chem. 39, 120 (1981).
2. R.M. Fleming, B. Batlogg, R.J. Cava, and E.A. Rietman, Phys. Rev. B 35, 7191 (1987).
3. R.J. Cava, R.B. van Dover, B. Batlogg, and E.A. Rietman, Phys. Rev. Lett., 58, 408 (1987).
4. G. Demazeau, C. Parent, M. Pouchard, and P. Hagenmuller, Mat. Res. Bull. 7, 913 (1972).
5. J.F. Bringley, B.A. Scott, S.J. Placa, R.F. Boehme, T.M. Shaw, M.W. McElfresh, S.S. Trail, and D.E. Cox. Nature 347, 263 (1990).
6. A.W. Webb, K.H. Kim, and C. Bouldin, Solid State Commun. 79, 507 (1991).
7. J.F. Bringley, B.A. Scott, S.J. Placa, T.M. McGuire, and F. Mehran, Phys. Rev. B 47, 15269 (1993).
8. S. Darracq, S. Matar, and G. Demazeau, Solid State Commun. 85, 961 (1993).
9. S.J. Laplaca, J.F. Bringley, B.A. Scott, and D.E. Cox, Acta Crystallogr. Sect. C 49, 1415 (1993).
10. K.R. Poeppelmeier, M.E. Leonowicz, J.C. Scanlon, and J.M. Longo, J. Solid State Chem. 45, 71 (1982).
11. C. Michel, L. Er-Rakho, and B. Raveau, Mater. Res. Bull. 20, 667 (1987).
12. A.N. Petrov, O.F. Kononchuk, A.V. Andreev, V.A. Cherepanov, and P. Kofstad, Solid States Ionics 80, 189 (1995).
13. L. Er-Rakho, C. Michel, J. Provost, and B. Raveau, J. Solid State Chem. 37, 151 (1981).
14. J. Remmel, J. Geerk, G. Linker, O. Meyer, R. Smithey, B. Strehlau, and G.C. Xiong, Physica C, 165, 212 (1990). 15. T. Siegrist, S.M. Zahurac, D.W. Murphy, and R.S. Roth, Nature
334,231 (1988).
16. D.M. DeLeeuw, C.A.H.A. Mutsaers, G.P.J. Geelen, and C. Langereis, J. Solid State Chem. 80, 276 (1989).
17. N. Murayama, S. Sakagugchi, F. Wakai, E. Sudo, A. Tsuzuki, and Y. Torii, Jpn. J. Appl. Phys. 27, L55 (1988).
18. H.C. Yu and K.Z. Fung, Mater. Res. Bull. 38, 231 (2003). 19. Y. Maeno, H. Teraoka, K. Matsukuma, K. Yoshida, K. Sugiyama,
F. Nakamura, and T. Fujita, Physica C 185-189, 587 (1991). 20. Z. Hiroi, J. Solid State Chem. 123, 223 (1996).
21. A.I. Nazzal, V.Y. Lee, E.M. Engler, R.D. Jacowitz, Y. Tokura, and J.B. Torrance, Physica C 153-155, 1367 (1988).
22. K. Otzschi, K. Koga, and Y. Ueda, J. Solid State Chem. 115, 490 (1995).
23. H. Takagei, T. Ido, S. Ishibashi, M. Uota, and S. Uchida, Phys. Rev. B 40, 2254 (1989).
24. K. Otzschi and Y. Ueda, J. Solid State Chem. 107, 149 (1993). 25. J.B. Torrance, P. Lacorre, A.I. Nazzal, E.J. Ansaldo, and
Ch. Niedermayer, Phys. Rev. B 46, 6382 (1992). 26. Z. Hiroi and M. Takano, Nature 377, 41 (1995).
27. S. Darracq, S.G. Kang, J.H. Choy, and G. Demazeau, J. Solid State Chem. 114, 88 (1995).
28. G. Ch. Kostogloudis and Ch. Ftilos, Solid State Ionics 109, 43 (1998).