Table of Contents
Table of Contents 1
List of Tables 3
List of Figures 5
致謝 7
Abstract 10
中文摘要 12
Chapter 1 General introduction 14
Chapter 2 Testing founder speciation in a wader: divergence genetics of the spoonbills (Platalea regia and P.
minor)
24
Chapter 3 Evaluating ancestral and extant effective population sizes of the spoonbills (Platalea spp.) with
implications to models of adaptive speciation
68
Chapter 4 Assessing the genetic impact of natural versus anthropogenic processes on endangered animals:
demographic history and current effective population size of the black-faced spoonbill (Platalea minor)
97
Chapter 5 General conclusion and perspectives 124
Appendix Publications
A. Yeung C, Yao CT, Hsu YC, Wang JP, Li SH (2006) Assessment of the historical population size of
130
an endangered bird, the black-faced spoonbill (Platalea minor) by analysis of mitochondrial DNA diversity. Animal Conservation 9:1–10
B. Yeung CKL, Hsu Y-C, Yao CT and Li SH (2009) Isolation and characterization of 23 microsatellite loci in the black-faced spoonbill ( Platalea minor ) and amplification in other Ciconiiformes waterbirds.
Conservation Genetics 10:1081-1084
141
List of Tables Chapter 2
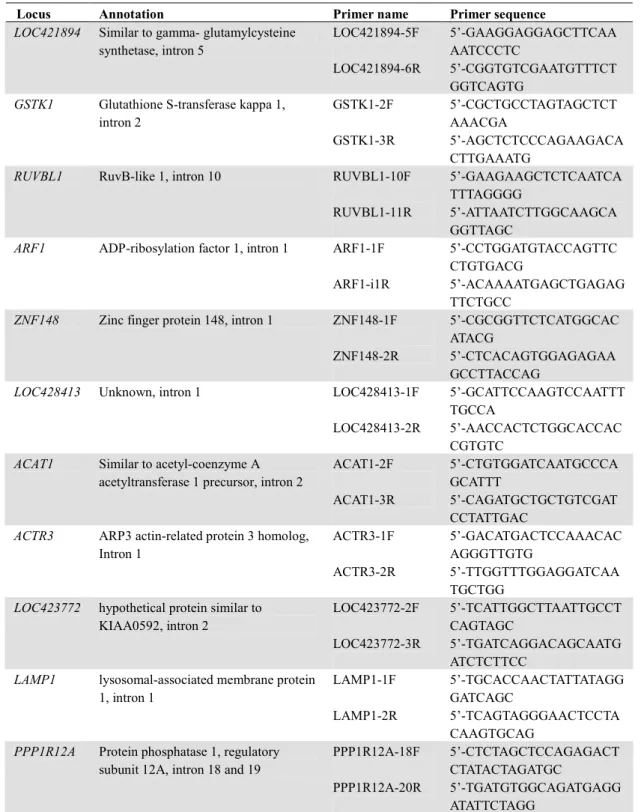
Table 2.1 Sequences of PCR primers used to amplify mitochondrial fragments in all Platalea species
54
Table 2.2 Sequences of PCR primers used to amplify the 20 nuclear fragments in this study
55
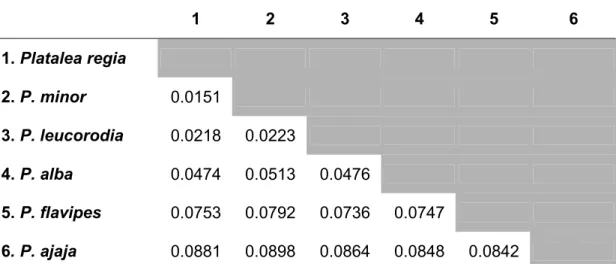
Table 2.3 Tamura-Nei distances between all spoonbill species
57
Table 2.4 Characterization of genetic polymorphism in the focal species pair P. regia and P. minor at 20 nuclear loci
58
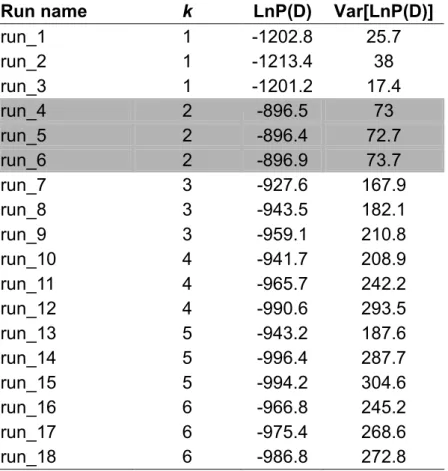
Table 2.5 Bayesian estimation (STRUCTURE 2.2) of the number of populations represented by the sampled genotype data of P. regia and P. minor at 17 nuclear loci.
60
Table 2.6 Calculating mutation rate for each of the 20 nuclear loci
61
Chapter 3
Table 3.1 The number of individuals sampled for Platalea minor (P.m.), P. regia (P.r.), P. leucorodia (P.l.), P. flavipes (P.f.), P. alba (P.a.) and Eudocimus rubber (E.r.) at each of the 91 genes.
88
Table 3.2 Gene specific θπ and θW and those averaged across all loci, proportion of monomorphic loci and Ne based onθπ in the five spoonbills.
91
Table 3.3 The ratio of θ’s of ancestral species of the spoonbills (at node N6 to N10) over that of its descendent spoonbill species (at node N1 to N5)
92
Chapter 4
Table 4.1 Sample size (N), the number of alleles (k), observed and expected heterozygosity (Hobs and Hexp), heterozygosity at mutation-drift
equilibrium (Heq) under the IAM model, and the degree of heterozygosity excess or deficiency at each of the nuclear intronic or microsatellite loci.
119
Table 4.2 Ncurrent estimated via linkage disequilibrium method using datasets varying in sample size and critical allelic frequency
120
List of Figures
Chapter 2
Figure 2.1 Distribution map of the P. regia, P. minor and P.
leucorodia (and subspecies P. l. archerii and P. l.
balsaci) following Hancock et al. (1992)
62
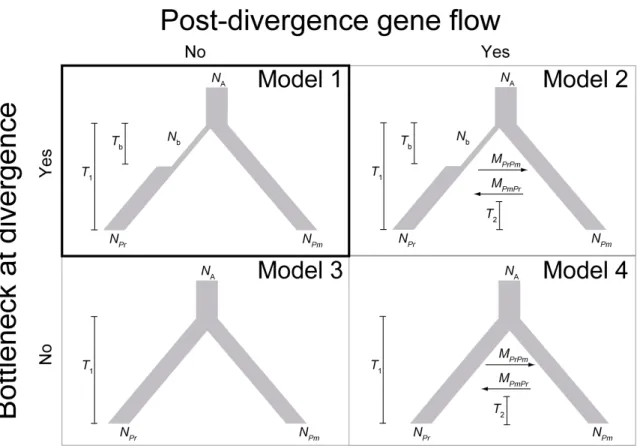
Figure 2.2 Proposed demographic models for the divergence of P. regia, and P. minor
63
Figure 2.3 Mitochodrial phylogeny of the spoonbills based on cytochrome b and ND2 gene reconstructed via the PHYML-aLRT method
64
Figure 2.4 Membership coefficient as estimated by STRUCTURE 2.2 for each sample of P. regia and P. minor
65
Figure 2.5 Smoothed posterior densities for demographic parameters of model 2
66
Figure 2.6 Smoothed posterior densities for demographic parameters of model 4
67
Chapter 3
Figure 3.1 Distribution of all Platalea species, including P.
minor, P. regia, P. leucorodia, P. alba, P. flavipes and P. ajaja (following Hanock et al. 1992)
93
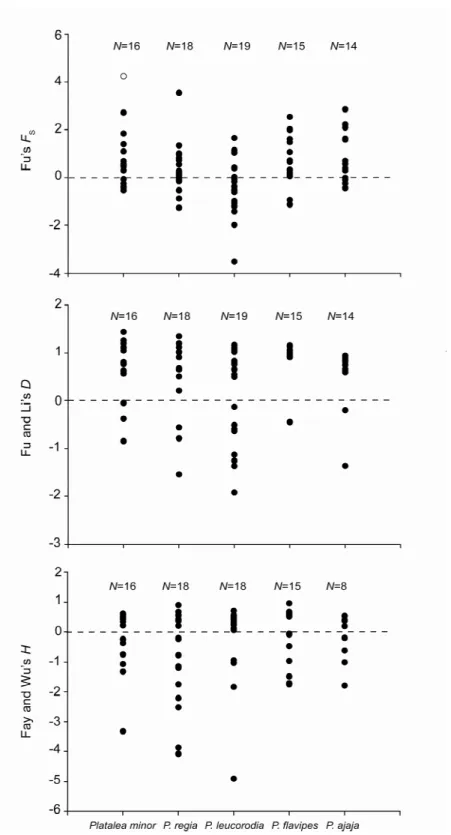
Figure 3.2 Fu’s FS, Fu and Li’s D* and Fay and Wu’s H value calculated from each of the N polymorphic genes in the five spoonbill species
94
Figure 3.3 Posterior distribution of the θ values estimated by MCMCcoal based on 91 genes
95
Figure 3.4 Mean Ne (with 95% CI), Ne/Nc ratio mapped to 96
the left of each node of a mitochondrial cladogram (according to chapter 2).
Chapter 4
Figure 4.1 Heterozygosity excess vs. allelic diversity at individual nuclear intronic and microsatellite locus
121
Figure 4.2 Comparison of the bottleneck model and constant size model via logit regression
122
Figure 4.3 Posterior densities of effective population size Npost-bottleneck and Npre-bottleneck and the time of change, in the number of generations, t of the bottleneck model
123
致謝
我要在這裡感謝一些人,沒有他們,我無法走完這趟旅程,當然,
這本論文也不會完成。
怎麼也想不到,第一次見到李壽先老師的時候,眼前這個穿著短 褲涼鞋、手裡拿著冰棒、表情一派輕鬆的教授,會對我未來幾年的研 究生活、甚至是世界觀產生如此重大的 影響。我身無長物地來到研 究所,對於理論和實驗都非常陌生,只有一顆好奇心。李老師卻並不 在意我毫無基礎,選擇相信我的潛力。他安排了讀書會,定期討論新 奇有趣的演化生態研究,參與的還有許育誠和林思民兩位學長。就這 樣,在他們三人的潛移默化下,漸漸地,那個壯麗的演化世界才向我 敞開了,我也才找到了精神 的立足點- 每次回想起曾經小辦公室裡 天馬行空的討論、和夾在其中生澀卻神往的自己,就會喚醒起初的熱 情! 另一方面,為了讓我操作實驗儘快上手,李老師請來師母黃儀君 博士一起帶著我完成了第一個篩選微衛星體的實驗; 謝謝他們鉅細 靡遺地傳授我實驗的技巧,並且耐心地陪我等待一次次的實驗結果。
我的第三個教室,在野外。多年來,主要是跟著李老師、姚正得和許 育誠,跑遍了 台灣的山林鄉野,體會書中所描述的演化如何在自然 中律動。野外跑多了,漸漸感到疲累,經過一陣子休息,當我再次漫 步於林間、體會到一種難以言喻的親切時, 才發現與自然已產生一 種本能的連結,這是書本上學不到的、更是一生至為寶貴的經驗。為 此,我深深感激他們長久的引領。
面對年輕學生所碰到的各種疑問和挫折,老師以智慧和耐心教導 我去解決;甚至在經歷誤會和衝突後,他寄予我的信任和希望竟是不 減反增。老師的治學和生活方式有著高度的一致性: 單純、自信、積 極、理性與感性兼具... 儘管有著疾風烈火的個性,行不改名坐不改 姓的態度卻贏得了我的尊重。我要謝謝他,始終如一地在資源和精神 上支持了我,以及他因為忠於自己、帶給了我許多雋 永的啟發。
研究生活有時候挺枯燥的,尤其實驗不順利的時候更容易沮喪。
幸運的我,遇見了一群很棒的夥伴,用無數的歡樂和溫暖點亮了實驗 室冰冷的空間,更不吝於提供我行政和 技術上的援助: 林美珠(多年 來陪我經歷大小 projects 的超強夥伴)、林容仟、葉佳芬、許育誠、
傅淑瑋、洪貫捷、洪心怡、羅文穗、李岱芬、李菁紋、李麗卿、黃傳 景、 林思民、許佑薰等人。尤其當論文進入最後階段時,容仟和佳 芬皆花了很多心力協助我完成實驗和分析數據,因此這本論文的成果 要特別歸功於她們。還曾經為了一 個實驗折騰了好長一段時間,期 間經常去請教蘇銘燦老師一些分子生物實驗的瑣碎問題,蘇老師對我 總是友善耐心、並且大方地出借器材供我使用,他無私的關照, 我 銘感於心。
這本論文能夠順利完成,還要感謝許多單位和人士各方面的協 助。感謝 American Museum of Natural History 及負責館藏的 Julie Felstein 和 Paul Sweet、Smithsonian National Museum of Natural History 的 Terry Chesser、University of São Carlos 的 Silvia Del Lama 教授、中國東北林業大學的田秀華教授及台灣國立成功大學的 王建平教授協助提供幾種琵鷺及外群物種的樣本。謝謝 Robin Waples、Jean-Marie Cornuet 及 Montgomery Slatkin 在資料分析
時透過通信詳盡且勤懇的指導。也感謝口試委員劉小如、黃士穎、丁 照棣、胡哲明、王弘毅、方淑老師針對論文的指正、和長久對我的鼓 勵。
最後,我要為我的家人喝采,他們對我不循常軌的生涯規劃、以 及當中的顛簸,始終抱持包容和樂觀的態度。儘管還是免不了偶爾的 焦慮,但他們確實給了我最大的自由、尊重、支持、和不懈的關愛。
寫到這裡,腦海中一一浮現了這些人的臉孔,心中無限溫馨。我 感到自己始終是很幸運的。但願這份學業的完成,能讓這些曾經啟發 我、幫助我、與我風雨同路的人,感到欣慰。
謝謝你們! WE did it!
Abstract
Avian speciation has long been attributed to spatial isolation that prohibits gene flow and that is accompanied by population size reduction, which enhances genetic drift. However, given that birds are the most mobile of terrestrial vertebrates, that geographic barrier should pose such
impediment to gene flow is rather counterintuitive and yet seldom
questioned. In Chapter 2 of my dissertation, I show that founder effect model, which assumes a small founder population size and the absence of post-divergence gene flow, failed to apply to the speciation of the royal spoonbill (Platalea regia) from the black-faced spoonbill (P. minor);
rather, models that considered post-divergence gene flow was much more probable for these species, and speciational bottleneck, if any, would have been very brief or the founder size would have needed to be much
larger. Furthermore, post-divergence gene flow was estimated to be quite substantial and span well over half of the divergence history. In Chapter 3, using the ratio of effective to census population size as a proxy for the level of population structuring, I showed that ancestral populations of the most recent common ancestors of some spoonbill species (Platalea spp.) may be large and panmictic. Furthermore, I found that population size of the common ancestor of all spoonbills and the common ancestor of the spoonbills and the scarlet ibis (Eudocimus ruber) was surprisingly large, and reasoned that this may be due to population structuring which can be depicted by a Wrightian island model- that is, one in which
populations exchange migrants persistently but remain genetically differentiated. Taken together, these results suggest a more important role for selection in promoting divergence despite gene flow. Finally, applying the ABC method to mirosatellite genotype data of the
endangered black-faced spoonbill, I found that rather than anthropogenic
disturbances, climatic changes at the onset of the last glacial period have had the greatest impact in reducing the species' effective population size. Moreover, current effective population size of black-faced spoonbill was estimated to be small, and calls for continuous human efforts to reduce environmental stochasticity in the hope of bettering the species' chances of persisting in the long run.
中文摘要 中文摘要中文摘要 中文摘要
長久以來,鳥類的種化被認為是由族群在空間上的隔離所啟動,
空間隔離所導致的族群間基因交流中斷及族群量的縮減,會進一步促 使各分隔族群內的遺傳漂變,而造成族群分化,最終以致種化。然而,
對於陸生脊椎動物中運動能力最強的鳥類而言,地理屏障對其族群間 基因交流所形成的障礙,是不近情理的假設,卻少被質疑。在我論文 的第二章,我以核基因片段為材料,說明分布於澳洲的皇家琵鷺
(Platalea regia)之物種形成,無法由前提是起始於少數奠基者及物種 形成過程中完全缺乏與其姊妹種黑面琵鷺(P. minor)間進行“分化後 基因交流“(post-divergence gene flow)的奠基者種化(founder speciation)模式加以解釋;相對的,我的遺傳證據較支持包含“分化 後基因交流“的種化模式; 我的結果也顯示 ,奠基者種化所需的瓶 頸事件(bottleneck)即便存在,所歷時間應極為短暫,或者所謂奠基者 的數目會遠大於一般所設想。我同時也發現皇家琵鷺與黑面琵鷺的祖 先在兩者開始分化之後,彼此間仍有相當程度的基因交流,而這樣交 流所歷時間,更佔兩者分化至今一半以上的時間。在第三章中,以核 基因序列所推估的遺傳有效族群量(effective population size)及普查 族群量(census population size)之比例,作為評估物種內族群結構化
(population structuring)的指標,我的結果顯示了部份琵鷺的祖先物 種可能是由數量大且不具明顯結構的族群所構成。我也發現所有琵鷺 的共祖及琵鷺與紅鹮(Eudocimus ruber)的共祖之有效族群量都出奇 的大,這現象可能是由於這兩者的族群結構是更近似於 Wright 所提 出的島嶼模式,也就是物種由幾個分化但彼此間持續進行基因交流的 族群所構成。這些結果都指出選汰壓力在物種的分化上,較阻斷基因 交流(地理隔離)扮演更重要的角色。最後在第四章中,我應用近似
貝氏計算(approximate Bayesian calculation)分析黑面琵鷺的微衛星多 態性數據,發現這個瀕危物種的有效族群量縮減,受到最近一次冰期 啟動之影響,遠勝於近期人為干擾的效應;而根據核基因座及微衛星 多態性所估出的現時黑面琵鷺有效族群量並不大,因此持續以人為努 力減少環境波動對其族群數量的影響,應為維持黑面琵鷺長期存續之 必要措施。在最末章中,我針對以上的結果作出綜合討論,並提出未 來研究的展望。
Chapter 1 General introduction
How species form is a question that has intrigued biologists since Darwin’s time. In this introductory chapter, I begin by providing a historical overview of the philosophical stances regarding the geography and demography of speciation, and the methods traditionally used to investigate the prevalence of these models in nature. I then review recent advents of divergence population genetics that are providing better means for testing some of the fundamental demographic assumptions of various geographic speciation modes, thereby introducing a shift in paradigm. My PhD studies are an exploitation of these theoretical frameworks in
investigating the speciation mode of the spoonbills and, as a byproduct, in resolving some of the demographic parameters critical for the
conservation of the endangered black-faced spoonbill, as introduced at the end of the chapter.
Speciation mode: from Darwin to the Modern Synthesis
Darwin’s observation of continuous phenotypic variation led him to
consider speciation more likely the product of natural selection that works gradually within large panmictic population over diverse environmental substrates (Darwin, 1859). His view remained popular for nearly a century since the publication of The Origin of Species. The first half of the 20th century saw the rediscovery of Mendel’s laws of inheritance and the emergence of theoretical as well as experimental population genetics, the foundation of the latter being laid largely by Fisher, Wright, Haldane
and Dobzhansky (reviewed in Crow 1990, 1992, Powell 1987, Orr 1996;
Wright 1968, 1969, 1977, 1978). Because their works bridged Darwin’s idea of genetic gradualism and natural selection with Mendelian genetics, the term ‘Modern Synthesis’ was coined by Huxley (1942) to collectively refer to these efforts. However, population geneticists at the time were so occupied with studying evolutionary forces- e.g. genetic drift, selection and mutation- that affect genetic variation at intra-specific level that no explicit connections were made between these processes and the origin of species.
It was not until later that the link between population genetics and speciation was illuminated and became influential. Bateson (1909), Dobzhansky (1937) and Muller (1942) independently developed the model of divergence characterized by the accumulation of genetic incompatibility between populations (later known as the
Bateson-Dobzhansky-Muller model), and thought that such kind of divergence most likely takes place in allopatry. In light of their works, Mayr, a naturalist and taxonomist by training, sought to synthesize population genetics with his observation of biogeographic patterns. In his Systematics and the Origin of Species (Mayr 1942), he noted that closely related species are mostly allopatric, and reasoned that spatial barrier must precede divergence event because the homogenizing effect of interbreeding is simply too powerful to be overcome by selection that causes differentiation. Also borrowing from Wright’s insights on the effect of genetic drift in his shifting balance model (Wright 1978), Mayr thought that divergence is most readily achieved in small populations, in which intensified genetic drift facilitates the formation of novel allelic combination. This eventually culminated in the proposal of founder principle and genetic revolution (Mayr 1942, 1954, 1963).
Mayr is arguably the first who made explicit connection between the geography and demography of speciation, and by providing intuitively plausible genetic models and large bodies of biogeographic patterns, he successfully championed allopatric speciation as the norm of speciation.
Phylogenies, divergence population genetics and changing paradigm Up to this point, discussion about speciation had been at best
qualitative. It was not until the early 1980s, owing to the emergence of molecular biology and phylogenetic analyses, that various speciation modes became statistically testable hypotheses. Note that by this time, speciation mode conventionally referred to the geography of speciation- the spatial setting had become an accepted proxy for the relative roles of various evolutionary forces, such as drift, gene flow and selection, during speciation. The most popular method of testing speciation mode was that of a phylogenetic comparative one (Lynch 1991). In practice, the degree of overlap of species range is mapped onto a phylogenetic tree, and the degree of spatial isolation and phylogenetic affinity among taxa is expected to differ according to different geographic speciation
mode. This practice opened a door to speciational studies that had not been possible except in few closely monitored species (e.g. the Darwin’s finches, Grant & Grant 2008) or model organisms (e.g. in the fruit flies Drosophila spp., reviewed in Moya et al. 1995). However,
phylogenetic comparative methods are not without limitation. The biggest problem is that the assumption of range immutability may often be violated in nature (reviewed in Losos & Glor 2003). Another equally fallacious assumption is that population splitting is instantaneous at the
time of divergence, when in fact a phylogeny says virtually nothing about the divergence process.
Towards the end of the 20th century and the beginning of the 21st century, aided by the advents in molecular genetic techniques, statistical methods and computation technology, theorists began to exploit the way DNA polymorphism is structured in extant species that potentially carries information regarding past demographic events (e.g. Hey 1994). Such 'paleo-population biology' (Takahata 1993) or divergence population genetics (DPG, Kliman et al. 2000) studies integrate classical population genetics, genealogical coalescence (Kingman 1982) and molecular phylogenetics in explaining variation observed among taxa at higher taxonomic levels than conspecific populations. Models built in such frameworks (reviewed in Kuhner 2008, Beaumont 2002) allow for the estimation of a suite of past demographic parameters including gene flow, effective population size or population size change and thus have
profound bearing on the geography and process of speciation.
To date, most application of DPG methods have concentrated on model organisms such as humans, chimpanzees and Drosophila fruit flies (e.g. Hey 2005, Machado et al. 2002, Won & Hey 2005), the genetic resource for which can be readily obtained in large quantity. One of the interesting patterns that emerged from these studies is that there had been residual gene flow during species divergence, suggesting that strict
allopatry may not be necessary for speciation. Nevertheless, whether such a pattern holds for the majority of life forms remains to be validated in more taxa, and with the growing availability of genetic resources, it is becoming increasingly feasible to apply DPG methods to study the divergence of non-model species.
Birds, perhaps owing to their diverse morphology, complex behavior and the fact that they are easily observed, have always been a major source of insights on evolution. For examples, Mayr’s works on Melanesian birds led to the emphasis on spatial isolation in speciation (Mayr, 1942), while long-term studies on the Darwin’s finches have revealed an important role of selection (e.g. reviewed in Grant & Grant 1997).
Despite that birds are perhaps the most mobile of terrestrial vertebrates avian speciation by and large has been considered to result in allopatry (Price 2008). Such a paradigm merits full re-examination for a number of reasons. First, literatures that champion such a stance draw heavily from observation of island taxa, for which geographic isolation may be more accountable for divergence (Mayr 1942, Price 2008). However, the majority of avian diversity resides in continents, as acknowledged by Mayr himself, where allopatry seems less likely a major impediment to gene flow. Climatic fluctuation, particularly recent glaciations, have also been suggested to promote avian speciation in allopatry as the result of habitat fragmentation during glacial period, rising of sea level during interglacial periods, or postglacial founder events (e.g. Lovette 2005, Weir & Schluter 2004). Yet most modern birds arise long after the formation of major geological barriers and even if these and climatic changes were important in impeding gene flow, the effect would not have been instantaneous since such processes would take place over a
protracted period. Phylogenetic comparative studies that suggest the prevalence of allopatric speciation in birds are also questionable due to the potential invalidity of the method's underlying assumptions, as mentioned earlier.
The spoonbills
The primary focus of my dissertation is to apply recently developed DPG methods in deciphering the speciational demography of the group of avian species known as the spoonbills. Spoonbills refer exclusively to all the members of the genus Platalea (family Threskiornithidae, order Ciconiiformes); these birds are characterized by bills that are long, straight and flatten and broad near the distal end, which are used to engage in a unique ‘sweeping’ forage activity. There are six spoonbill species currently identified worldwide: the black-faced spoonbill (P.
minor) distributed in Eastern Asia, Eurasian spoonbill (P. leucorodia) distributed across the entire Palearctic region, African spoonbill (P. alba) in the African continent, royal spoonbill (P. regia) and yellow-billed spoonbill (P. flavipes) in Australasia, and roseate spoonbill (P. ajaja) in the Americas (Figure 3.1). Substantial morphological and ecological variation exists among the spoonbills. For example, P. minor, P. regia, and P. leucorodia are strikingly similar in terms of the overall white plumage and black bill and legs, which is drastically different from P.
alba, P. flavipes and P. ajaja whose plumage bill and legs are red or yellow, the latter is also conspicuously characterized by the overall pink plumage. While most spoonbills utilize freshwater habitat, P. minor is entirely coastal and utilize saline habitats and P. ajaja has a close affinity to mangroves.
Given that morphologically similar spoonbills occur mostly in near paraptry or complete allopatry (for example, P. minor, P. regia, and P.
leucorodia), it is natural to assume that some of these species arose via founder event. Chapter 2 of my thesis provides a test of founder
speciation in P. regia. Specifically, I asked whether divergence between P. regia and its sister species was associated with the absence of gene flow and initial population bottleneck. I also sought to evaluate the likelihood of alternative scenarios and estimate the values of relevant demographic parameters. In Chapter 3, I focused on the demographic state that preluded spoonbill divergences, i.e. the effective population of the ancestral species. Chapter 4 provides a case of conservation genetic study of the black-faced spoonbill. In short, my works can be viewed as a concerted effort in exploiting the genomic signal of past demographic events that could shed light on important evolutionary issues, such as the process of speciation, and provide useful information in conserving endangered species.
Reference
Bateson W (1909). Heredity and variation in modern lights. In Darwin and Modern Science (ed. Seward AC). Cambridge University Press, Cambridge.
Beaumont MA, Zhang W, Balding DJ (2002) Approximate Bayesian Computation in Population Genetics. Genetics, 162,: 2025-2035.
Crow JF (1990) R. A. Fisher, a centennial view. Genetics, 124, 207-211.
Crow JF (1992) Centennial: J. B. S. Haldane, 1892-1964. Genetics, 130, 1-6.
Darwin C (1859). The Origin of Species by Means of Natural Selection, or the Preservation of Favoured Races in the Struggle for Life.
John Murray, London.
Dobzhansky T (1937). Genetic and the Origin of Species. Columbia University Press, New York.
Grant PR, Grant BR (1997) Genetics and the origin of bird species. PNAS, 94, 7768-7775.
Grant BR, Grant PR (2008) Fission and fusion of Darwin's finches populations. Philosophical Transactions of the Royal Society of London B, 363, 2821-2829.
Hey J (1994). Bridging phylogenetics and population genetics with gene tree models. In Molecular Ecology and Evolution: Approaches and Applications (eds. Schierwater B, Wagner GP, DeSalle R) Birkhâuser, Basel, Verlag, Switzerland.
Hey J (2005) On the number of New World founders: a population genetic portrait of the peopling of the Americas. PLoS Biology, 3(6): e193.
Huxley J (1942) Evolution, the Modern Synthesis. Allen & Unwin, London.
Kingman JFC (1982) The coalescent. Stochastic Process and their Applications, 13, 235-248.
Kliman RM, Andolfatto P, Coyne JA et al. (2000). The population genetics of the origin and divergence of the Drosophila simulans complex species. Genetics, 156, 1913-1931.
Kuhner, M. K. (2008). "Coalescent genealogy samplers: windows into population history." Trends in Ecology & Evolution, 24: 86-93.
Lynch M (1991) Methods for the analysis of comparative data in evolutionary biology. Evolution, 45, 1065-1080.
Losos JB, Glor RE (2003) Phylogenetic comparative methods and the geography of speciation. Trends in Ecology and Evolution, 18, 220-227.
Lovette IJ (2005) Glacial cycles and the tempo of avian speciation.
Trends in Ecology and Evolution, 20, 57-59.
Machado CA, Kliman RM, Markert J et al. (2002) Inferring the history of
speciation from multilocus DNA sequence data: the case of
Drosophila pseudoobscura and close relatives. Molecular Biology and Evolution, 19, 472-488.
Mayr E (1942) Systematics and the Origin of Species. Columbia University Press, New York.
Mayr E (1954) Changes in genetic environment and evolution. In Evolution as a process (eds. Huxley JS, Hardy AC, Ford EB) Allen & Unwin, London.
Mayr E (1963) Animal species and evolution. Harvard University Press, Cambridge.
Moya A, Galiana A, Ayala FJ (1995) Founder-effect speciation theory:
failure of experimental corroboration. PNAS, 92: 3983-3986.
Muller HJ (1942) Isolating mechanisms, evolution, and temperature.
Biology Symposium, 6, 71-125.
Orr HA (1996) Dobzhansky, Bateson, and the genetics of speciation.
Genetics, 144, 1331-1335.
Powell JR (1987) “In the Air"- Theodosius Dobzhansky's Genetics and the Origin of Species. Genetics, 117, 363-366.
Price T (2008) Speciation in Birds. Roberts & Company, Greenwood Village, CO.
Takahata N (1993) Mechanisms of molecular evolution: introduction to molecular paleopopulation biology. Sinauer Associates,
Sunderland, MA.
Wakeley J, Hey J (1997) Estimating ancestral population parameters.
Genetics, 145, 847-855.
Weir J, Schluter D (2004) Ice sheets promote speciation in boreal birds.
Proceedings of Biological Sciences, 22, 1881-1887.
Won YJ, Hey J (2005) Divergence population genetics of chimpanzees.
Molecular Biology and Evolution, 22, 297-307.
Won YJ, Sivasundar A, Wong Y et al. (2005) On the origin of Lake Malawi cichlid species: A population genetic analysis of divergence. Proceedings of the National Acadmy of Sciences, 102(suppl. 1), 6581-6586.
Wright S (1968) Evoution and the Genetics of Populations, Vol. 1:
Genetics and Biometric Foundations. University of Chicago Press, Chicago.
Wright S (1969) Evolution and the Genetis of Populations, Vol. 2: The Theory of Gene Frequencies. University of Chicago Press, Chicago.
Wright S (1977) Evolution and the Genetics of Populations, Vol. 3:
Experimental Results and Evolutionary Deductions. University of Chicago Press, Chicago.
Wright S (1978) Evolution and the Genetics of Populations, Vol. 4:
Variability Within and Among Populations. University of Chicago Press, Chicago.
Chapter 2 Testing founder speciation in a wader: divergence genetics of the spoonbills (Platalea regia and P. minor)
Abstract
Although founder speciation has been a popular theoretical model for the origin of species featuring marked geographic isolation from its closest relative, its empirical importance has remained controversial and difficult to evaluate. In this study, using approximate Bayesian computation, I tested the validity of two major assumptions of founder speciation- that is, small founder population size (speciational bottleneck) and the absence of post-divergence gene flow- in a disjunctively distributed spoonbill
species Platalea regia, which occurs in Australasia, with its sister species P. minor, which occurs in eastern Asia. My results suggest that models which allowed for post-divergence gene flow was significantly more probable than those that did not. Intriguingly, post-divergence gene flow between the two spoonbills persisted for a considerably long period of time (roughly 70% of divergence time) and its magnitude was likely substantial (most probable estimate of asymmetrical gene flow 4Nm ranged from 59 to 66 from P. regia to P. minor and 49 to 70 from P.
minor to P. regia). On the other hand, speciational bottleneck was not necessary in explaining the observed extant genetic variation; even if there was a bottleneck, it would have lasted for a short period (≈100 generations) or else the size of founder population would have needed to be much larger (>50 effective individuals). Based on these results, I was able to reject the founder speciation for the two spoonbills, and propose that their divergence should have been driven mainly by selection acting
on evolutionarily labile trait with significant fitness consequences, such as migratory behavior.
Introduction
The primacy of various geographic speciation modes has been hotly contested in the field of evolutionary biology. Specifically, founder speciation (Mayr 1942), a variant of allopatric speciation also known as peripatric speciation, is arguably the favored explanation for the origin of species featuring geographic isolation (e.g. organisms on the Hawaiian archipelago, reviewed by Coyne & Orr 2004) or that disperse rarely but survive well in new environment (e.g. Paulay 2002). In a founder speciation scenario, a characteristically small number of individuals colonize distant area where they remain isolated from the parental population; initially, increased genetic drift as a result of extreme
bottleneck accelerates the formation of novel allelic combination that is adapted to the new environment, where selective pressures may be
relaxed or different, consequently allowing the population to grow rapidly (e.g. Mayr 1942, 1954; Carson 1968; Kaneshiro 1980; Templeton 1980;
Carson and Templeton 1984). Such ‘genetic revolution’ (Mayr 1954), however, has been subjected to long-run theoretical debates (e.g.
Charlesworth & Smith 1982; Barton & Charlesworth 1984; Rouhani &
Barton 1987, Charlesworth & Rouhani 1988; Barton 1996; Slatkin 1996;
Gavrilets & Hastings 1996) that are little resolved due to the lack of consistent model assumption regarding selection intensity or the genetic basis of divergence, which is difficult to verify in the wild.
On the other hand, founder speciation may be more conveniently validated by examining speciational demography of natural
populations. Close monitoring of the consequences of founding event has only been possible in very few studies. For example, testing founder speciation in model organisms with short life span such as the fruit fly (Drosophila spp.) has generated inconsistent results wherein reproductive isolation had a chance of arising only after multiple bottlenecks in the laboratory (e.g. as reviewed in Moya et al.1995). In the wild, a nearly 30 years study on the large ground finch Geospiza magnirostris indicated active and prolonged dispersal into the presumably isolated population on the island of Daphne Major, and failed to find predicted decrease in
genetic diversity (Grant et al. 2001). In fact, in a population founded by two mouflon Ovis aries individuals, heterozygosity had actually
increased possibly owing to selection (Kaeuffer et al. 2007). In most other cases, empirical validation of founder speciation had relied on phylogenetic comparative methods (reviewed in Losos & Glor 2003)- specifically, founder effect is deemed responsible for speciation if sister taxa are currently allopatric and the range of one taxon is small relative to the other. Applying these methods have yielded results in support of (in birds, Friesen & Anderson 1997; Barraclough & Vogler 2000) or against (e.g. in birds, Chesser & Zink 1994; in felids, Mattern & McLennan 2000) founder speciation, but re-evaluation may be necessary because extant species range as the key evolutionary trait in such studies is highly labile and therefore unreliable (reviewed in Losos & Glor 2003). More
importantly, emphasizing the spatial setting of divergence often assumes prejudiced demographic correlates that are in fact grounded on little evidences- there is no good reason to believe that small species range necessarily results from founding event, and that colonization occurs once and for all.
The advents of population and evolutionary genetics in the past couple of decades encourage a return to addressing the demographic attributes of speciation that may yield more insights into the geography of speciation than, ironically, extant geography itself. For instance, genetic analyses have shown that population size at incipient speciation could be quite large (e.g. Darwin’s finches, Vincek et al. 1997; the silvereye species complex Zosterops lateralis, Clegg et al. 2002; Lagenorhynchus dolphins, Hare et al. 2002; the auklets Aethia spp. Walsh et al. 2005), as opposed to the 100 or fewer individuals generally expected for a founder population (Coyne & Orr 2004); nevertheless, historical bottleneck could not be rejected in some cases (e.g. Z. lateralis population on Norfolk Island, Clegg et al. 2002; beetle Orphraella bilineata, Knowles et al. 1999). On the other hand, the amount of historical gene flow between species is also of growing interest (e.g. in Drosophila fruit flies, Machado et al. 2002; in human, Hey 2005; in chimpanzees,Won & Hey 2005; in Icterus orioles, Kondo et al. 2008; in cave salamanders Gyrinophilus spp., Niemiller et al.
2008; in Solanum wild tomatoes, Stadler et al. 2008), but has not been studied in the context of founder speciation- that is, the complete
cessation of gene flow since the founding of geographically disjunctive taxa.
In this study, a recently developed DPG method was used to investigate the speciational demography of a pair of closely related spoonbill species. The spoonbills (genus Platalea) are waders
distributed globally and appear nearly allopatric from one another. In particular, the royal spoonbill (P. regia) that is mainly resident in
Australasia bears great morphological resemblance to its two
non-Australasian congeners, the black-faced spoonbill (P. minor) and the Eurasian spoonbill (P. leucorodia) which is distributed in eastern Asia and the Palearctic region, respectively (Figure 2.1, distribution according to Hancock et al. 1992). The fact that P. regia and its relatives occur on vastly separated continents which were never in physical proximity
throughout geological history suggest the potential for founder speciation.
I specifically sought to validate the two pillars of founder speciation in a strictly allopatric sense, namely speciational bottleneck and the absence of post-divergence gene flow in the speciation of P.
regia. Genetic variation at up to 20 independent nuclear fragments, which summed up to 11,941 bp, was used to infer the likelihood of demographic models, each differing in its allowance for speciational bottleneck and/or post-divergence gene flow, as well as to estimate model parameters for the divergence between P. regia and its sister species via coalescent simulation and appoximate Bayesian computation (ABC, Beaumont et al. 2002). My results suggest that P. regia did not
experience bottleneck upon divergence from its closest relative P. minor and that gene flow was continuous between these two species throughout most of the divergence period. Such a study should yield insights into a framework for explaining the global diversity and distribution of
waterbirds.
Materials and Methods
Sampling and DNA Preparation
Blood, feathers, muscle or liver tissues or toepad tissues of museum skins were sampled for all six species of spoonbills: the royal spoonbill (P.
regia), black-faced spoonbill (P. minor), Eurasian spoonbill (P.
leucorodia and P. l. archerii), African spoonbill (P. alba), yellow-billed spoonbill (P. flavipes) and roseate spoonbill (A. ajaja) (from the
American Museum of Natural History, voucher no.: AMNH35621 and AMNH37454). The scarlet ibis (Eudocimous rubber;
AMNH-PAC1060), sacred ibis (Threskiornis aethiopicus; AMNH-JJ002), great egret (Ardea alba; AMNH-JJW002) and black-crowned night heron (Nyctiorax nycitcorax) were also included as outgroup taxa for
phylogenetic analysis. Fresh tissue samples were stored in 100% ethanol in the field and transferred to a -80oC freezer for long term
storage. Genomic DNA was extracted from fresh tissue samples using a modified LiCl method (Gemmell & Akiyama 1996). DNA of toepad samples was extracted, using the Qiagen DNeasy Kit (QIAGEN), in a separate room located on a different floor from the main laboratory to avoid aerosol contamination of amplicons, followed by ‘primerless’
polymerase chain reactions (PCR) (Stemmer 1994) to recover historical DNA fragments in greater length.
Mitochondrial and nuclear DNA amplification and sequencing To identify the sister species of P. regia, molecular phylogenetic
relationships among all spoonbills were reconstructed with sequence data of the nearly complete mitochondrial NADH dehydrogenase subunit 2 (ND2) gene (1024 bp) and part of the cytochrome b (CYTB) gene (769 bp). Sequences of primers used to amplify these two mitochondrial regions are provided in Table 2.1. A touchdown PCR scheme was
employed to amplify these fragments in reaction volumes of 12μL
containing around 50 to 200 ng of template DNA, 0.2μM of each primer, 0.5 mM dNTP, 10 mM Tris-HCl, pH9.0, 50 mM KCl, 0.4 U Taq DNA polymerase (GE Biosciences) and 1.5 mM MgCl2. PCR thermoprofile included denaturing at 94oC for 2 minutes, followed by 10 cycles of 94 oC for 30 seconds, 30 seconds at 60 oC ramping down to 50 oC at 1 oC per cycle, and then 72 oC for 45 seconds, and 30 cycles in which annealing temperature remained at 50 oC, then a final extension at 72 oC for 7 minutes. Sequencing reactions were performed using DYEnamic ET Dye terminator cycle Sequencing Kit for MegaBACE (GE Biosciences) and electrophoresised on MegaBACE 1000 autosequencer (GE
Biosciences). Sequences were proofread, assembled, and indels were removed with the aid of software SEQUENCHER (V4.7, Gene Codes Corporation).
Multiple individuals of P. regia and its sister species, as well as single samples of other spoonbills would be genotyped at 17 nuclear autosomal loci and a Z-linked locus which was derived from a vinous-throated
parrotbill Paradoxornis webbianus spleen cDNA library and annotated by blasting against the chicken genome (Yeung and Li, unpublished data), and two additional published Z-linked genes (Backström et al. 2006;
Table 2.1). We employed PCRs the same as those described earlier except that extension time was increased to 1 min 30 sec. To determine individual haplotypes, genotypes of nuclear loci were phased into
different haplotypes for each individual using the software PHASE (Stephens & Scheet 2005; Stephens et al. 2001) which implemented likelihood reconstruction of haplotypes from population data. For each
locus, 20000 iterations were performed while thinning at every 20 steps and discarding the first 100 samples as burn-in; individuals whose
genotypes at a given locus could not be phased with probability of >60%
were excluded at that locus. To ascertain the number of chromosomes sampled at Z-linked loci, we sex-typed individuals which were typed at the three Z-linked loci using primers published in Hornfeldt et al. (2000) and PCR conditions in Yeung et al. (2006).
Data Analyses
Mitochondrial genetic distances and phylogeny reconstruction of Platalea spp.: For the total 1793 bp mitochondrial fragment, Tamura-Nei distance (Tamura & Nei 1993) was calculated for each Platalea species pair using MEGA 4 (Tamura et al. 2007) to account for the base inquality and unequal substitution rates among bases. Phylogenetic relationships among the spoonbills and outgroup were reconstructed via Bayesian analysis using MrBayes 3.1.2 (Ronquist & Huelsenbeck 2003) under the GTR+I+G model, which permitted more thorough exploitation of
parameter sample space (Huelsenbeck & Rannala 2004); two independent Markov chains, each of 10,000,000 steps, were run while sampling at every 1,000 steps and discarding the first 2,500,000 sampled trees as burn-in. A maximum likelihood method was also employed using software PHYML-aLRT (Guindon & Gascuel 2003; Anisimova &
Gascuel 2006), prescribing a TVM+I+G model as proposed by
MODELTEST 3.7 (Posada & Buckley 2004): base frequency for A, T, C and G was 0.2564, 0.2032, 0.2479 and 0.2926, respectively; rates of
nucleotide substitution relative to that between G\T was 1.2059 between A\C, 4.9892 between A\G, 0.3715 between A\T; 0 between C\G, and 4.9892 between C\T; proportion of invariable sites was 0.4004 and gamma distribution shape parameter was 0.9022 (-lnL=6497.1245, K=9, AIC=13012.2490); non-parametric branch supports were based on
Shimodaira-Hasegawa-like procedure. Royal spoonbill’s sister species was identified according to mtDNA phylogeny; together these two species formed the focal species pair of this study.
Genetic diversity, recombination, linkage, neutrality tests: For each of the two focal species, I calculated genetic diversity indices such as the number of segregating sites (s) and haplotypes (h), pair-wise nucleotide diversity (π), Tajima's D (TD) and Fu and Li's D* (FLD) using DnaSP 4.90.1 (Rozas et al. 2003). In addition, I used the program LDhat2.1 (McVean et al. 2002) to estimate recombination paramter ρ (ρ=4Nr where N is the effective population size and r is the recombination rate) for each locus in the two respective species. Interspecific summary statistics were also calculated, including the number of polymorphic sites that are shared between the two species (Ss), unique to each species (e.g. SPr for P. regia), or fixed in either species (Sf) as defined in Wakeley & Hey (1997); a mutation was identified as ancestral or derived by comparing with sequences of P. leucorodia, P. flavipes and P. ajaja using parsimony criterion (Madison & Madison 2000) within a phylogenetic
framework. Gametic disequilibrium was tested between each pair of loci, using the program GENEPOP (Raymond & Rousset 1995) with
Bonferroni correction for bias of type I error arising from 136 pairwise comparisons (hence α value was adjusted to 0.000238). For all loci
except those that are Z-linked, conformation to Hardy-Weinberg (HW) equilibrium tested using software CERVUS 2.0 (Kalinowski et al. 2007).
For each locus, the average number of nucleotide differences between the two focal species was calculated for each locus using MEGA 4
(Tamura et al. 2007); this information was then used in a multi-locus HKA test for molecular neutrality (Hudson et al. 1987) across all loci, as implemented in the program HKA (available from Jody Hey at
http://lifesci.rutgers.edu/~heylab/HeylabSoftware.htm).
Current gene flow: To ascertain that there is no current gene flow between the two focal spoonbill species, which could confound
estimation of historical gene flow, we inferred the likelihood that dataset of the two species combined represents K populations at HW equilibrium using the program Structure 2.2 (Pritchard et al. 2000). The three
Z-linked loci were excluded from analysis due to non-diploid inheritance;
also excluded was any locus at which observed heterozygosity differed significantly from HW expectation. To avoid assignment errors, only individuals that were typed at least nine out of a total of 17 loci were included in analysis. I performed three runs of identical model assumptions, which were ancestral admixture and correlated allele frequencies among populations, for each of the proposed number of
populations (K= 1 to 6) with 20000 MCMC (Monte Carlo Markov Chain) replications recorded after burn-in of the first 2000 replications.
Approximate Bayesian inference of speciational demography: I used the ABC method to infer the likelihood of alternative demographic models (Beaumont 2007) as well as relevant demographic parameters (Beaumont et al. 2002) for the divergence of royal spoonbill and its sister species. I began by constructing four models of interest (Figure 2.2), each characterized by essential parameters such as the long-term equilibrium effective population size of each of the two species (e.g. NPr
for P. regia), population size of the ancestral species (NA) and divergence time in generations (T1). Additionally and more importantly, the four models differed in their allowance for asymmetrical post-divergence gene flow between the focal species pair (M = 4Nm, where m is the proportion of migrants each generation) until some time ago (T2) and/or speciational bottleneck experienced by P. regia (Nb, size of bottlenecked population) for a period of time (Tb).
To select the model that best explains genetic polymorphism observed in the two focal species, one million multilocus dataset were simulated, using the program MSNSAM (Hudson 2002; Ross-Ibarra et al. 2008), given parameter values randomly drawn from uniform prior distributions while assuming the observed sample size for each locus. For all
equilibrium effective population sizes, N was a random variable between 100 and 100,000, and scaled by locus-specific mutation rate to yield θ value required by the program. Substitution rate of each locus was deduced by first calculating divergence between each of the focal species and a more distantly related spoonbill in average net Tamura-Nei
distances; average of the two pairwise comparisons was then compared with averaged divergence at mitochondrial CYTB gene
(0.0849). Mutation rate per gene per generation was obtained by multiplying this ratio by the Ciconiiformes CYTB molecular clock of 1.05x10-8 substitution per site per year (Weir & Schluter 2008), locus length and generation time of 10.7 years (deduced for the black-faced spoonbill, Yeung et al. 2006)(Table 1.7). M for both directions was between 0.0001 and 100. Tb in the royal spoonbill was assumed to be between 50 to 500 generations. Prior for Nb was set as one to 50 effective individuals; note that this may be a conservative range because the size of bottleneck in a founder speciation scenario is generally accepted as
between one to 100 census individuals (Coyne & Orr 2004), whose Ne is likely to be much smaller than 50 given that Ne is on average 1/10 of census size in natural populations (Frankham 1995). For T1 I assumed a naïve prior between 51 and 467,290 generation; the lower bound ensures that it is at least one generation longer than the duration of bottleneck, while the upper bound is equivalent to the time divergence from the most distantly related spoonbill as suggested by mitochondrial
phylogeny. The time T2 at which gene flow completely ceased was taken to be a fraction of divergence time T1. All time estimates were scaled to be in units of 4NPr generations as required by the
program. Prior for ρ was set as uniformly distributed between the lowest and highest value estimated in the two species.
The ABC procedure basically followed that described in Beaumont, Zhang and Balding (2002). Ten summary statistics, namely π, TD, FLD for each species and Ss, S in each species when compared to the other and Sf was calculated for each simulated dataset. Data whose
summary statistics were among the 0.01 percentile closest to the observed
statistics in terms of Euclidean distances were retained, weighted by the Epanechnikov kernel, and adjusted using local-linear regression. The posterior probabilities of models were then estimated by treating each model as a categorical variable in a weighted multinomial logistic
regression procedure (Beaumont 2007). For the model with the highest posterior probability, demographic parameters were tangent-transformed before estimation to ensure that posterior density falls within the range of prior distribution (Hamilton et al. 2005).
Results
Mitochondrial divergence and phylogeny
Genetic distances among spoonbill species is as given in Table 2.3. The greatest differences were observed between P. ajaja and all the other spoonbills (average Tamura-Nei distances was around 0.087); the least difference was observed between P. minor and P. regia (Tamura-Nei distance = 0.015). Topology of phylogenetic trees deduced by Bayesian and ML analyses were identical (Figure 2.3) and appeared ladder-like: the most basal species is P. ajaja, followed by P. flavipes, P. alba, P.
leucorodia and the two terminal species P. minor and P. regia. Nodal support was high (Bayesian posterior probability was 100%; bootstrap value in PHYML analysis was 79-100%; aLRT value was 90-100%) for all nodes except for containing all the other spoonbills except P. ajaja (Bayesian posterior probability= 55%; bootstrap value in PHYML analysis= 49%; aLRT value= 43%). P. ajaja has formerly been
suggested to comprise a separate genus Ajaja due to the species' distinct morphology (Allen 1942; Vestjens 1975), but my result here suggests that
P. flavipes is just as likely to be more closely related to P. ajaja than to other spoonbills, it seems the most appropriate to retain P. ajaja within the genus Platalea.
Both trees suggest the P. minor to be P. regia's sister taxon, hence forming the focal species pair in this study. In addition, P. flavipes was used in some of the following analyses as an outgroup species to the focal species pair.
Genetic diversity, recombination, linkage and neutrality test
Characteristics of nuclear fragments for both P. regia and P. minor are summarized in Table 2.4. Although there were about twice as many samples of black-faced spoonbill as royal spoonbill, levels of genetic diversity are similar between the two species (e.g. average for P. regia was 0.00136 and for P. minor was 0.00188). None of the loci appeared to be in linkage disequilibrium with another. All loci conformed to HW equilibrium with the exception of the FBP1 gene (p=0.0004), the LPL gene (p=0.0003) and ABCE1 gene (p=0.0297) in the P. regia, which is not surprising given that the former two genes are Z-linked and therefore were nevertheless kept in coalescent analyses since no other tests
suggested that they depart from neutrality; ABCE1 was excluded from allelic frequency-based Structure analysis but kept it in the coalescent analyses. Results of HKA test indicated that none of the loci is under selection since the ratio of within species polymorphism and between species divergence is relatively constant across all loci in both species (p=0.598). No significant departure from neutrality was detected for any locus using the Tajima's D and Fu and Li's D* test (Table 2.4).
Current gene flow
Structure analysis showed that dataset containing ten P. regia and 19 P.
minor individuals typed for at least nine loci most likely consisted of two populations (for K = 2, LnP(D) ranged from -896.4 to -896.9. with small variance of about 73, Table 2.5). Conspecific individuals were assigned to the same population with very high probabilities (over 90%; Figure 2.4). I therefore conclude that there is no current gene flow between these two species and that any sharing of genetic polymorphism should be attributed to historical event(s).
Evolutionary and speciational demography
Results of model selection indicated that the posterior probability was the highest for model 4 (0.473) and slightly lower for model 2 (0.449),
both characterized by post-divergence gene flow but differed in its allowance for speciational bottleneck; posterior probability was much lower for model 1 (0.004) and model 3 (0.007), the two models that did not allow for post-divergence gene flow. Therefore, posterior densities of demographic parameters were estimated only for model 2 (Figure 2.5) and model 4 (Figure 2.6). For both models, posterior distribution of NPr
(mode= 2334, mean=9556, 95% credible interval, CI:9372-9741 in model 2; mode=7204, mean=7949, 95%CI: 7891-8007 in model 4), NPm
(mode=6447, mean=6806, 95%CI: 6751-6861 in model 2; mode=6453, mean=6507, 95%CI: 6435-6579 in model 4), T1 (mode=67275,
mean=83063, 95%CI: 82094-84033 in model 2; mode=61179,
mean=156559, 95%CI: 153751-159367 in model 4) and T2 (mode=13555,
mean=14475, 95%CI: 14364-14585 in model 2; mode=14059, mean=16581, 95%CI: 16426-16737 in model 4) was completely
bell-shaped with significantly non-zero mode value. On the other hand, that of NA (mode=34767, mean=48511, 95%CI: 47963-49059 in model 2;
mode=45483, mean=46973, 95%CI: 46450-47497 in model 4), Nb
(mode=44, mean=26, 95%CI: 25-26 in model 2), Tb (mode=102, mean=
267, 95%CI: 265-270 in model 2), MPmPr (mode=59, mean=48, 95%CI:
48-49: in model 2; mode=66, mean=55, 95%CI: 55-56 in model 4) and MPrPm (mode=49, mean=50, 95%CI: 49-50 in model 2; mode=70,
mean=52, 95%CI: 52-53 in model 4) was less clearly resolved around a moderately sloped non-zero peak. With respect to distribution mode, all parameters were similar between the two models except for NPr that was about three times larger in model 4 than that in model 2. Effective population size of the ancestral species appeared much larger than that of the extant species: NA was about 14.9 and 5.4 times larger than NPr and NPm, respectively, in model 2 and 5.7 to 7.0 times larger in model 4. By multiplying time estimates (the mode of model 2 and model 4) by the generation time of 10.7 years, divergence time was estimated to be around 654,615 to 719,842 years ago (ya) while post-divergence gene flow ceased around 145,038 to 150,431 ya.
Discussion
Nullifying founder speciation and alternative speciational process
In this study, I was able to reject founder speciation as the most probable model for the divergence between P. regia and P. minor, because two models with post-divergence gene flow (model 2 and 4) had significantly higher posterior probabilities than those without (model 1 and 3). More intriguingly, after the divergence of P. regia and P. minor around 0.6 to 0.7 mya, gene flow persisted for a considerably long period and ceased as late as about 0.15 mya. On the other hand, extremely small speciational bottleneck was not necessary in explaining extant genetic
polymorphism. Had a bottleneck indeed existed, my result suggests that its duration would have been short, or else bottleneck size would have needed to be larger than that defined in the current study, which was set in the spirit of founder speciation to be no more than 50 effective
individuals. That substantial and persistent post-divergence gene flow would blur the genetic signal of bottleneck may also be responsible for failing to distinguish model 2 and model 4. Taken together, these results overrule the importance of the two pillars of founder speciation- that is, the complete cessation of gene flow since divergence and small founding population size.
A prolonged period of post-divergence gene flow that spans nearly 70% of the divergence history suggests a more important role for selection (e.g. sexual or differential adaptive selection) than physical separation in causing speciation- i.e. speciation was parapatric or sympatric. Indeed, in an early meta-analytical account of North American amphibians and reptiles, Endler (1977) revealed more incidences of geographic differentiation among spatially continuous populations than those with disjunct distribution, undercutting its
differentiation has been supported by studies identifying ecology-based divergent selection that facilitates reproductive isolation between closely related taxa (e.g. reviewed in Rundle & Nosil 2005; Hendry et al.
2007). In these cases, differentiation is initiated at some traits that, depending on their degree of association with reproductive isolation, may lead to further reduction in gene flow. Such a process may be the most conceivable at the genic level. Wu (2001) proposed the concept of 'porous genome' in which gene flow between two populations ceases earliest at loci where selection is the strongest while the rest of the genome is free for introgression; as genomic regions of differential adaptation expand and develop linkage through coadaptation with one another, gene flow between the two genomes is reduced until
reproductive isolation arises to prevent further genetic
exchanges. Owing to the growing availability of genomic data, this concept has been corroborated empirically, for example, by the higher divergence level observed at genes of putative adaptive values (e.g.
candidate genes, coding or regulatory sequences) relative to that at
presumably neutral loci (e.g. non-coding sequences, (Osada & Wu 2005) or variable degree of isolation across different genomic regions (e.g.
Turner et al. 2005).
A scenario of gradual divergence may be further corroborated by the possible existence of population structure in the ancestral species. Here I found that the effective size of the ancestral species was about 5.4 to 14.9 times larger than that of extant species. A possibility for the
discrepancies between the size of ancestral and extant species, assuming no population size change since divergence (as suggested by the results of
was structured. It has been suggested that if structured subpopulations were taken as a whole, genetic estimate of population size would be much larger than that of either individual subpopulation, resulting in an
observed size ‘inflation’ (Wakely 1999, 2000). In another word, an unusually large ancestral population size may imply that the ancestral species consisted of differentiated populations (Zhou et al.
2007). Indeed, the ubiquity of population structure in birds (e.g. in chestnut-backed chickadee Poecile rufescens, Burg 2007; in satin bowerbirds Ptilonorhynchus violaceus, Nicholls et al. 2007; in black-capped vireos Vireo atricapill, Barr et al. 2008) reinforces a
pre-divergence population differentiation scenario in the spoonbills. The phenomenon of NA inflation has also been found in a few other studies (e.g. humans and chimpanzees, Chen & Li 2001; grass finches Poephila spp. Jennings & Edwards 2005; Lake Malawi cichlid Tropheops, Won et al. 2005; humans, Hey 2005; mangroves Sonneratia spp. Zhou et al.
2007).
Speciation in the spoonbills: divergence in habitat usage and migratory behavior
When looking for traits responsible for the initiation or aggravation of divergence, those that are conspicuously different between the species in question and are likely to be evolutionarily labile should be among the first to consider. For P. regia and P. minor, speciation could have been driven by differentiation through change in migratoriness. Mapping migratoriness within a phylogenetic framework, both P. leucorodia and P.
minor are long distance migrants, therefore it appears most parsimonious that long distance migration was lost in the P. regia. The ancestral
species of P. minor and P. regia may have been a seasonal migrant between temperate and subtropical East Asia; at some point in time a novel migration route developed which led to wintering quarters near or in Australasia. Divergence between populations at either side of the migratory divide may have been driven partially by reproductive isolation arising via assortative, possibly allochronic mating- for example, in the blackcap Sylvia atricapilla, individuals from different wintering quarters return to the same breeding site at different times, hence showing
temporal segregation in breeding (Bearhop et al. 2005). Selection may then have favored increased residency via assortative mating in
population that winter in Australasia, where the tropical to subtropical climates likely created environments of reduced seasonality in resources and intraspecific competition.
Although the genetic basis of migratoriness remains a mystery, the evolutionary lability of migratory behavior is hypothesized to be under the control of the threshold model of quantitative genetics, which posits that variation at relevant genes can be easily sustained within population (reviewed in Pulido 2007). The inherent variation of migratory
characteristics that probably prompted the divergence of P. minor and P.
regia may be analogous to those evident in contemporary
populations. Even though P. regia is largely known as resident in Australia, migration across the Torres Straits in large flocks has been reported; even within Australia, banding records have revealed highly variable movement distances ranged between 277 and 1472 km (as reviewed in Hancock et al. 1992). Migratory variation is also known in the focal species’ immediate kin P. leucorodia: multiple geographic
European, Western European and Eastern Asian regions, each with distinctive migratory route (Figure 2.1). Migratoriness is known to be polymorphic as resident populations are known at the southern or coastal rim of wintering ranges, such as in central India and Sri Lanka (P. l.
leucorodia), islands off the coast of West Africa (P. l. balsaci) and on the coasts and islands of the Red Sea (P. l. archerii). According to
mitochondrial phylogeny (Figure 2.3), P. l. archerii has a very recent divergence from the nominate species, suggesting that the loss of
migratoriness could be easily achieved. The body size of P. l. archerii is also smaller than the nominate species, implying that a change in migratory behaviour can potentially be accompanied or caused by other morphological/physiological modification.
Conclusion
Here I show that rather than founder speciation, a speciation model that involves protracted period of post-divergence gene flow is much more probable for P. regia and P. minor. As post-divergence gene flow is being reported in an increasing number of cases, a future direction for speciation study may be for biologists to shift their focus from the spatial setting of divergence to the mechanism that underlies adaptive
differentiation in the face of gene flow. Migratory birds, which are the most mobile of terrestrial vertebrates and therefore least limited by geographic barriers, would potentially yield many insights into such a speciation process.
Acknowledgment
I would like to thank PW Tsai and D Chang for their assistance with the ABC analysis. I thank T Chesser and CT Yao for providing samples in this study. I also thank members of the Genetic Diversity Lab for their technical support.
Reference
Allen RP (1942) The Roseate Spoonbill. Research Report No. 2. National Audubon Society, New York.
Anisimova M, Gascuel O (2006) Approximate likelihood ratio test for branches: A fast, accurate and powerful alternative. Systematic Biology, 55, 539-552.
Backström N, Brandström M, Gustafsson L, et al. (2006) Genetic
mapping in a natural population of collared flycatchers (Ficedula albicollis): conserved synteny but gene order rearrangements on the avian Z chromosome. Genetics, 174, 337-386.
Barr KR, Lindsay DL, Athrey G, et al. (2008) Population structure in an endangered songbird: maintenance of genetic differentiation despite high vagility and significant population recovery.
Molecular Ecology, 17, 3628-3639.
Barraclough TG, Vogler AP (2000) Detecting the geographical pattern of speciation from species-level phylogenies. The American
Naturalist, 155, 419-434.
Barton NH (1996) Natual selection and random genetic drift as causes of evolution on islands. Philosophical Transactions of the Royal Society of London B, 351, 785-795.
Barton NH, Charlesworth B (1984) Genetic revolutions, founder effects, and speciation. Annual Reviews of Ecology and Systematics, 15, 133-164.
mechanism for rapid evolution of a migratory divide. Science, 310, 502-504.
Beaumont MA (2007) Simulations, genetics and Human Prehistory- A focus on Islands University of Cambridge, Cambridge, UK.
Beaumont MA, Zhang W, Balding DJ (2002) Approximate Bayesian computation in population genetics. Genetics, 162, 2025-2035.
Burg T (2007) Phylogeography of chestnut-backed chickadees in western North America. In Ecology and Behavior of Chickadees and
Titmice: An Integrated Approach (ed. Otter KA). Oxford University Press, Oxford.
Carson HL (1968) The population flush and its genetic consequences. In Population Biology and Evolution (ed. Lewinton RC). Syracuse University Press, Syracuse, NY.
Carson HL, Templeton A (1984) Genetic revolutions in relation to
speciation phenomena: The founding of new populations. Annual Reviews of Ecology and Systematics, 15, 97-131.
Charlesworth B, Rouhani S (1988) The probability of peak shifts in a founder population. II. An additive polygenic trait. Evolution, 42, 1129-1145.
Charlesworth B, Smith DB (1982) A computer model of founder effect speciation. Genetics Research Cambridge, 39, 227-236.
Chen F-C, Li W-H (2001) Genomic divergence between humans and other hominoids and the effective population size of the common ancestor of humans and chimpanzees. American Journal of Human Genetics, 68, 444-456.
Chesser RT, Zink RM (1994) Modes of speciation in birds: a test of Lynch's method. Evolution, 48, 490-497.
Clegg SM, Degnan SM, Kikkawa J, et al. (2002) Genetic consequences of sequential founder events by an island-colonizing bird.