Original Article
Evaluation of folate status by serum and erythrocyte
folate levels and dietary folate intake in Taiwanese
schoolchildren
Kuan-Ju Chen
PhD1, Ning-Sing Shaw
PhD2, Wen-Harn Pan
PhD2,3,4and Bi-Fong Lin
PhD21
Department of Hospitality Management, Chung-Hwa University of Medical Technology, Tainan, Taiwan 2
Department of Biochemical Science and Technology, Institute of Microbiology and Biochemistry, National Taiwan University, Taipei, Taiwan
3
Institute of Biomedical Science, Academia Sinica, Taipei, Taiwan 4
College of Public Health, National Taiwan University, Taipei, Taiwan
The folate status and dietary folate intake of Taiwanese schoolchildren was investigated by analysis of both se-rum and red blood cell (RBC) folate levels and dietary folate intake in 1105 boys and 958 girls aged 6-13 years sampled from the Nutrition and Health Survey in Taiwan Elementary School Children 2001-2002 (NAHSIT Children 2001-2002). Mean serum folate levels were 18.3±8.8 nmol/L (8.1±3.9 ng/mL) in boys and 20.3±9.7 nmol/L (9.0±4.3 ng/mL) in girls. Mean RBC folate levels were 700±320 nmol/L (308±141 ng/mL) in boys and 751±347 nmol/L (331±153 ng/mL) in girls. The prevalence of serum folate deficiency was 1.4% in boys and girls, and the prevalence of marginal serum folate deficiency (7-14 nmol/L) was 31.1% in boys and 25.8% in girls. In addition, 8.5% of boys and 7.4% girls had RBC folate deficiency (RBC folate < 318 nmol/L), and 17% of chil-dren had marginal RBC folate deficiency (RBC folate of 318-454 nmol/L). Our results suggesting that Taiwanese schoolchildren have poor folate status especially during periods of rapid growth and development such as the transition from childhood to early adolescence (boys at age 12-12.9, girls at age 11-12.9). The average estimated folate intakes were 269±9 µg/d in boys and 259±9 µg/d in girls, and 42% of Taiwanese schoolchildren had a die-tary folate intake below 2/3 of the RDA, indicating a poor diedie-tary folate intake in this population. This study shows that the folate status of Taiwanese schoolchildren is currently inadequate and strategies are needed for im-provement.
Key Words: folate intake, serum folate, RBC folate, schoolchildren, NAHSIT 2000-2001
INTRODUCTION
Recent studies have shown that elevated plasma homocys-teine (Hcy) is associated with cardiovascular disease and venous thrombosis in children.1-3 Much previous research
has focused on adults and only a few studies have found a strong inverse association between plasma Hcy levels and folate levels in children.4-6 Adequate folate nutrition has
been emphasized in recent years because of the association of low folate status with neural tube defects,7 brain
devel-opment, and emergent cognitive functions in children.8,9
Such findings have led to increased attention being placed on folate status in children. However, data on folate status in children are sparse.
Folate status may be evaluated from serum/plasma, RBC levels and dietary folate intake.10 The serum/plasma folate
reflects recent folate status, whereas RBC folate levels are considered to be an indicator of long term status.11
There-fore, in addition to the evaluation of folate status in school-children by serum folate levels, RBC folate level is an important indicator for those children entering adolescence. Serum folate may be lower due to rapid tissue growth and development, but low RBC folate levels would imply poor body stores of folate which may affect adolescent devel-opment. Folic acid fortification has markedly improved
serum folate, and RBC folate levels in the United States and Canada.12,13 Recommended dietary intakes for folate
differ among countries and range from 300 to 400 µg/day.14,15 In Taiwan, the recommended Dietary
Allow-ance (RDA) for folate is 200 μg/d for children aged 6-6.9 yrs, 250 μg/d for 7-9.9 yrs, and 300 μg/d for 10-12.9 yrs.16
A previous survey, the Nutrition and Health Survey in Taiwan (NAHSIT 1993~1996), revealed that 9-13% of Taiwanese children aged 7-12 yrs had plasma folate insuf-ficiency, or RBC folate insufficiency.17 The NAHSIT
1993~1996, however included only 400 children, and the database on folate content for most vegetables and food produced in Taiwan was not fully available at the time.
Corresponding Author: Kuan-Ju Chen, Associate professor,
Department of Hospitality Management, Chung-Hwa University of Medical Technology, No.89, Wen-Hwa 1st st, Jen-Te Hsiang, Tainan 717, Taiwan, ROC; Bi-Fong Lin, Professor, Department of Biochemical Science and Technology, National Taiwan Uni-versity, No.1, Sec.4, Roosevelt Road, Taipei 10617, Taiwan, ROC
Tel: +886- 6-267-4567 ext.750; Fax: +886-6-2903181 (K.-J. Chen) Tel: +886-2-3366-4451; Fax: +886-2-2362-1301 (B.-F. Lin) Email: d89623701@ntu.edu.tw (K.-J. Chen); bifong@ntu.edu.tw (B.-F. Lin) Accepted 28 June 2007
In this NAHSIT Children 2001-2002 survey, we evalu-ated dietary folate intakes using the folate composition databank for a Chinese-style diet as described previ-ously.18,19 In addition, we also measured serum and RBC
folate to more comprehensively evaluate folate status in the Taiwanese schoolchild population.
MATERIALS AND METHODS
Subjects
The subjects in this study were schoolchildren sampled from the Taiwanese population using a multistage, strati-fied sampling method, as part of the NAHSIT Children 2001-2002. Detailed description of sampling design can be found in Tu et al’s report.20 The sample included 2063
subjects, comprising 1105 boys and 958 girls aged 6 to 13 yrs.
Measurement of folate
Serum and RBC folate were measured by a combined system of competitive immunoassay and chemilumi-nesence (IMMULITE 2000 analyzer, Diagnostic Products Corporation, LA, CA). This procedure involved the use of monoclonal antibodies, paramagnetic particles, and a chemiluminesence substrate. The light emitted was in-versely proportional to the concentration of folic acid. A series of quality control tests were performed to evaluate the precision of this assay. These tests confirmed both the between- and within-run consistency of this method. The Coefficients of Variation (CV) for serum folate, and RBC folate were 8.5 %, and 6.8%, respectively. Folate defi-ciency was defined as a serum folate < 7 nmol/L (3.0 ng/mL) or RBC folate < 318 nmol/L (140 ng/mL). Mar-ginal folate deficiency was defined as a serum folate tween 7-14 nmol/L (3.0-6.0 ng/mL) or RBC folate be-tween 318-454 nmol/L (140-200 ng/mL). Folate
insuffi-ciency was defined as a serum folate ≤ 14 nmol/L (6 ng/ml) or RBC folate ≤ 454 nmol/L (200 ng/ml).21-23
Dietary assessment
Dietary folate intake was assessed using data from 24-hour dietary recalls and calculated the dietary folate in-take using the folate composition databank.18 This was
prepared by integrating the information on food folate contents from 1) USDA Nutrient database for standard reference, 24 2) Bowes & Church’s food values of
por-tions commonly used25 3) folate contents for vegetables
commonly used in Taiwan.26 The RDA in Taiwan for
folate is 200 μg/d for children aged 6~6.9 yrs, 250 μg/d for 7~9.9 yrs, and 300 μg/d for 10~12.9 yrs.16 Subjects
with daily folate intake below 2/3 of the RDA were con-sidered to have inadequate folate intake.
Statistical analysis
Statistical analysis was carried out with the SAS program (SAS/STAT Version 8.2, SAS Institute, Cary, NC). As the NAHSIT Children 2001-2002 was conducted using a stratified, multistage probability design, sample weighting was used to account for the complex survey design in the variance estimates, using SUDAAN, SAS-callable ver-sion 8.2. The data were analyzed by gender, and age was divided into one year intervals (6-6.9, 7-7.9, 8-8.9, 9-9.9, 10-10.9, 11-11.9, and 12-12.9 yrs). Trends across tertiles were evaluated with linear regression. Student’s t test was used for the analysis of differences in continuous vari-ables, and the chi-squared test was used for analysis of differences in categorical variables. Differences were considered significant if p < 0.05.
RESULTS
Data presented in Table1 show that serum folate levels
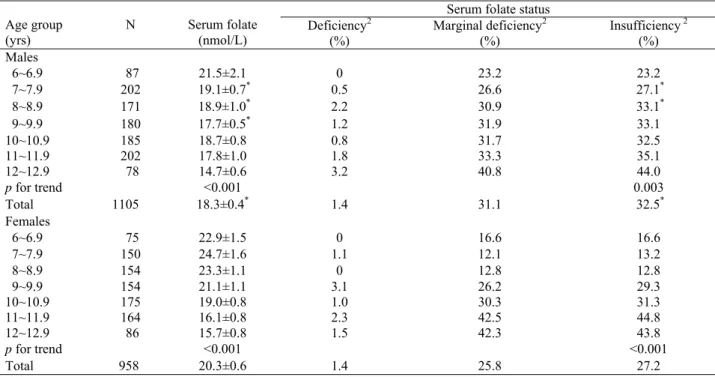
Table 1. Serum folate levels and folate status of Taiwanese schoolchildren by age1
Serum folate status Age group (yrs) N Serum folate (nmol/L) Deficiency 2 (%) Marginal deficiency2 (%) Insufficiency 2 (%) Males 6~6.9 87 21.5±2.1 0 23.2 23.2 7~7.9 202 19.1±0.7* 0.5 26.6 27.1* 8~8.9 171 18.9±1.0* 2.2 30.9 33.1* 9~9.9 180 17.7±0.5* 1.2 31.9 33.1 10~10.9 185 18.7±0.8 0.8 31.7 32.5 11~11.9 202 17.8±1.0 1.8 33.3 35.1 12~12.9 78 14.7±0.6 3.2 40.8 44.0 p for trend <0.001 0.003 Total 1105 18.3±0.4* 1.4 31.1 32.5* Females 6~6.9 75 22.9±1.5 0 16.6 16.6 7~7.9 150 24.7±1.6 1.1 12.1 13.2 8~8.9 154 23.3±1.1 0 12.8 12.8 9~9.9 154 21.1±1.1 3.1 26.2 29.3 10~10.9 175 19.0±0.8 1.0 30.3 31.3 11~11.9 164 16.1±0.8 2.3 42.5 44.8 12~12.9 86 15.7±0.8 1.5 42.3 43.8 p for trend <0.001 <0.001 Total 958 20.3±0.6 1.4 25.8 27.2
1 All valuesare shown as mean ± SE or percentage of participants. 2 Serum folate levels < 7 nmol/L (3.0 ng/mL) indicate deficiency;
between 7-14 nmol/L (3.0-6.0 ng/mL) indicate marginal deficiency; and ≤ 14 nmol/L (7.0 ng/mL) indicate insufficiency. *Significantly
tended to decrease with age in both sexes (p for trend < 0.001). Boys had significantly lower serum folate levels than girls, especially between the ages of 7 to 9.9 yrs. The prevalence of serum folate deficiency (< 7 nmol/L) was
the same for boys and girls, but the prevalence of mar-ginal serum folate deficiency (7-14 nmol/L) was 31.1% in boys and 25.8% in girls. Thus, the overall prevalence of serum folate insufficiency (≤ 14 nmol/L) was signifi-cantly higher in boys (32.5%) compared to girls (27.2%). The prevalence of serum folate insufficiency also in-creased with age in both sexes.
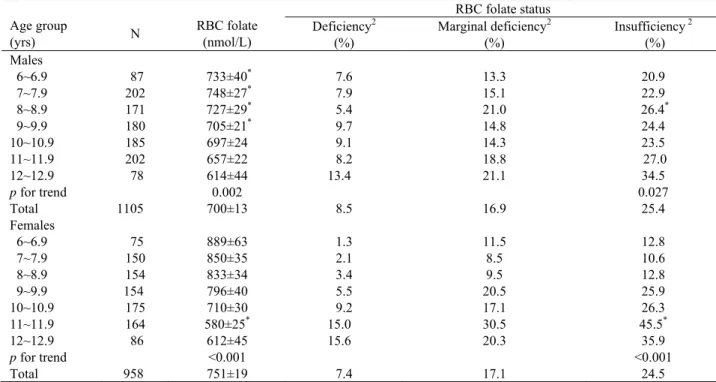
Data presented in Table 2 show that RBC folate levels also tended to decrease with age in both sexes (p for trend < 0.001). Boys still had significantly lower RBC folate levels between the ages of 6 to 9.9 yrs. However, at age 11-11.9 yrs girls had significantly lower RBC folate lev-els than boys. The prevalence of RBC folate deficiency (318 nmol/L, i.e. 140 ng/mL) and marginal RBC folate deficiency (RBC folate of 318-454 nmol/L, i.e.140-200 ng/mL) was similar for boys and girls. However, a higher prevalence of folate deficiency was observed when de-fined by RBC folate than by serum folate level. The over-all prevalence of RBC folate insufficiency also increased with age in both sexes (p for trend < 0.05). The highest prevalence of serum or RBC folate insufficiency in girls occurred at ages 11-11.9 (44.8 % for serum folate, 45.5% for RBC folate), and 12-12.9 yrs (43.8% for serum folate, 35.9% for RBC folate), but in boys occurred at age 12-12.9 yrs only (44.0% for serum folate, 34.5% for RBC folate).
Estimated dietary folate intake is shown in Table 3. The folate intake levels in the schoolchild population in-creased with age due to inin-creased dietary intake for growth. However, the average dietary intake did not meet RDA requirements in any age group, except in boys aged 12~12.9. There was no significant difference between boys and girls in dietary folate intake. The prevalence of low folate intake among Taiwanese schoolchildren was
Table 2. RBC folate levels and folate status in Taiwanese schoolchildren by age1
RBC folate status Age group (yrs) N RBC folate (nmol/L) Deficiency 2 (%) Marginal deficiency2 (%) Insufficiency 2 (%) Males 6~6.9 87 733±40* 7.6 13.3 20.9 7~7.9 202 748±27* 7.9 15.1 22.9 8~8.9 171 727±29* 5.4 21.0 26.4* 9~9.9 180 705±21* 9.7 14.8 24.4 10~10.9 185 697±24 9.1 14.3 23.5 11~11.9 202 657±22 8.2 18.8 27.0 12~12.9 78 614±44 13.4 21.1 34.5 p for trend 0.002 0.027 Total 1105 700±13 8.5 16.9 25.4 Females 6~6.9 75 889±63 1.3 11.5 12.8 7~7.9 150 850±35 2.1 8.5 10.6 8~8.9 154 833±34 3.4 9.5 12.8 9~9.9 154 796±40 5.5 20.5 25.9 10~10.9 175 710±30 9.2 17.1 26.3 11~11.9 164 580±25* 15.0 30.5 45.5* 12~12.9 86 612±45 15.6 20.3 35.9 p for trend <0.001 <0.001 Total 958 751±19 7.4 17.1 24.5
1 All valuesare shown as mean ± SE or percentage of participants. 2 RBC folate levels< 318 nmol/L (140 ng/mL) indicate deficiency;
between 318-454 nmol/L (140-200 ng/mL) indicate marginal deficiency; and ≤ 454 nmol/L (200 ng/mL) indicate insufficiency.
*Significantly different between sexes (p<0.0001).
Table 3. Dietary folate intake in Taiwanese
school-children by age1 Age group (yrs) n Folate intake 1 (µg/d) Inadequate intake 2 (%) Males 6~6.9 87 244±20 31.6 7~7.9 202 261±22 37.0 8~8.9 171 244±19 40.6 9~9.9 180 276±18 38.0 10~10.9 185 282±18 41.2 11~11.9 202 264±14 44.1 12~12.9 78 322±31 35.9 p for trend 0.001 Total 1105 269±9 41.3 Females 6~6.9 75 234±23 35.0 7~7.9 150 210±15* 45.6 8~8.9 154 247±20 39.7 9~9.9 154 255±14 34.2 10~10.9 175 285±22 45.1 11~11.9 164 290±20 38.2 12~12.9 86 286±19 42.2 p for trend <0.001 Total 958 259±9 41.8
1 All values are shown as mean ± SE or percentage of
partici-pants. 2 Inadequate folate intake is indicated by a dietary folate
intake <2/3 RDA in Taiwan. The RDA in Taiwan for folate is 200 μg/d for 6~6.9 yrs, 250 μg/d for 7~9.9 yrs, and 300 μg/d for
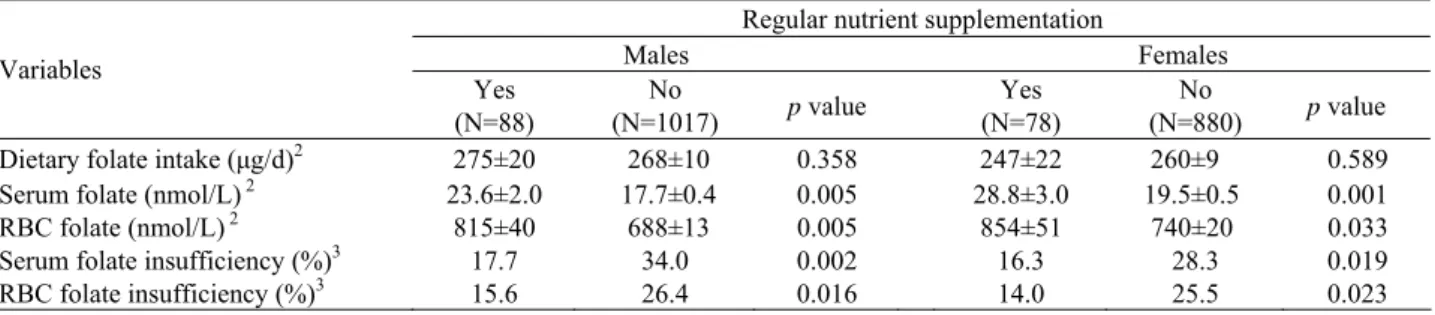
assessed and it was found that about 41% of subjects had a daily folate intake below 2/3 of the RDA (2/3 of RDA in Taiwan for folate is 133 μg/d for children aged 6~6.9 yrs, 167 μg/d for 7~9.9 yrs, and 200 μg/d for 10~12.9 yrs). The effect of nutrient supplementation on dietary folate intake and biochemical indicators of folate status is shown in Table 4. About 9% of schoolchildren took nu-trient supplements in this nation-wide survey. After ad-justment for age, there was no difference between nutrient supplement users and non-supplement users in dietary folate intake for both sexes. However, the subjects who took nutrient supplements had significantly higher serum and RBC folate levels, and a lower prevalence of serum or RBC folate insufficiency than those who did not take nutrient supplements.
To investigate the effect of dietary folate intake on folate status, the association between dietary folate intake and serum and RBC folate levels among subjects not tak-ing nutrient supplements was analyzed by tertile of die-tary folate intake. The results are shown in Table 5. For both boys and girls, serum folate levels significantly in-creased with dietary folate intake (p for trend < 0.03), however, RBC folate levels did not significantly increase with dietary folate intake.
DISCUSSION
Early studies of folate nutritional status focused mostly on pregnant women and elderly people,27 but children’s
folate status has become of increasing importance due to changes in dietary habits. In our previous study on the Taiwanese elderly, we showed that the elderly population consuming a Chinese-style diet and benefiting from the
abundance of a diverse variety of fruit and vegetables in Taiwan had no folate deficiency.19 However, as
Western-style diets with increased amounts of fast-food become popular among the young generation in Taiwan, the folate status of children may be adversely affected. Some stud-ies have indicated that folate deficiency can be found in schoolchildren. 17,28-33 In our study, the average serum
folate concentration in Taiwanese schoolchildren was 18.3±8.8 nmol/L (8.1±3.9 ng/mL) in boys and 20.3±9.7 nmol/L (9.0±4.3 ng/mL) in girls, which are lower than the average plasma folate levels in elderly people from the Elderly Nutrition and Health Survey in Taiwan (1999-2000) (Elderly NAHSIT). 19 Our results showed that
se-rum folate was significantly lower in boys than in girls, which is consistent with other studies.31 This suggests that
folate status in the male population is an important issue, particularly as folate status is a strong determinant of total homocysteine in children.4
To compare our blood folate levels with those in other studies, we stratified age according to the age groups used in these studies and then reanalyzed our data. The mean serum folate level in Taiwanese schoolchildren was higher than figures in Dutch children (20.4 vs. 17.9 nmol/L, aged 6-10 yrs; 16.6 vs. 15.9 nmol/L, aged 11-13 yrs) and in the USA before folic acid fortification (20.3 vs. 19.3 nmol/L, aged 6-11 yrs, NHANES III),28,31 but lower
than figures in Greek children (16.8 vs. 21.6 nmol/L for boys, 16.1 vs. 20.9 nmol/L for girls, aged 10-13 yrs),30 in
northeast Thailand (19.4 vs. 21.4 nmol/L, aged 6-13 yrs),33 and in the USA after folic acid fortification (20.3
vs. 43.8 nmol/L, aged 6-11 yrs, NHANES 1999-2000).31
The mean RBC folate level in Taiwanese schoolchildren
Table 4. Effect of nutrient supplementation on dietary folate intake and folate status in Taiwanese schoolchildren1
Regular nutrient supplementation
Males Females Variables Yes (N=88) No (N=1017) p value Yes (N=78) No (N=880) p value
Dietary folate intake (μg/d)2 275±20 268±10 0.358 247±22 260±9 0.589
Serum folate (nmol/L) 2 23.6±2.0 17.7±0.4 0.005 28.8±3.0 19.5±0.5 0.001
RBC folate (nmol/L) 2 815±40 688±13 0.005 854±51 740±20 0.033
Serum folate insufficiency (%)3 17.7 34.0 0.002 16.3 28.3 0.019
RBC folate insufficiency (%)3 15.6 26.4 0.016 14.0 25.5 0.023
1 All values are shown as mean ± SE or percentage of participants. 2 Adjusted for age. 3 Serum folate levels ≤14 nmol/L (6.0 ng/ml)
indi-cate serum folate insufficiency. RBC folate levels≤ 454 nmol/L (200 ng/mL) indicate RBC folate insufficiency.
Table 5. Serum and RBC folate levels in Taiwanese schoolchildren not taking nutrient supplements by tertile of
die-tary folate intake 1
Folate intake (µg/d) Variables
<153 153~293 > 293 p for trend
Males
N 333 342 342
Serum folate (nmol/L) 2 17.0±0.5 17.9±0.6 18.8±0.8 0.010
RBC folate (nmol/L) 2 697±19 719±21 724±23 0.173
Females
N 312 300 268
Serum folate (nmol/L)2 18.6±0.7 19.1±1.0 20.4±0.8 0.027
RBC folate (nmol/L) 2 720±27 731±31 744±20 0.634
was also lower than that found in northeast Thailand (755 vs. 842 nmol/L, aged 6-13 yrs),33 and higher than those
found in the USA before and after folic acid fortification (762 vs. 444 nmol/L, aged 6-11 yrs, NHANES III; 762 vs. 643 nmol/L, aged 6-11 yrs, NHANES 1999-2000).31
The prevalence of folate deficiency among children in different countries has varied substantially due to the use of different ranges of age. After reanalysis to take this into account, our data showed that the overall prevalence of RBC folate deficiency (< 318 nmol/L) in Taiwanese children is probably lower than that in Mexican children (6.4% for Taiwanese aged 6-11 yrs vs. 10% for Mexican aged 5-11 yrs).32 In our study, there was a 1.4%
preva-lence of serum folate deficiency and 7-9% prevapreva-lence of RBC folate deficiency in Taiwanese schoolchildren. The prevalence of marginal serum folate deficiency was also higher among schoolchildren than the elderly (26-31% vs.12-18%).19 It should be mentioned that serum and
RBC folate decreases with age without decrease of folate intake, suggesting folate requirement for adolescence may be higher. It is not known whether the same cut-offs are appropriate for children as little information is available to establish such cut-offs for this age group. The use of the adult cut-offs for folate status in schoolchildren may underestimate the extent of inadequate folate status in childhood. Our data indicated that schoolchildren in Tai-wan have poorer folate status than the TaiTai-wanese elderly. More attention should therefore be given to this important issue, especially as folate is associated with brain devel-opment and emergent cognitive function in children.8,9
Our results showed that serum and RBC folate levels significantly decreased and the prevalence of insuffi-ciency significantly increased with age in both sexes. This tendency is consistent with other surveys indicating an age-related reduction in serum and RBC folate during periods of rapid growth and development, such as the transition from childhood to early adolescence.31,34
There-fore, lower levels of serum and RBC folate in boys com-pared to girls was noted between ages 7-9.9 yrs, while girls had significantly lower RBC folate levels than boys at age 11-11.9 yrs. Importantly, the highest prevalence of serum (~44%) or RBC (36~46%) folate insufficiency and the highest dietary folate intake occurred in girls at age 11-12.9 yrs, and occurred in boys at age 12-12.9 yrs. The-se data suggests that folate status is particularly poor in those children entering adolescence, as a result of in-creased folate requirements due to rapid tissue growth and development. Therefore, adequate dietary folate in-take is vitally important for such schoolchildren. Higher intakes of dark green vegetables, citrus fruits, gourds, pickled vegetables, seaweed are significantly correlated to higher RBC folate in our previous survey.17 Therefore,
these food, as well as folate supplement, may be recom-mended to improve the folate status of schoolchildren. The USA (NHANES III) study reported that average dietary folate intake was 270 µg/day in boys and 235 µg/day in girls aged 6-11 yrs, and 382 µg/day in boys and 220 µg/day in girls aged 12-15 yrs.35 In our study, the
estimated average dietary folate intake in a nation-wide survey of 2063 schoolchildren was 261 µg/d in boys and 246 µg/d in girls aged 6-11 yrs, and 322 µg/d in boys and 286 µg/d in girls aged 12~13 yrs using the folate
compo-sition databank described previously.18 The data suggest
that Taiwanese male schoolchildren have lower folate intake, but that girls have higher folate intake compared to the folate intakes of US schoolchildren before folate fortification. In addition, 42% of the Taiwanese school-child population are at risk of inadequate dietary folate intake (dietary folate intake below 2/3 RDA values). This suggests that greater attention should be given to this im-portant health issue.
The folate fortification program has increased concen-trations of serum and RBC folate in the entire population and substantially eliminated folate deficiency in the US.31
Our study has shown that nutrient supplement users and nonusers did not differ in dietary folate intake at baseline, however nutrient supplements users had higher serum and RBC folate concentrations and better folate status than nonusers. This suggests that nutrient supplements may have a beneficial effect on folate status although there was limited information collected about the types of sup-plements taken by subjects in the survey. This study also showed that serum folate, but not RBC folate, of school-children who did not take nutrient supplements increased with dietary folate intake, consisting with the fact that serum folate is highly influenced by current dietary in-take.11 RBC folate levels were also positively correlated
with serum folate levels (r = 0.6501, p < 0.0001) in our study, suggesting that recent folate status may be associ-ated with long term folate status.36,37 Our results showing
a higher prevalence of folate deficiency evaluated by RBC folate level than by serum folate level indicate that the folate status of schoolchildren needs to be improved. In summary, this study provides information on the folate status of Taiwanese schoolchildren. Boys had poorer folate status than girls. It should be noted that up to 32% of boys and 27% of girls had serum folate insuffi-ciency, and 25% of children had RBC folate insufficiency. In addition, 42% of Taiwanese schoolchildren had inade-quate folate intake. Those who took nutrient supplements had significantly better serum and RBC folate status. Those who did not take nutrient supplements had higher serum folate levels, but not RBC folate levels, with in-creased folate intakes. This study suggests that the folate status of Taiwanese schoolchildren is inadequate, and is particularly poor in those children entering adolescence. It is important to prevent the development of folate insuf-ficiency in this potentially vulnerable population.
ACKNOWLEDGEMENTS
This study was supported by the Department of Health, Taiwan (DOH 95-TD-F-113-030). Data analysed in this paper were collected by the research project "Nutrition and Health Survey in Taiwan (NAHSIT)" sponsored by the Department of Health in Taiwan (DOH-88-FS, DOH89-88shu717, DOH90-FS-5-4, DOH91-FS-5-4). This research project was carried out by the Institute of Biomedical Sciences of Academia Sinica and the Research Center for Humanities and Social Sciences, Center for Survey Research, Academia Sinica, directed by Dr. Wen-Harn Pan and Dr. Su-Hao Tu. The Center for Survey Research of Academia Sinica is responsible for data distribution. The assis-tance provided by the institutes and aforementioned individuals is greatly appreciated. The views expressed herein are solely those of the authors.
AUTHOR DISCLOSURES
Kuan-Ju Chen, Ning-Sing Shaw, Wen-Harn Pan, and Bi-Fong Lin, no conflicts of interest.
REFERENCES:
1. Cardo E, Vilaseca MA, Campistol J, Artuch R, Colome C, Pineda M. Evaluation of hyperhomocysteinaemia in chil-dren with stroke. Eur J Paediatr Neurol. 1999;3(3):113-7. 2. Cardo E, Monros E, Colome C, Artuch R, Campistol J,
Pineda M, Vilaseca MA. Children with stroke: polymor-phism of the MTHFR gene, mild hyperhomocysteinemia, and vitamin status. J Child Neurol. 2000;15(5):295-8. 3. Koch HG, Nabel P, Junker R, Auberger K, Schobess R,
Homberger A, Linnebank M, Nowak-Gottl U. The 677T genotype of the common MTHFR thermolabile variant and fasting homocysteine in childhood venous thrombo-sis. Eur J Pediatr. 1999;158 (Suppl 3):S113-6.
4. Ueland PM, Monsen AL. Hyperhomocysteinemia and
B-vitamin deficiencies in infants and children. Clin Chem Lab Med. 2003;41(11):1418-26.
5. Delvin EE, Rozen R, Merouani A, Genest J Jr, Lambert M. Influence of methylenetetrahydrofolate reductase genotype, age, vitamin B-12, and folate status on plasma homocysteine in children. Am J Clin Nut. 2000;72 (6):1469-73.
6. Chen KJ, Pan WH, Yang FL, Wei IL, Shaw NS, Lin BF.
Association of B vitamins status and homocysteine levels in elderly Taiwanese. Asia Pac J Clin Nutr. 2005;14 (3):250-5.
7. Antony AC, Hansen DK. Hypothesis: Folate-responsive neural tube defects and neurocristopathies. Teratology. 2000;62(1):42-50.
8. Bryan J, Osendarp S, Hughes D, Calvaresi E, Baghurst K, van Klinken JW. Nutrients for cognitive development in school-aged children. Nutr Rev. 2004;62(8):295-306. 9. Barbaux S, Plomin R, Whitehead AS. Polymorphisms of
genes controlling homocysteine/folate metabolism and cognitive function. Neuroreport. 2000;11(5):1133-6. 10. Bailey LB. Folate status assessment. J Nutr. 1990;120
(suppl 11):1508-11.
11. Senti FR, Pilch SM. Analysis of folate data from the na-tional health and nutrition examination survey (NHANES II). J Nutr. 1985;115(11):1398-402.
12. Jacques PF, Selhub J, Bostom AG, Wilson PWF,
Rosenberg IH. The effect of folic acid fortification on plasma folate and total homocysteine concentrations. New Engl J Med. 1999;340(19):1449-54.
13. Ray JG, Vermeulen MJ, Boss SC, Cole DE. Increased red cell folate concentrations in women of reproductive age after Canadian folic acid food fortification. Epidemiology. 2002;13(2):238-40.
14. Sandstrom B. A framework for food-based dietary guide-lines in the European Union. Public Health Nutr. 2001;4 (2A):293-305.
15. Bailey LB, Gregory JF 3rd. Folate metabolism and re-quirements. J Nutr. 1999;129(5):779-82.
16. Department of Health: Dietary Reference Intakes (DRIs). Taipei, Taiwan; 2002. (In Chinese)
17. Lin BF, Lin RF, Yeh WT, Pan WH. The folate status in Taiwan population from the NAHSIT 1993-1996. Nutr Sci J. 1999;24(1):99-117. (In Chinese)
18. Lee CH, Lee FY, Wong J, Tzeng MS, Hung S RF.
De-sign of food frequency questionnaire for assessing dietary folate: It’s application to study consumption frequency of folate-rich foods in ischemic stroke patients. Nutr Sci J. 2003;28(4):210-7. (In Chinese)
19. Chen KJ, Pan WH, Shaw NS, Huang RF S, Lin BF.
As-sociation between dietary folate intake and folate status of elderly Taiwanese. Asia Pac J Clin Nutr. 2005;14 (3):244-9.
20. Tu SH, Hung YT, Chang HY, Hang CM, Hsiao NH, Lin
W, Lin YC, Hu SW, Yang YH, Wu TT, Chang YH, Su SC, Hsu HC, Pan WH. Nutrition and Health Survey in Taiwan Elementary School children 2001-2002: research design, methods and contents. Asia Pac J Clin Nutr. 2007;16(S2):507-17.
21. Raiten DJ, Fisher KD. Assessment of folate methodology used in the third National Health and Nutrition
Examina-tion Survey (NHANES , 1988Ⅲ -1994). J Nutr.
1995;125(5):1371s-1398s.
22. Gibson RS. Principles of nutritional assessment. New York, NY: Oxford University Press, 1990.
23. Herbert V. The 1986 Herman Award Lecture. Nutrition science as a continually unfolding story: the folate and vi-tamin B-12 paradigm. Am J Clin Nutr. 1987;46(3):387-402.
24. USDA Nutrient database for standard reference, Release 14. Folate, DEF (mcg) content of selected foods per common measure, sorted alphabetically. 2002.
25. Pennington JAT, Church HN. Bowes and Church’s food values of portions commonly used. 1998; 17th edition: pp. H-1-H-87.
26. Leh J, Lin BF. Determination of folate contents of com-monly consumed vegetables in Taiwan. Nutr Sci J. 2000; 25(1):29-37. (In Chinese)
27. Chen KJ, Shaw NS, Lin BF. The folate status of prenatal follow-up pregnant women at hospital in Taipei. Nutr Sci J. 2006;31(1):8-16. (In Chinese)
28. van Beynum IM, den Heijer M, Thomas CM, Afman L,
Oppenraay-van Emmerzaal D, Blom HJ. Total homocys-teine and its predictors in Dutch children. Am J Clin Nutr. 2005;81(5):1110-6.
29. Monsen AL, Refsum H, Markestad T, Ueland PM.
Co-balamin status and its biochemical markers methylmalo-nic acid and homocysteine in different age groups from 4 days to 19 years. Clin Chem. 2003;49(12):2067-75. 30. Papoutsakis C, Yiannakouris N, Manios Y,
Papaconstan-tinou E, Magkos F, Schulpis KH, Zampelas A, Matalas AL. Plasma homocysteine concentrations in Greek chil-dren are influenced by an interaction between the methyl-enetetrahydrofolate reductase C677T genotype and folate status. J Nutr. 2005;135(3):383-8.
31. Pfeiffer CM, Caudill SP, Gunter EW, Osterloh J,
Sampson EJ. Biochemical indicators of B vitamin status in the US population after folic acid fortification: results from the National Health and Nutrition Examination Sur-vey 1999-2000. Am J Clin Nutr. 2005;82(2):442-50.
32. Rivera JA, Sepulveda-Amor J. Conclusions from the
Mexican National Nutrition Survey 1999: translating re-sults into nutrition policy. Salud Publica Mex. 2003;45 Suppl 4:S565-75.
33. Thurlow RA, Winichagoon P, Green T, Wasantwisut E, Pongcharoen T, Bailey KB, Gibson RS. Only a small pro-portion of anemia in northeast Thai schoolchildren is as-sociated with iron deficiency. Am J Clin Nutr. 2005;82(2):380-7.
34. Wright JD, Bialostosky K, Gunter EW, Carroll MD,
Najjar MF, Bowman BA, Johnson CL. Blood folate and vitamin B12: United States, 1988-94. Vital Health Stat 11. 1998;(243):1-78.
35. Alaimo K, McDowell MA, Briefel RR, Bischof AM, Caughman CR, Loria CM, Johnson CL. Dietary intake of vitamins, minerals, and fiber of persons ages 2 months and over in the United States: Third National Health and Nutrition Examination Survey, Phase 1, 1988-91. Adv Data. Nov 1994;14: -28.
36. Magnus EM. Folate studies. Folate and vitamin B12 val-ues in relation to bone marrow pattern. Scand J Haematol suppl. 1975;24: III-VII,1-110.
37. Jaffe JP, Schilling RF. Erythrocyte folate levels: a clinical study. Am J Hematol. 1991;36(2):116-21.
Original Article
Evaluation of folate status by serum and erythrocyte
folate levels and dietary folate intake in Taiwanese
schoolchildren
Kuan-Ju Chen
PhD1, Ning-Sing Shaw
PhD2, Wen-Harn Pan
PhD2,3,4and Bi-Fong Lin
PhD21
Department of Hospitality Management, Chung-Hwa University of Medical Technology, Tainan, Taiwan 2
Department of Biochemical Science and Technology, Institute of Microbiology and Biochemistry, Na-tional Taiwan University, Taipei, Taiwan
3
Institute of Biomedical Science, Academia Sinica, Taipei, Taiwan 4
College of Public Health, National Taiwan University, Taipei, Taiwan