Marine Chemistry 41 (1993) 343-351 343 Elsevier Science Publishers B.V., Amsterdam
Determination of dissolved oxygen in seawater by direct
spectrophotometry of total iodine
Su-Cheng Pai a, G w o - C h i n g G o n g "'b a n d K o n - K e e Liu ~x "lnstitute t)f Oceanography. National Tahvan University. Taipei, Taiwan hDepartment of Oceanography, National Taiwan Ocean University, Keelung, Taiwan
~lnstitute of Earth Sciences, Academia Sinica, Taipei, Taiwan (Received 20 January 1992; revision accepted 6 May 1992)
ABSTRACT
Pai, S.-C., Gong, G.-C. and Liu, K.-K., 1993. Determination of dissolved oxygen in seawater by direct spectrophotometry of total iodine. Mar. Chem., 41: 343-351.
A modified procedure has been proposed for the colorimetric determination of dissolved oxygen in seawater to improve its precision and accuracy. When a pickled sample is acidified, iodine liberated in the iodometric reaction is measured by direct spectrophotometry at 456 nm. Loss of molecular iodine by volatilization is eliminated by transferring the sample to a flow cuvette without contact with air. The method was calibrated for oxygen by spiking ktown amounts of potassium iodate. Precision was found at better than 0.2% r.s.d. (full scale). Evaluation of accuracy was made by comparison with calculated oxygen solubilities, which shows a relative bias of no more than 0.5% for oxic waters. The analytical throughput was much faster than that of the standard titration procedure.
INTRODUCTION T h e W i n k l e r t i t r a t i o n m e t h o d ( t 888) h a s b e e n the d o m i n a n t t e c h n i q u e f o r t h e d e t e r m i n a t i o n o f d i s s o l v e d o x y g e n in s e a w a t e r for o v e r a c e n t u r y . I t is g e n e r a l l y b a s e d o n t h e r e a c t i o n o f o x y g e n w i t h M n -'+ in a l k a l i n e m e d i u m f o l l o w e d b y r e a c t i o n o f the M n 3+ o r M n 4+ w i t h a c i d i c i o d i d e s o l u t i o n . T h e r e s u l t a n t i o d i n e is d e t e r m i n e d t i t r i m e t r i c a l l y w i t h s t a n d a r d t h i o s u l p h a t e . A l t h o u g h i n t r i c a t e m o d i f i c a t i o n s h a v e b e e n m a d e t o i m p r o v e c o n s i d e r a b l y t h e p r e c i s i o n o f t h e o r i g i n a l m e t h o d (see e.g. C a r p e n t e r , 1965a, b; G r e e n a n d C a r r i t t , 1966), l a t e r i n c l u d i n g t h e u s e o f p h o t o m e t r i c a n d p o t e n t i o m e t r i c e n d - p o i n t d e t e c t i o n devices, t h e a c c u r a c y o f t h e r o u t i n e
Correspondence to: Dr. S.-C. Pal, Institute of Oceanography, National Taiwan University, P.O. Box 23-13, Taipei, Taiwan. Tel: 886-2-362-7358; Fax: 886-2-363-5165. t i t r a t i o n p r o c e s s is still m u c h t h e s a m e as it w a s t w o o r t h r e e d e c a d e s ago. R i l e y (1975), w h o h a s s u m m a r i z e d t h e r e s u l t s o f i n t e r c a l i b r a t i o n w o r k o n t h e W i n k l e r t i t r a t i o n m e t h o d s , h a s s u g g e s t e d t h a t a v e r y i m p o r t a n t s o u r c e for the e r r o r is the u n c e r t a i n t y in t h e s t a n d a r d i z a t i o n o f t h i o s u l p h a t e , b e c a u s e o f its i n s t a b i l i t y . A n a c c u r a c y o f b e t t e r t h a n 1% c a n be a c h i e v e d b y t i t r a t i o n b u t o n l y u n d e r e x t r e m e l y careful conditions. A m u c h simpler non-titrimetric a p p r o a c h , a v o i d i n g t h e use o f t h i o s u l p h a t e , h a d b e e n p r o p o s e d b y several w o r k e r s . T h i s i n v o l v e s d i r e c t m e a s u r e m e n t o f t h e b r o w n c o l o r w h i c h is g e n e r a t e d w h e n t h e p i c k l e d s a m p l e is acidified ( B r o e n k o w a n d Cline, 1969). T h e c o l o r r e s u l t s f r o m a m i x t u r e o f m o l e c u l a r i o d i n e a n d tri- i o d i d e ion. T h e l a t t e r is g e n e r a t e d b y t h e c o m - p l e x a t i o n r e a c t i o n o f m o l e c u l a r i o d i n e w i t h excess i o d i d e ( B u r g e r a n d L i e b h a f s k y , 19731: 12 + I - ~ l j - K = 725 (1)
344
Tri-iodide ion exhibits a strong absorption with a maximum at 350-360nm. Wong (1982) has estimated the molar extinction coefficient of tri4odide to be 1.9 × 104M ~cm -~ at 353nm. The sensitivity is sufficient to cover the oxygen concentration range found in natural samples. However, a review of the literature shows that direct photometry may not be as precise as titri- metric methods (Riley, 1975). For this reason Broenkow and Cline (1969) suggested that it is useful only with low oxygen concentrations. The reason why direct colorimetry is not sufficiently precise at high oxygen concentration is probably due to the unavoidable loss of volatile molecular iodine during the manual transfer of the sample into then colorimeter cell. Green and Carritt (1966) have shown that such loss may be the major source of error in the Winkler procedure. If the exposure of the liquid to air could be controlled, then the colorimetric determination could be made with much better precision and accuracy than that claimed by earlier invesiti- gators.
A preliminary attempt was made to use a Brinkmann PC°900 optical fiber colorimeter (Brinkmann Instruments, New York, USA) for making the measurement. The optical probe was dipped into the iodine-containing liquid con- tained in then sample bottle and the absorbance was recorded. Although a precision of ca. 0.6% (at 420 nm) was achieved for replicated measure- ments, the calibration curve was found to be non-linear compared with results obtained from a parallel Winkler titration. This might be a result of the broad band pass of the light filter in the colorimeter. For this reason, a regular spectrophotometer would be preferable. An attempt to use a flow injection system similar to that suggested by Novic et al. (1988) coupled with a spectrophotometer was unsatisfactory, because the resulting precision was no better than that of the normal titration.
The pregent study describes a simple fast flow sipper system u-;ed in conjunction with a wide- bore flow cm,~tte installed in a double beam spectrophotometer. Measurements of the iodine
S. C. PAl, G,-C. GONG AND K,-K. LIU
color appeared much more stable than those with a flow injection system using a narrow bore flow cell. A precision of ca. 0.2% can be readily achieved. After comparison of hundreds o f parallel determinations, the authors are con- fident that the proposed spectrophotometric method can replace the standard Winkler titra- tion for routine field work.
M A T E R I A L S A N D M E T H O D S
Reagents
Manganese chloride (MnCI_~ " 4H20 600 g 1 - i), alkaline iodide reagent (NaOH 320g and Nal 600gl -~) and sulphuric acid (concentrated sul- phuric acid 280 roll -j ) were prepared following the suggestion by Carpenter (1965). The con- centrations of these reagents were 3 M for Mn 2+ ; 8 M for O H - ; 4 M for I- and 10M for H ~.
Potassium iodate: prepared by dissolving 1.0665 g of potassium iodate (dried at 130°C) in 11 of distilled water. Each ml of this standard contains 5.000#tool of iodate.
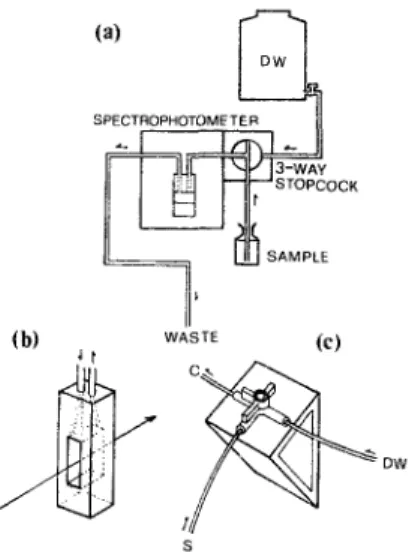
Sample bottles: approximately 360 Wheaton BOD bottles (ca. 60ml capacity, Cat. no. 227494-00, Wheaton, Millville, N J, USA) were routinely used in this laboratory. All were care- fully calibrated and had an average volume of 59.43 __+ 0.34ml (ca. 0.6%). Spectrophotometer: a Shimadzu 160A double beam spectrometer was fitted with a wide bore quartz flow cell (10ram light path, with an aperture of 11 mm x 4ram, Cat. no. 176.000-QS, Hellma, Germany) having a total chamber capacity of 450~d. A three-way stopcock was fixed outside the spectrophoto- meter, with connections of Teflon tubing as shown in Fig. 1. The spectrophotometric reading was zeroed against another cuvette when both were filled with distilled water.
Laboratory test samples: distilled water and filtered seawater were prepared in 10 or 251 polyethylene carboys immersed in a large thermo- statically controlled water bath. The water was aerated continuously except for a few minutes before samples were withdrawn.
DETERMINATION OF DISSOLVED OXYGEN IN SEAWATER ( s OCK ! (b) WASVE (e) J t s
Fig. 1. (a) The sipper/flow cuvette system coupled with a spectrophotometer for the measurement of total iodine. Flow rate was adjustable by changing tne diameter or level of the waste tube, (h) The large-bore flow cuveUe having a chamber capacity of 450/A. (c) The three-way stopcock can be turned to select LOAD, FLUSH, or STOP functions.
Procedure
A n u m b e r o f 60 ml B O D bottles were flushed a n d filled with the water samples in the n o r m a l way. T h e y were pickled immediately by the addition o f 0.5 ml o f m a n g a n e s e chloride a n d 0 . 5 m l o f alkaline iodide reagents by m e a n s o f dispensers. After being allowed to s t a n d for at least l h, the bottle was opened and 0 . 5 m l o f sulphuric acid was added. The bottle was left open and the mixture was gently stirred with a magnetic stirring bar until all the precipitate h a d dissolved. The solution was then withdrawn into the spectrophotometer by m e a n s o f the non- powered sipping flow system (Fig. 1). The end o f the inlet tube was placed near the b o t t o m o f the bottle to avoid influence from the exposed surface layer. A flow rate o f ca. 2 0 m l m i n ~ was f o u n d to be sufficiently fast to allow the photometric reading to reach a steady state within 20s. After the a b s o r b a n c e had been
345 recorded the cuvette was flushed with distilled water by turning the three-way valve.
Calibration o f the procedure was carried out by adding potassium iodate standard solution to a series o f replicate samples. After acidification the bottles were added with 0, 1, 2, and 3ml o f 5 m M KIO3 and the absorbances were then measured. Since the addition o f iodate changes the total volume, the absorbance readings were normalized by a dilution factor before the cali- bration was constructed. A n empirical extinction coefficient k was evaluated from the slope o f the curve. Alternatively, the calibration could be m a d e using a series o f reagent blanks~ prepared by putting 6 0 m l aliquot o f distilled water or seawater in the bottle a n d mixed in sequence with sulphuric acid, alkaline iodide and m a n - ganese reagents. T h e y were spiked with k n o w n a m o u n t s o f iodate. Details o f the calculation are given in the following section.
RESULTS AND DISCUSSION
Volatilization of iodine
Loss o f iodine v a p o u r is a major source o f error in the colorimetric determination o f oxygen if the sample is transferred m a n u a l l y to a col- orimeter cuvette. A l t h o u g h K n a p p et al. (1991) suggested such loss might not be i m p o r t a n t for titration, we have f o u n d that each transfer step could cause 1-3% decrease on the absorbance reading. T h e following experiment was carried o u t to evaluate the m a g n i t u d e o f the volatiliza- tion loss when the sample is in a colorimetric cuvette. A sample solution was p u t into an ordinary 1 0 m m cuvette (capacity 4 m l ) , a n d covered by a Teflon cap, leaving no air gap between the liquid and the cap. U n d e r these conditions the absorbance was stable for hours. However, when the cap was removed loss o f iodine occurred immediately at a rate which was strongly dependent on temperature a n d salinity (Fig. 2). The absorbance for a seawater sample was f o u n d to decrease at a rate o f - 0 . 0 3 0 A h -* ( - 6% h - * ) at 22.5°C, a n d could reach as high as
346 S.-C. PAL G.-C. GONG AND K.-K. LIU 0.650 E u~C 0.600 t.~ .4" 0.550 O 0.500 < 0.450 D W SW
1'o 2'0 3'o 4'o 5o
Time (min) - ~ ' 2.0
~
1.0 350 DW ~6o ~5o 500 Wavelength (nm) Fig. 2. Va',~)rization of iodine demonstrated by placing theacidified sr~mple into a standard I cm cuvene (capacity 4 ml), and mon{1oring the variation of absorbance at 456 nm. ?,ir- saturated distilled water and seawater were pickled at 18.5°C. The temperature in the cuvette chamber of the spec- trophotometer was 22.5°C. a, covered by Teflon cap; b, without cover.
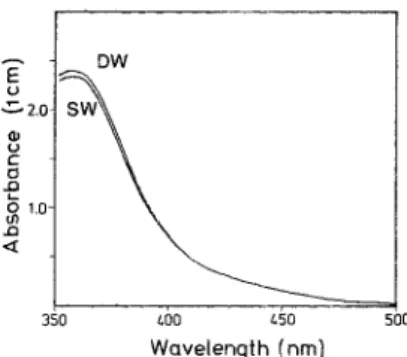
Fig. 3. Absorption spectra of the brownish iodine color in freshwater and seawater media. Aliquot of 0.05 ml of 50 mM iodate was added to 61.5 ml sample (added previously with 0.5 ml of acid, and then 1 ml of the pickling reagents), The final concentrations of iodide and total iodine in both media were ca. 32.5mM and 122#M, respectively.
- 0.150 A h - ~ (ca. - 25- - 30% h - t ) at tempera-
ture above 30°C. T h e rate o f volatilization for freshwater was approximately halved. However, the loss rate was almost negligible (less t h a n
- 0.2% h-~ ) when the sample was contained in a
60 ml bottle (with free surface o f ~ 2.5 cm 2) as long as the liquid was not poured out. T h u s the bottle containing the acidified sample can be left open for several minutes without detectable loss. To avoid loss on transfer o f the acidified sample to the cuvette the sipper system (Fig. 1) was designed. With this the sample is withdrawn from the lower part o f the bottle. A n y possible loss o f iodine at the surface would n o t affect the concentration in the b o t t o m layer. If the sipping was stopped, the absorbance reading for the trapped sample could be maintained u n c h a n g e d for over 1 h. A wide bore cuvette was preferable to one o f narrow bore, as the reading is m o r e stable. In addition it can be adapted to a variety o f spectrophotometers. In order to reduce the tailing effect arising from the large c h a m b e r capacity (450#1), a high flow rate o f ca. 15- 2 5 m l m i n -~ was appfied. T h e absorbance then reached a steady state within ~ 20 s and returned to zero in the same time when flushing with
distilled water. A further a d v a n t a g e o f using the non-powered sipper system is that it eliminates the pulsations in flow which are usually associated with a peristaltic p u m p .
Choice of wavelength
A l t h o u g h the wavelength o f the m a x i m u m absorption o f the tri-iodide is at ca. 353-356 n m (Fig. 3). a wavelength o f 4 5 6 n m (near the m a x i m u m a b s o r p t i o n for molecular iodine) was chosen for the following reasons: (1) T h e sen- sitivity at 353 n m is too great to cover the n o r m a l range o f oxygen concentrations in oxic waters. The m e a s u r e m e n t at the shoulder o f the peak is less sensitive yet it provides e n o u g h resolution for oceanographic use. (2) Dissolved organic m a t t e r usually s h o w s high absorption in the UV and n e a r - U V ranges. There is negligible absorp- tion at 4 5 6 n m a n d the interference is n o t as severe as at the lower wavelength. (3) T h e m o l a r absorptivities o f molecular iodine a n d the tri- iodide ion are similar at 450-460 nm. T h e extinc- tion coefficients ibr the mixture are identical in both freshwater a n d seawater. (4) In practice, absorbances m e a s u r e d at 456 n m provide a quick estimate o f the oxygen concentration, thus, a
DETERMINATION OF DISSOLVED OXYGEN IN SEAWATER 347
reading o f 0.5 A in 1 0 m m cuvette c o r r e s p o n d s with a n oxygen concentration o f ca. 5.2 ml 1-~. A resolution o f 0.001 A corresponds to an oxygen concentration o f ca. 0.01 ml 1- ].
Quantification
In the Winkler technique, each mole o f mol- ecular oxygen in sample will produce two moles o f molecular iodine after acidification. M o s t o f this will further complex with excess ioside to f r o m tri-iodide ions. In the procedure described, the final concentration o f excess iodide is ca. 0.0167 M. U n d e r these conditions ~ 96% o f the total iodine would be tri-iodide and ~ 4 % would be molecular iodine. O t h e r species o f iodine such as I O - a n d H I O will be negligible under acidic condition. In evaluating the absorbance at 456 n m the contributions from the tri-iodide ion as well as the free iodine m u s t be taken into account.
W h e n doing the calibration, s t a n d a r d potas- sium iodate was added to the sample. Each mole o f iodate added would produce three moles o f additional total iodine.
10 3 + 51- + 6 H + ~ 312 + 3 H 2 0 (2) It should be noted that the capacities o f the B O D bottles will vary. I f a bottol o f volume V b is filled with a sample having dissolved oxygen concentration of[O2] and treated with the pickling reagents (volume Pc), a n d sulphuric acid (volume 1/~), the concentration o f total iodine (Cj) in the solution will be:
c, = 2 x [o:1 x
(v~
- Pc)/(~ + Pc.) (3) which will produce an absorbance reading of: Abs = k x b × C~, where k is the empirical extinction ceofficient, a n d b is the cuvette length. Since the value of(V~ - Pc)/(Vb + V~) is almost c o n s t a n t with bottles having capacities ranging from 58-62 ml, the initial concentration C~ in all bottles was a s s u m e d identical (with an error no m o r e t h a n 0.02%). If this sample was spiked with an aliquot (V,) o f p o t a s s i u m iodate (eoncentra-1,5- 1.0 d v t~ 0.5 j z < j
J
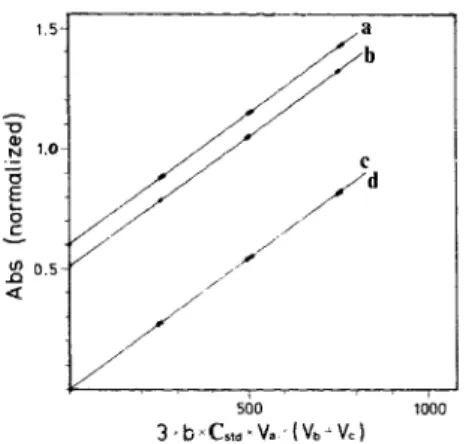
500 1000 3, b~C,,, ~V,, (Vb- Vc)Fig. 4. Typical calibration curves for evaluating the empiri- cal coefficient k. Triplicate spikings w e r e made to: a. a pickled distilled water sample; b, a pickled seawater sample; c, a distilled water reagent blank; d, a seawater reagent blank. The slopes (k values} of the four sets of curve were 1084, 1086, 1087. 1084M ~cm ~, respectively.
tion C,,d), the absorbance could be derived as: Abs(spiked) = k x b
C i b< ( V b Jr Pc) Jr 3 x C q d ~. ~,/l
x (4)
14+ Pc+ v,,
It is convenient to normalize the spiked absor- bance by a factor to compensate for the dilution effect.
Abs(normalized) = Abs(spiked)
x
( ~ + pc + V.)/(Vb + PC) = Abs(initial) + k x b x 3 x C~,jx v./(v~ + PC)
T h u s the empirical coefficient k can then be obtained from the slope by linear regression o f a series o f normalized absorbances plotted against increments o f total iodine concentration.
Typical cafibration curves are s h o w n in Fig. 4. Aliquots o f standard iodate solution were added to (a) a pickled distilled water, (b) a pickled seawater, (c) a distilled water reagent blank a n d (d) a seawater reagent blank. (The reagent blanks were prepared by adding the pickling reagents to
3 4 8 S-C. PAl, G - C G O N G A N D K - K . Lit!
a sample in the reverse order.) The k values calculated from the four sets o f data are: 1084, 1086, 1087 and 1084M ' c m -~, respectively, showing that there was no salt effect on the absorptivity. The empirical k value could change from one batch o f reagent to another because the concentration o f iodide ( 4 M ) in reagent might not be exactly the same.
Turbidity blank
For waters containing suspended particles, a turbidity blank was obtained by treating the sample with excess thiosulphate or by adding a few drops o f sodium sulphite, and then measur- ing the absorbance again (Riley, 1975). In our experience the turbidity blank o f open ocean water rarely exceeds 0.001 A.
Reagent blanks
At least two possible sources o f error can be induced by the addition o f reagents: the impurities and the oxygen contained in the pickling reagents. The effect o f the former is termed as 'reagent blank" in this work, which is caused by the small a m o u n t o f iodine or iodate in the K ! reagent as well as other interfering c o m p o u n d s . It was measured by a procedure similar to that described by Broenkow and Cline (1969): to ~- B O D bottle fillted with 6 0 m l o f disillted water were added consecutively 0 . 5 m l o f sulphuric acid, 0,5ml o f alkaline iodide reagent (mixed thoroughly) and 0.5mi of m a n g a n e s e reagent. The absorbance measured at 456 n m was accounted for the "reagent blank', which should be deducted from the raw absorbance.
The oxygen contained in the pickling reagents was found s o m e w h a t difficult to measure. The m o s t practical way to compensate such effect is to subtract an empirical value from the final concentration, as de,~cribed in eqn. (7). Cak'ulation
The absorbance or Abs(raw) found for a
sample is corrected by:
Abs(corr) = Abs(raw) -- Abs(turb) - Abs(rea)
W h e r e Abs(turb) a n d Abs(rea) are readings for turbidity a n d reagent blanks. Tile oxygen con- centration o f a sample is then calculated by the following equation:
[O2](pM) - Abs(corr) x 2 x k x h
v~+v~
x 0.5 (pM) (7)
where k is the empirical extinction coefficient at 456 n m for the mixture o f molecular iodine and tri-iodide; l/~ is the volume (ml) o f the bottle, 58-61 in this study; V~ is the total volume (ml) o f pickling reagents (0.5 + 0.5 ml); V~ is the volume (ml) o f sulphuric acid (0.5 ml). The final correc- tion term: - 0.5 p M (or - 0.01 roll -~ ) was added to c o m p e n s a t e for the oxygen contained in the pickling reagents. This value was adopted from the estimation by M u r r a y et al. (1968).
Since(l/h + 1(.)/(~, - VD is nearly a c o n s t a n t (1.0258 + 0.0002), and k, h are fixed values, a simplified equation was used in quick calculations: [O2](vM) = F × Abs(corr) - 0 . 5 p M o r
[O~](mll i) = F x Abs(corr) - 0.01roll i
(8)
where F is a calibration factor, equivalents to (Vh + Vc)/(2 × k × b)/(Vh -- VD. F v a l u e m a y change slightly for differe~at batches o f alkaline iodide reagent, usually a r o u n d 4 7 3 p M (or 10.59mi1 t) for I cm cuvette depending on the final concentration o f iodide present.
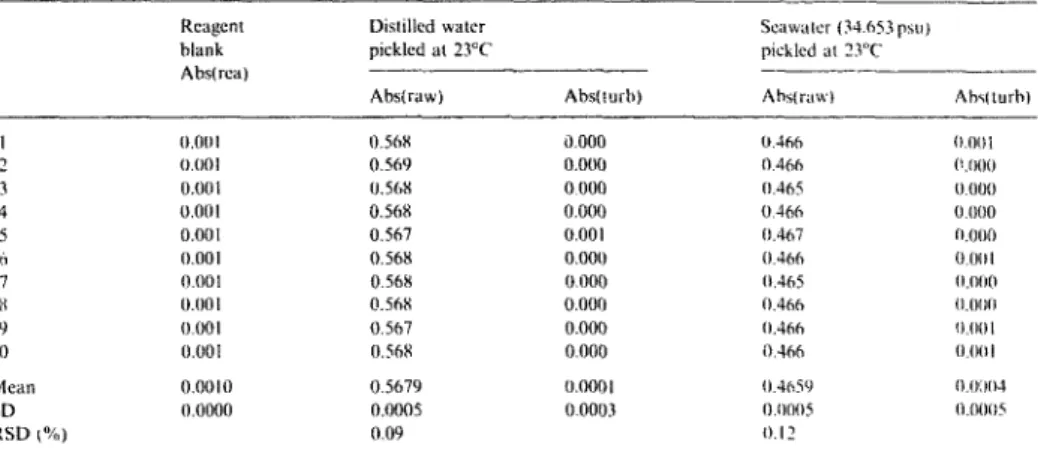
Precision and accuraQ'
T h e precision o f the present m e t h o d is d e m o n - strated in Table 1 For an air-saturated distilled water pickled at 23°C, the average a b s o r b a n c e was 0.568 + 0.0005 (n = 10). For a seawater
I)ETERMINATION DI" DISSOLVED ()XY(JEN IN SEAWATER T A B L E I
Laboratory evaluation of the precision of the spectropholometric method
349
Rcagem Distilled water blank pickled at 23°C Abslrea)
Seawater (34.653 psu) pickled at 23°C
Abslraw) Abs(turb) Abs(raw) Abslturb)
I 0.001 0.568 0.000 0.466 0,0()1 2 0.001 0,569 0.000 0.466 0.000 3 0.001 11.568 0000 0.465 0.000 4 0.001 0.568 0.000 (1466 0.000 5 0.001 0.567 0.001 O. 467 f).O00 6 0.001 0.568 0.000 0.466 0.0{)1 7 0.001 0.568 0,000 (I,465 0.000 ~ 0.001 0.568 0.000 0,466 (I.0110 9 0.001 0.567 0.000 0.466 0.001 I0 0.001 0.568 0.000 0.466 0.001 Mean 0.0010 0.5679 0.0001 0A659 0.09{)4 SD 0.0000 0.0005 0.0003 0.c)005 0.0005 RSD i % ) 0.09 0.12
Absorbances were measured al 456 nm in a I cm flo~ cuvette.
pickled at the same temperature, the mean result was 0.466 _+ 0.0005 0l = 10). Both show a relative standard deviation about 0.1%. In con- sidering the minimum resolution of the spectro- photometer (0.001 A), a precision of better than 0.2% shuuld be reported. The reagent blank was 0.001 A 01 = 101. The turbidity blank for the water test was almost zero. A similar set of measurements was carried out on board a research vessel for at least 30 samples of different depths, each replicated six times. An average relative standard deviation of less than 0.12% has been consistently obtained over an oxygen concentration of 2.5-4.8 mll ~.
The accuracy of the procedure was evaluated by comparing the results with the oxygen solubil- ity taken from the U N E S C O tables (Fig. 5). Although it was difficult to verity the exact oxygen concentration for the sample tested, the difference between the measured results and the calculated values was within 0.5% over a range of 3 - 6 ml 1-~. For oxic waters, a relative bias of less than 0.5% could be readily achieved. Detec- tion limit was estimated by taking 4.65 times the standard deviation of the reagent blank, to be Jess thall 0.51,M or 0.01 mll ~.
Comparison witk f i { r a i i o n met~'""~,i,*
A number of parallel determination was carried out on board R/V "Ocean Researcher I'" using both spectrophotometric and titrimetric methods. The results are plotted in Fig. 6. G o o d agreements between the two methods have been found. 7 >., "5 E 6 o *d
g~
m"t
(5 ? D 2 / / " 2 ,,a " o" S" od K i 3 z, 5 6Oxygen solubility (mL/L)
Fig. 5. Comparison on the results for a number of air- saturated samples using the proposed procedure and cal-
culat ed oxygen solubilities. Test range tbr temperature: 2 0 -
350 Dissolved oxygen (mL L) I 2 3 L. i I , , i
1000
t
E'i
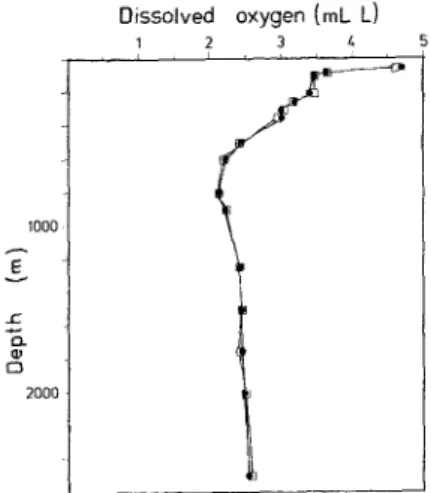
0 2000Fig. 6. Comparison of oxygen proliles obtained by (O) Winkler titration and (OI direct spectrophotometry. Both measurements were carried out on board R/V "Ocean Researcher |". Cruise OR1-246, at Station 1090 in the South China Sea on 18 July 1990.
The proposed spectrophotometric method has the following advantages over traditional Winkler titration method: (1) The Winkler titra- tion measures the amount o f total iodine in the bottle. The precision and accuracy o f the deter- m!- ~tion are very dependent upon the precision : , a accuracy of volumetric measurements, i.e. bottle volume, burette volume, and the decision of the end-point for the titre. The spectro- photometric method measures the concentration o f total iodine without titration, and therefore those volumemc errors do not exist. (2) The quantification o f oxygen in Winkler titration is based on the concentration o f thiosulphate, which needs to be standardized by potassium iodate. The spectrophotometric method is more straightforward because it uses potassium iodate directly for calibration, and therefore is more accurate, (3) The preparation work for titration is comparatively tedious and time consuming. The titration throughput is slow even aided by computerized end-point detection devices. The calculation o f oxygen concentration requires a
S.-C. PAL G.-C. G O N G A N D K.-K, LIU
note to be made o f the volume o f each individual sample bottle. In contrast, the spectrophotometric method is much simpler, quicker, and easier to learn. The results are calculated using a simple equation, and there is no need to know the exact volume o f each sample bottle. (4) The resolution o f the titration method depends on the sample volume used (normally 120-140 ml). The proposed spectrophotometric method measures the con- centration o f iodine, therefore smaller bottles (60 ml or even less) can be used without losing sensitivity.
However, ~ince the final concentration o f iodide can slightly affect the accuracy o f the spectrophotometric method, and the alkaline iodide reagent is difficult to prepare at required precision owing to its high viscosity, several pre- cautions should be taken: (1) the alkaline iodide reagent may undergo oxidation; this causes a high background value. The reagent blank should be measured frequently. The empirical extinction coefficient k (or factor F ) for different batches o f alkaline iodide reagent should be carefully cal- culated by the iodate spiking experiment just before the determination, or whenever the reagent bottle is refilled. (2) The addition o f alkaline iodide solution must be performed carefully to ensure that the volume (0.5ml) is added to all samples with a precision o f less than _+ 5%. Frequent checks on the tip of the dispenser to avoid bubbles is necessary.
C O N C L U S I O N
lodometry is still the most precise way o f determining dissolved oxygen in seawater. Improvements have been made to the colori- metric determination o f liberated iodines by use o f an air-contact-free sipper system m conjunc- tion with a wide bore flow cuvette coupled with a regular spectrophotometer. These modifi- cations stabilized the absorbance readings by completeiy eliminating iodine loss and manual movement o f the cuvette alignment. They enable a precision o f ca. 0.2% (full scale) to be easily attained even by i n inexperienced analyst. The
DETERMINATION OF DISSOLVED OXYGEN IN SEAWATER 351 s p e c t r o p h o t o m e t r i c m e t h o d is v e r y c o n v e n i e n t f o r o n - b o a r d o p e r a t i o n . I t s q u i c k t h r o u g h p u t is o f g r e a t v a l u e in t h e c a l i b r a t i o n o f t h e C T D o x y g e n sensor. T h e p r o p o s e d p r o c e d u r e h a s b e e n n a m e d t h e ' S h i b a l a ' m e t h o d in o u r l a b o r a t o r y a f t e r a l o c a l dice g a m e w h i c h s h o w s c o n c s e c u t i v e n u m b e r s o f 4, 5, 6 in a row. ACKNOWLEDGEMENTS T h e a u t h o r s w o u l d l i k e to t h a n k T i n g - Y u K u o , Y i h - C h u n g Li, C h u n g - C h e n g Y a n g , K w u n g - L u n g Jeng, W e n - J o n g Y a n g , the C a p t a i n a n d c r e w o f t h e R / V " O c e a n R e s e a r c h e r F ' for t h e i r k i n d a s s i s t a n c e w i t h the s h i p - b o a r d e x p e r i m e n t s . T h e a u t h o r s a r e a l s o g r a t e f u l t o Prof. J.P. R i l e y for his c r i t i c i s m a n d s u g g e s t i o n s o n t h e m a n u - script. T h i s project w a s s u p p o r t e d by the N a t i o n a l Science C o u n c i l , T a i w a n ( # N S C 80-0209- M002a-20),
REFERENCES
Broenkow, W.W. and Cline, J.D., 1969. Colorimetric deter- ruination of dissolved oxygen at low concentrations, Limnnl. Oceanogr,, 14(3): 450-454.
Burger, J.D. and Liebhafsky, H.A., 1973. Thermodynamic data for aqueous iodine solutions at various tempera- lures, An exercise in analytical chemistry, Anal. Chem., 45: 600-602.
Carpenter, J.H., 1965a. The accuracy of the Winkler method for dissolved oxygen analysis, Limnot. Oceanogr., 10( 1 ): 135-140.
Carpenter, J.H., 1965b. The Chesapeake Bay Institute technique for the Wink!er dissolved oxygen method, Limnol. Oceanogr.. 10(1): 14t-143.
Green, EJ. and Carritt, D,E., 1966. An improved iodine determination flask for whole-bottle titrations. Analyst, 91 : 207-208.
Knapp, G.P., Stalcup, M.C. and Stanley, R.J., 1991. Iodine losses during Winkler titrations. Deep-Sea Res., 38(t): 121-128.
Murray, CM.. Riley, J.P. and Wilson. T.R.S., 1968. The solubility of oxygen in Winkler reagents used for the determination of dissolved oxygen. Deep-Sea Res., 15: 237-238.
Novic, M.. Pihlar, B. and Dular, M., 1988. Use of flow injection analysis based on iodometry for automation of dissolved oxygen (Winkler method) and chemical oxygen demand (dichromate method) determinations, Fresenius Z. Anal. Chem., 332: 750-755.
Riley, J.P., 1975. In: Analytical Chemistry of Seawater. Chemical Oceanography, Vol. 3. Academic Press. London, 258 pp.
Wong, G.T.F., 1982. The stability of molecular iodine in seawater, Mar. Chem., 11: 91-95.