Biomaterials 16 (1995) 793-802 0 1995 Elsevier Science Limited Printed in Great Britain. All rights reserved 014%96X2/95/$10.00
Mechanical properties and histological
evaluation of sintered /.?-Ca2P207 with
Na4P207*
1 OH,0
addition
Feng-Huei Lin, Chi-Chang Lin” , Chung-Ming Lu, Haw-Chang Liu”,
Jui-Sheng Sun* and Cheng-Yi Wang
Center for Bio%edical Engineering and ‘&artmenf of C&hopaedics, College of Medicine, Nafional Taiwan University, Taipei, Taiwan, ROC
The ultimate goal of implantation of biomaterials in the skeleton is to reach full integration of the non- living implant with the living bone. The biomaterial can be used much as a bone graft, resorbing or dissolving as bone growth occurs, and the end result is a new remoulded bone. Calcium pyrophos- phate, Ca2P207, is one of the intermediate products of bone mineralization. /I-Dicalcium pyrophos- phate (/I-DCP) doped with certain amounts of Na4P207.10H20 was prepared as the developed material. Na4P207.10H20 was used as a liquid-phase additive to improve the sintering process and promote physiological bioresorbability. Compressive strength and four-point bending strength were measured by the Bionix test system 858. The mechanical strength of the sintered jI-DCP increased with the addition of Na4P207.10H20 up to 5wt%, but thereafter decreased. The microstructure and crystal structure were analysed by the techniques of SEM, EPMA, TEM and XRD. The relationship between the mechanical strength of the sintered bioceramics and the Na4P207.10H20 dopant was examined in terms of the presence of NaCa(PO&, grain growth and abnormal grain coalescence while the dopant increased. Preliminary in viva evaluation was studied by rabbit femur condyle implantation. There was no inflammation or any toxic sign during the experimental period. The histological section of intraosseous implantation revealed that the new bone deposited directly on the surface of the material in the fourth week after operation. The implant gradually decreased in volume and was replaced by the surrounding regenerated bone in the rabbit condyle in vivo environment. The results led us to conclude that the developed material has great potential as a biodegradable bone substitute.
Keywords: Bone substitute, ceramics, biodegradation, mechanical properties, histological evaluation
Received 10 April 1994; accepted 15 October 1994
The capacity of the human body to generate bone components that are lost, damaged or diseased is
limited. Consequently, surgeons have long
endeavoured to find or develop materials that might adequately replace bone tissue, especially minera- lized tissues such as bone and teeth’,‘. Autogenous bone graft is considered to be the most suitable transplant material, because differences in histocom- patibility and the risk of transferring a disease from one individual to another are non-existent. However, removal of the bone graft creates additional surgical trauma, and its supply may not be available in sufficient quantity, particularly during childhood. Moreover, the risks of autograft for all patients are longer operations and anaesthesia, high blood loss, infection, damage to nerves and blood vessels, thrombosis and fracture. Allogenic and xenogenic bone transplants represent the alternatives to Correspondence to Professor Jui-Sheng Sun.
autogenic bone transplants for certain indications. However, they require considerable care, involve very high costs, cause problems by resorption and disease transfer, and regularly provoke an immunode- fensive reaction, which limits their efficiency, application and availability3*4.
To overcome these problems, various artificial materials to fill bone defects have been developed. Having the same chemical composition as natural
bone, calcium phosphate ceramic products are
regarded as promising bone substitute materials in the orthopaedic field. The aim of a bone substitute is initially to refill the defective bone structure and finally to be replaced by the host new bone4V5. In order to reach this expectation, the implant has to be bioactive and biodegradable. Most of the products of
calcium phosphate ceramics were found to be
bioactive so that osteogenic mesenchymal tissues can be attracted and proceed to form new bone. Hydroxy- apatite (HA) and tricalcium phosphate (TCP) are two 793 Biomaterials 1995, Vol. 16 No. 10
794 Sintered fl-DCP with Na4P207.10H20 addition: F.-H. Lin et al. major products commonly used in the conventional
market. Because their calcium/phosphate (Ca/P) ratios resemble that of natural bone, they are mostly stable in the physiological environment. HA is the major consti- tuent of bone materials and is almost non-degradable even after a long period of implantation, while TCP is more or less resorbable. However, the extent of degradation of TCP mostly depends on its structure. It was reported to be completely resorbed in the powder form but only partially resorbed in a block form. In practice, the granular or block form of the bone substi- tute is more compatible for clinical usezS6-*. Thus, searching for a new biodegradable ceramic material is the primary goal of this study.
HA and other complex calcium phosphate salts are the end products of the biological mineralization process. /?-Dicalcium pyrophosphate (J-DCP), formula CazP207, is one of the intermediate products in this processlS4. Its Ca/P ratio is 1, far less than TCP, so that it is potentially biodegradable in comparison to TCP. The biological response for new bone development is quite similar to HA in spite of the low Ca/P ratio’-“. Because of these advantages, we selected fl-DCP as the raw material for the new bone substitute.
However, the development of the sintering technique for this /I-DCP ceramic is not that easy. The sintering temperature of fi-DCP is about 1000°C. The extent of volume reduction during sintering will affect its microstructure and mechanical strength. In addition, the cost of the heating facility is rather high for sinter- ing calcium phosphate ceramics in a block form at temperatures higher than around 1000°C. In order to reduce the sintering temperature, enhance the volume reduction and improve the initial mechanical strength of the developed material, our modification was to add some sintering additives. One of the sodium polyphos- phates, Na4Pz0,.10Hz0, was chosen as a sintering additive in developing our new product.
Pure b-DCP and some compositions of fi-DCP and Na4P207.10H20 were built up in disc-shaped block forms. The volume reduction during sintering and the mechanical strength were measured by using a dilatometer and a Bionix test system 858, respectively.
In vivo evaluations including biocompatibility, bioresorbability, new bone regeneration and response in rabbit femur condyles are described in this study. The results of the study of the development of the bone substitute are supported by its positive perfor- mance in the in viva experiments.
MATERIALS AND EXPERIMENTS
Material preparation
P-DCP powder of mean grain size 0.1 pm was used in the experiment. The specific area determined by BET analysis was 51 f 0.2 cm’g-‘. Trace elements that might be connected with biocompatibility were detected by atomic absorption analysis. The concentra- tion of the trace elements was much lower than the maximum tolerable level”.
The g-DCP powder was mixed with Na4Pz07.10Hz0 in water and dried for 3 days at 70°C. The well-mixed
and dried cake was ground and sieved into 40-60 meshed particles. The sieved particles were then compacted in a stainless steel die under a hydrostatic pressure of 270MPa and a green density of about 60% T.D. was obtained. Na4Pz07.10Hz0 was added to /?- DCP in a series of compositions of 1, 2.5, 5, 7.5, 10, 12 and 15% weight ratio. A group of the test samples with a stepwise series of compositions of the sintering additive, Na,P,07.10H20, were also manufactured in a dense block form which was more suitable for mechan- ical strength measurement. The implant samples for the biological test were 6 mm in diameter and 4 mm thick and were made with 48% porosity by mixing with poly(ethylene glyco1.4000) of particle sizes of 5 and 500pm to produce macro- and micropore structures after decomposition”. The prepared green material was placed on a platinum sheet and heated to various temperatures at a heating rate of 3”Cmin-’ in a program-computerized Ni-Cr coiled furnace, and then maintained for about 1 h after the sintering tempera- ture was reached.
Measurement of mechanical strength
In the compressive mode, a parallel cylinder of the materials was prepared and external load applied so that the specimen was macroscopically in a state of uniaxial stress. The height/diameter ratio was lower than a critical value in order to eliminate the possibi- lity of instability (bucking). Owing to the anisotropy of the individual grains, the state of stress is not uniaxial at the microscopic level; stress and strain inhomogene- ities establish themselves inside the individual grains. However, in the treatment given here, these localized variations were not consideredl’.
Bending strength was measured by a four-point
loading method using rectangular specimens
5 mm x 5 mm x 40 mm abraded with alumina powder and diamond paste. The lower and upper span lengths were 32 mm and 16mm, respectively. A cross-head speed of 0.5 mm min-’ was used at room temperature. Ten specimens were prepared for each condition to measure the compressive strength and four-point bending strength, respectively’3.
Operation and implantation
New
Zealand white male rabbits with an average weight of 3-3.5 kg were used in the study. They were delivered to the laboratory not later than 1 week before the start of the test, and acclimatized to the housing conditions, tap water and the standard dry feed diet. The animals were kept in single stainless steel cages at 22’C and 60% relative humidity in natural daylight with a day-night rhythm.The surgical operations were carried out in a conven- tional operating theatre with the animals lying supine on an operating table. They were anaesthetized by intravenous injection of pentobarbital (50 mg kg-‘) and local administration of 0.5% lidocaine. After shaving, disinfecting and sterile draping of the operation site, the femoral condyles were exposed by means of a medical longitudinal incision. Initially, a bone defect was created by a 3.2mm drill and was subsequently expanded with a 6mm drill. All the drill holes were
Sintered a-DCP with Na4P207.10H20 addition: F.-H. Lin et al. 795
carefully rinsed with Ringer’s solution and cleaned out, so that any abraded particles formed during drilling were removed. These defects were then completely filled with the impla.nt materials. The periosteum and fascia were sutured .with catgut; the skin was sutured with silk. The animals were administered antibiotic prophylaxis orally for 4 days after the implantation procedure.
After 1, 2, 4, 6, 8 and 12 weeks, the animals were
killed with an overdlose of intravenous pentobarbital. A total of 36 rabbits divided into six groups for the above experimental time periods was used in the study. Twelve legs were examined for each time period. Six legs were used for the fl-DCP ceramic implantation and six were saved as a control group. The control group was an empty defective cavity without any bone substitute inside. The two groups were randomly applied to 12 legs with no rabbit receiv- ing the same treatment to both legs. Double labelling was performed in all rabbits with tetracycline (intramuscular administration) and calcine green (subcutaneous administration), which were injected alternately every other week after the operationl’.
Measurements and analysis
Histological evaluations
The legs were harvested from the treated animals at the end of the mentioned time periods after operation. Implants and surrounding tissues were removed en bloc, washed in normal saline and fixed in 10%
neutral buffered formalin to be processed for histology. The specimens were dehydrated in an ascending series of alcohol and embedded in a methyl methacrylate resin, After polymerization, the specimens were sectioned with a diamond saw at about 200pm and ground down to about 30pm. About four sections were cut for each implant, parallel to the major axis. All the slices were histologically examined under optical transmission and fluorescence microscopes’4”5.
Quantitative evaluation was then performed using a semiautomatic histomorphometric method. The system consisted of a microscope with cross-polarization filters, a digitizing platen, a digitizer and a microcom- puter. The microscope was equipped with a drawing tube, through which the image of the digitizing platen was projected over the optical field. By moving a cursor on the digitizing platen, which was visible by its projection over the histological field, the areas of the regenerated bone, implanted materials and pores in the defective cavity were traced and calculated by the microcomputer, and expressed as a percentage of the area of the defective cavity12*‘4.
Specimen characterization
The specimens were characterized by means of a number of techniques, including X-ray diffraction (XRD), scanning electron microscopy (SEM), transmis- sion electron microscopy (TEM) and dilatometry.
The microstructures of the sintered P-DCP were observed by SEM. The surfaces (2mm thick) were coated with a thin layer of carbon, after being polished with diamond paste and etched with 48% HF for about 10s. They were then observed by SEM and
analysed using an energy-dispersive electron-probe X-
ray microanalyser. P, Ca and Na were analysed across the grains and grain boundaries. An electron beam maintained at 2 x 10W1’ A was used and X-ray intensi-
ties in counts per second (cps) were recorded. The accelerating voltage was 12 kV. Ultra-thin sections for
TEM were obtained from the sintered specimen by slicing with a diamond blade saw and an ultrasonic cutter. The slices were polished with diamond abrasive to a thickness of 30pm on the dimple grinder and then mounted on a copper ring. The specimens were finally thinned by ion-beam milling. The crystal structure of the intergrain material was investigated using a Hitachi-700 STEM operating at 175 kV. Selected area diffraction patterns were recorded with photographic plates. Tilting the crystal from one orientation to another was carried out in the selected area diffraction mode using the double tilt holder.
The optimum sintered temperature and duration were decided according to the analysis result of the dilatometer. A specimen 5 mm in diameter and 7mm in length was placed in the dilatometer, where the sintered shrinkage curve was recorded on an X-Y pen recorder; X and Y axes were displacement (in terms of linear shrinkage, AL/Lo) and temperature, respec- tively. All crystalline phases existing in the sintered specimens were identified by the X-ray diffractometer. The phase contents of the sintered /I-DCP were detected by the relative intensities of the (008) and (132) reflections of /3-CaZP20, and CaNa(PO&, respec- tively15.
RESULTS AND DISCUSSION
Mechanical strength and microstructures
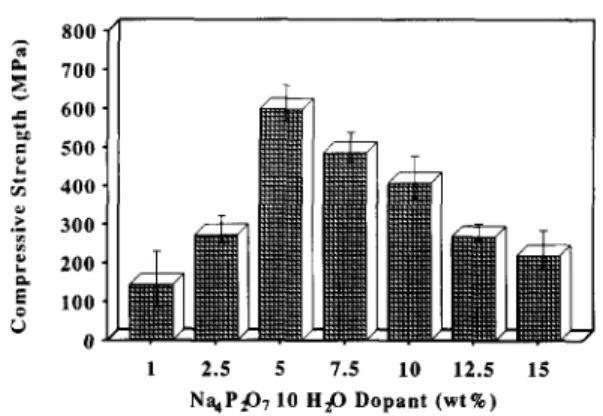
Figures I and 2 show the compressive strength and
four-point bending strength, respectively, of sintered p- DCP bioceramics prepared by adding different quanti- ties of Na4Pz07.10H20. The values for the compressive and four-point bending strengths of pure /I-DCP bioceramic without addition of Na4P207~10H20 were only 159 and 35MPa, respectively, much lower than
the values for sintered /I-DCP with 5 wt%
Na4P207~10H20 (589 and 128MPa). It is clear that the strength of the fl-DCP dense material initially increased on addition of Na4P207~10H20 up to 5wt%, but thereafter decreased.
Densification and growth of the solid particles in the liquid-phase sintering process have been analysed by dividing it into three steps16-“:
1. The liquid flow or rearrangement stage. On formation of a liquid phase, there is a rearrange- ment of particles to give a more effective packing. This process can lead to complete densification if the volume of liquid present is sufficient to fill the interstices completely. In this stage, densification is brought about under the action of capillary pressure by the collapse of melt bridges between particles and by the arrangement in which solid particles slide over one another.
2. The solution-reprecipitation or accommodation process. In this stage further densification and
796 Sintered /&DCP with Na4P207.10H20 addition: F.-H. Lin et al. 800 -
2
r_
700. c 600. L E 500. z 400. F ‘;: 300. P k 200. E 6 100. O- 1 2.5 5 7.5 10 12.5 15 NqP@, 10 H@ Dopant (wt%)Figure 1 Compressive strength of sintered /Gdicalcium pyrophosphate on addition of Na4P207.10H20.
_~ G E 140 z 120 F 100 % 80 $ 60 2 40 ‘5 L 20 vi 0 1 2.5 5 7.5 10 12.5 Na$,O 710 HP Dopant (wtC)
Figure 2 Four-point bending strength of 15
sintered /I- dicalcium pyrophosphate on addition of Na4P207.10H20.
3.
growth of particles of the solid phase are achieved by solution, reprecipitation and coalescence.
The solid-state sintering stage. In many cases of liquid-phase sintering, complete densification is achieved during the first two sintering stages. Prolonged holding of compacts at the sintering temperature may lead to microstructural changes in the dense compacts, including further growth of the particles and formation of a rigid skeleton of solid phase in the system (abnormal grain growth)l’. The microstructure of the sintered p-DCP ceramic containing 2.5 wt% Na4P207.10H20 is shown in
Figure 3~. Porosity was seen to remain in the sintered /3-DCP bioceramic. Liquid-phase sintering was applied in the sintering process during the addition of Na4Pz07.10H20. However, insufficient Na,P,O,~lOH,O was added to fl-DCP, so it was not easy to bring about liquid-phase sintering for densification. In this case, the /?-DCP bioceramic could not densify completely and remove porosity; thus a lower mechanical strength was obtained.
The linear shrinkage curves of pure sintered P-DCP and sintered /?-DCP doped with different quantities of
Na4P207.10H20 were recorded by using the
dilatometer and are shown in Figure 4. Pure /3-DCP ceramic had the lowest linear shrinkage of only S%, whereas P-DCP ceramic doped with Na4P207.10H20 up to 5 wt% demonstrated the greatest linear shrinkage of 13.1%. In Figure 3b, the sintered j-DCP bioceramic with 5wt% Na4P,07.10H20 addition showed a dense
microstructure with a uniform grain size correspond- ing to the higher mechanical strength179’g.
As seen in Figure 5, the grain size of sintered fi-DCP increased with the addition of Na4P207.10H20. The effect of grain size on the fracture strength has been generally interpreted in terms of the dependence” ,J = f(G)-“‘, where cr is the fracture strength, G is the grain size and f is a constant. Consequently, the larger the grain size of the ceramics, the lower the fracture strength obtained. Moreover, the higher quantities of Na,P,07~10H,0 doped into the j-DCP bioceramics would speed up atomic diffusion between the grain boundaries and promote a sintered process or densifi- cation rate that might lead to an abnormal grain growth or rigid skeleton formation in the sintered system, as shown in Figure 6. Growth by coalescence in liquid-phase
postulatedr6, ‘s.
sintering has been repeatedly The process has also been treated analytically. If the dihedral angle in the sintering system is positive, grain boundaries between coales-
Figure 3 The microstructure of sintered j?-DCP with addition of: a, 2.5wt% and b, 5wt% Na.,P207.10H20. The former shows a smaller average grain size of about 0.75pm and pores remain between the intergranular area. The latter has an average grain size of about 1.3pm and shows a much more compact microstructure.
Sintered fl-DCP with N,a4P207.10H20 addition: F.-H. Lin et al. 797
Curve A,: Pure (Owt%) Curve FL: Z.Swt% Curve c: Swt%
Curve o: lSwt%
200 400 600 800
Temperature (“(2)
Figure 4 Linear shrinkage of pure sintered /3-DCP and /3- DCP doped with different amounts of Na4P207.10H20.
12 5 s g ;ij .I! 6 e v 3 0 2.!5 5 7.5 10 12.5 15 NaJ CJ lOH,O Dopant (wt%)
Figure 5 The mean grain size of sintered p-DCP biocera-
mics plotted against thfe amount of Na4P207.10H20.
cence particles will be formed and an aggregate of two, three or more grains will be established. These aggregates may result in the formation of a rigid skeleton or abnormal large grains. Figure &I shows the microstructure of /LDCP bioceramic doped with 7.5 wt% Na4Pz07.10Hz0, where the coalescence grains and coalescence lines were observed. The microstruc- ture of the P-DC:P bioceramic with 12.5wt%
Na4PZ07.10Hz0 addition shows (Figure 6b) the
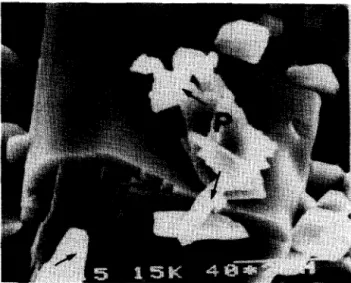
existence of the rigid. skeleton and the abnormal large grains. Stress would concentrate on these abnormal large grains when the specimen was under mechanical loading. This stress-concentration effect might lead to fracture generated along the abnormal lkrge grains or rigid skeletons. Figure 7 shows a typical fracture
generating along an abnormal large grain for the fi-DCP bioceramic with addition of 15 wt% Na4P207.10H20.
Crystalline identification
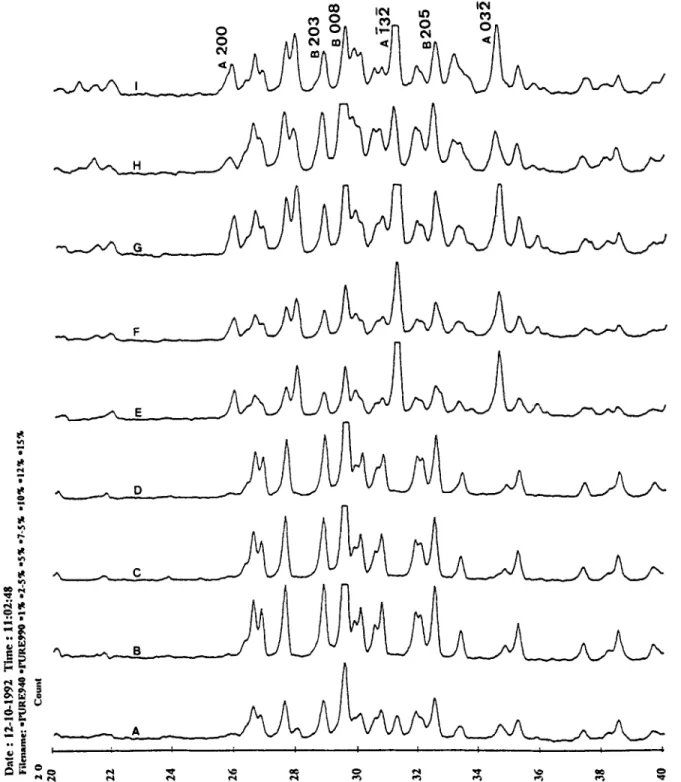
Figure 8 shows the X-ray diffraction pattern of sintered
P-DCP bioceramics with different quantities of Na,P,07.10HZ0. No phase other than B-DCP is identi- fied up to 5wt% addition of Na4Pz07.10Hz0. After addition of 5wt% N+Pz07.10H20, the second phase of NaCa(PO& is observed where the leading peak of NaCa(PO,), is A(132), as shown in Figure 8. If R is the
ratio of peak height of NaCa(POJ, A(132) to that of the sintered /GDCP bioceramic B(008), then the larger the value of R, the larger the amount of NaCa(PO,), obtained in the matrix. The values of R have a clear
tendency to increase with the amount of
Na.+P207.10Hz0 added. The existence of a second
Figure 6 The microstructure of sintered /?-DCP biocera- mics with addition of a, 7Swt% and b, 12.5wt%
Na4P207.10H20. The formation of coalescence grains (C) and coalescence lines (arrows) was observed. Abnormal grains are indicated by AG.
Figure 7 Micrograph of sintered /3-DCP bioceramic with 15 wt% Na4P207.10H20 addition, demonstrating a typical fracture due to the stress concentrating on the abnormal grain. The second $phase observed in the matrix is identi- fied as NaCa(PO.& and is indicated by NP and arrows.
798 Sintered /I-DCP with Na4P20,~10H20 addition: F.-H. Lin et a/.
Figure 8 X-ray diffraction patterns of sintered p-DCP with different amounts of Na4P207.10H20: A, pure a-DCP sintered at
940°C; B, pure /I-DCP sintered at 990°C; C, 1 wt%; D, 2.5wt%; E, 5wt%; F, 7.5wt%; G, lOwt%; H, 12.5wt%; I, 15wt% addition.
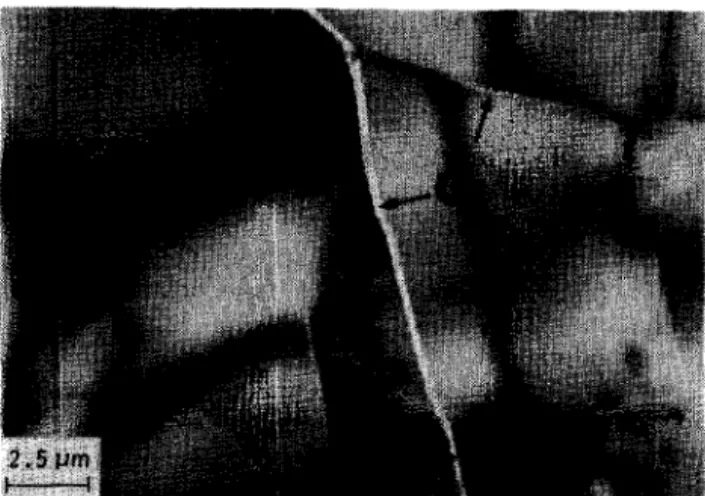
phase in sintered fl-DCP might lead to the initiation of fracture growth13*20. The glassy phase distributed along the grain boundaries and the second phase precipitated in the matrix could be observed using TEM of the sintered fl-DCP bioceramic doped with 7.5wt% Na,P,O,.lOH,O, as shown in Figure 9. The second phase was identified as the NaCa(P03), crystal by the selected area diffraction pattern. The spot pattern was the (231) orientation of the NaCa(PO& crystal and was analysed by the equivalent superim- posed projection pattern shown in Figure 10. The
NaCa(PO,), crystal was precipitated as a spindle- shaped grain (Figure 7) when the N~P207.10H20 addition was increased further. The chemical composi- tion of the spindle-shaped grains was mkasured by EPMA-WDS and was in good agreement with that of the NaCa(P03)3 crystal.
The above facts can be summarized as follows: the mechanical strength of sintered /I-DCP bioceramics after addition of 5 wt% N%P207-10HzO is probably due to the presence of a second phase, increasing grain size and abnormal grain growth.
Sintered b-DCP with NaqPp0,.10H20 addition: F.-H. Lin et a/. 799
Figure 9 Transmission electron micrograph of sintered B- DCP bioceramic with addition of 7.5 wt% Na4P207.10H20: G, glassy phase; NP, NaCa(PO&.
Since /?-DCP sintered with 5 wt% NQP~O,~~OH~O had a
better mechanical strength, it has been chosen as a potential material to implant into the rabbit femur condyle and to evaluate the possibility of use as a bone substitute. Most pora’us calcium phosphate implants currently being invesitigated have been fabricated to have interconnecting macropores in the range from 100 to 400pm in order to meet the requirements for bone ingrowth21V22. One interesting and guiding sugges- tion, proposed by de Grootz3, is that it is actually the micropores in these structures which determine the rate of bioresorbability. In the designed P-DCP implant,
interconnected micropores (about 5pm) and
macropores (100400~m) were prepared by adding two different sizes of poly(ethylene glycol) particles as foaming agent. The pores were left in the matrix after the foaming agent decomposed at the volatilization temperature; they were uniformly distributed in the gross polycrystalline structure and frequently localized at the grain boundaries which connected the indivi- dual crystallites.
The amounts of neyly grown bone, implants and pores were expressed as a percentage of their area relative to the area of the defective cavity; the area of defective cavity was defined as 100%. The area occupied by the pores included areas of soft tissue, connective tissue, bone marrow, osteoids, etc.
Figde 11 summarizes the results of the quantitative
assessment. One week postoperatively, histomorpho- metric quantitation of the P-DCP bioceramics demonstrated a new bone area fraction of l.3%, an implant traction of 50.4% and a pore fraction of 48.3%. The area of regenerated bone gradually increased from 1.3 to 38% during the postoperative
period from 1 week up to 12 weeks. The fraction of
pore area, however, had a negative tendency while the postoperative period increased; the area fraction decreased from 48.3% (week 1 postoperatively) to
27.3% (week 32 postoperatively). The area fraction of
the designed bioceramic also decreased as the experi- mental period increased; the area fraction decreased from 50.4% in the first week down to 34.7% at the end of the twelfth week after operation. The results of the quantitative assessment showed significant proof of the new bone growing well into the macropores, the implant gradually dissolving in the in viva environ- ment, and the materials progressively being replaced by the new regenerated bone.
Histo~ogicd evaluation
The formation of new bone in the empty cavities [control group) was restricted to the edge of the defect. The large part at the centre of the defect remained free of bone during the entire course of the test up to 12 weeks, and was filled with bone marrow rich in fat cells. When the results obtained were evaluated as a function of time, it became apparent that between 2 and 12 weeks, the time elapsed after implantation had practically no influence on the amount of regenerated bone present. In other words, progressive bony reconstruction of the defect cavity with the course of time was not detected. In long-term experiments, the bone defect was still clearly visible after 6 months.
Figure 10 The NP grain indicated in Figure 9 was identified as the Na4P207.10H20 crystal in the (231) orientation by the selected area diffraction pattern.
800 Sintered [GDCP with Na4P207.10H20 addition: F.-H. Lin et al.
1 2 4 6
Weeks
8 12
Figure 11 The variations of occupied area of regenerated bone, implant and pores plotted against the implantation period.
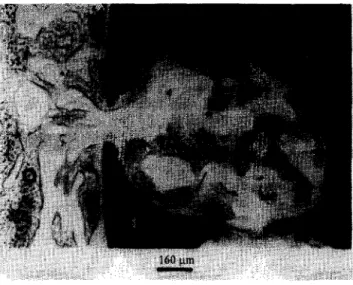
Figure 12 The immature woven bone was growing into the defect cavity in the fourth week after operation: N, new bone; 0, original bone; I, implant.
Microscopically, the sintered P-DCP bioceramics showed no intervening soft tissue and directly contacted the bone structure. In the first week after operation, the defective cavity was filled with a haematoma and a small amount of connective tissue was observed around the implant.
Two weeks postoperatively, the regenerated bone started growing into the macropores and immature woven bone was generated along the surface of the /I- DCP implant materials. After the fourth week postoperation, immature woven bone was found in the entire defect cavity, as shown in Figure 12. Figure 13 shows the results of histological examination at 6 weeks postoperation. The breakdown and dissolution of the sintered ,!I-DCP bioceramics were observed in the histological sections at this stage. The regenerated bone was vigorously growing into the macropores of the developed bioceramics. In the eighth week after operation, the implant gradually dissolved and was replaced by the bony structure. The laminar bone and Have&m system appeared at this stage. The sintered /I-DCP bioceramics continued to be dissolved, digested and replaced in the physiological environment in the twelfth week postoperation. It was apparent that bone regeneration was occurring in connection with, and
influenced by, the original bone and the P-DCP biocera- mics as incorporation of fluorescent dye into the regenerated bone surrounding the material was observed (Figure 14). The sintered /I-DCP with 5wt%
Na,P,O,~l0H,O addition bone substitute was thought to be a degradable ceramic. It was expected to be progressively phased out in the physiochemical environment and subsequently replaced by the regener- ated bone structure as the bone reconstruction and bone remodelling process occurred.
An understanding of this diverse bioresorbability behaviour can be gained by considering the biocera- mics from two points of view21~22~24-their crystal/ chemical composition and their structure as materials. Several workers have expressed the opinion that
calcium phosphates with Ca/P 6 1 could be
resorbed21*22S24. Microporosity, however, played a more dominant role in the resorption than macroporos- ity. Microporosity determined the geometry of ‘necks’
Figure 13 The material’s dissolution and breakdown debris were observed in the section 6 weeks postopera- tion: D, dissolution and breakdown debris; N, new bone; I, implant.
Figure 14 Incorporation of a fluorescent dye into the regenerated bone surrounding the implant; intensive bone regeneration can bb seen. There is no separating layer of connective tissue between the bone and the implant: N, new bone area: 0, original bone; I, implant.
Sintered /?-DCP with Na4P207.10H20 addition: F.-H. Lin et al. 801
between sintered particles, while macroporosity determined the number of ‘necks’ in contact with the environment. The ‘neck’ formation was dependent on the preparation technique, i.e. on the sintering tempera- ture and the pressure applied to compress the powder into a tablet before sintering21~22. In addition, the assumption was made that two different biological resorption pathways existed, solution-mediated processes (the implants dissolved in the physiological solution) and cell-mediated processes (phagocyto-
5,25
sis) .
It is quite possible that this microporosity could aid
in bioresorption by causing microscopic ‘break-up’ secondary to solution-mediated resorption. Thus, partially dissolved rnaterials could slough off indivi- dual crystals or fragments, as shown in Figure 13, that are sufficiently small to allow for aggressive cell- mediated removal. Moreover, much of the sodium- calcium-phosphate glassy phase was detected on the grain boundary and intergranular area in the developed bioceramic, which was described in the previous section. This would enhance the material’s dissolution process because the glassy phase was easily attacked by the in vivo fluid. Evidence in support of this hypothesis has been the observation of multinuclear-type cells in close proximity to digest the developed calcium phosphate implant. In some cases, ceramic fragments were indeed found to be present in vesicles of these cells. To summarize, from the observed biodegradable behaviour of the sintered /3- DCP with 5wt% addition of Na4P207~10H20, the conclusion can be drawn that biodegradation occurs in two steps. The first and most important step is the extracellular dissolution of the implant. The second step is the digestion and migration of the particles.
CONCLUSIONS
The compressive strength and four-point bending strength of sintered fi-DCP bioceramics with addition of Na4P207~10H20 initially showed a tendency to increase with the increasing addition of N~P207~10H20 up .to 5 wt%, after which the trend reversed. This could be interpreted as the presence of a second phase, increasing the grain size and abnormal grain growth while the Na4P207~10H20 dopant was increasing in the matrix.
The results of the quantitative assessment and histological evaluatioln showed significant evidence of the new bone prosperously growing into the macropores of the materials, the implant gradually dissolving in the i.n vivo environment and being progressively replaced by the regenerated bone. The bioresorbable behaviour of the sintered P-DCP biocera- mic doped with !jwt% N~P207~10H20 can be explained by a two-step process. The first step is the extracellular dissolution of the implant. The second step is the digestion and migration of the particles. The developed material will be phased out in the physio- chemical environment and subsequently replaced by the regenerated bone tissue. This is thought to have great potential in the field of orthopaedic applications in the near future.
ACKNOWLEDGEMENT
The authors sincerely thank the National Science Council (ROC) for their financial support of this research. We dedicate this paper with gratitude to the National Science Council, ROC.
REFERENCES 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21
Ducheyne P. Bioceramics: material characteristics versus in vivo behavior. j Biomed Mater Res: Appl
Biomater 1987; HA: 219-236.
Bucholz RW, Holmes RE. Hydroxyapatite and trical- cium phosphate bone graft substitute. Orthop Clin North Am 1987;18:323-334.
Stupp SI, Hanson JA, Eurell JA, Ciegler GW, Johnson A. Organoapatites: materials for artificial bone. III. Biologi- cal testing. JBiomed Mater Res 1993; 27: 301-311. Aebi M, Regazzoni P. Bone Transplantation, Updating on Osteochondral Auto- and Allografiing. Berlin:
Springer, 1987.
Klein CPAT, Lubbe HBM, de Groot K, Hooff A. Biodegradation behavior of various calcium phosphate materials in bone tissue. J Biomed Mater Res 1983; 17: 769-784.
Jarcho M. Calcium phosphate ceramics as hard tissue prosthetics. Clin Orthop Rel Res 1981; 158: 259-278. Klein CPAT, de Groat K, Driessen AA, Lubbe HBM. Interaction of biodegradable /3-TCP ceramics with bone tissue: an in vivo study. Biomaterials 1985; 8: 189-192. Kitsugi T, Yamamuro T, Nakamura T, Kotani S, Kokubo T. Four calcium phosphate ceramics as bone substitutes for non-weight-bearing, Biomaterials 1993; 4: 216-224. Weng J, Wang J, Chen W. In vivo response of biophasic calcium phosphate in dogs. In: Xingdong Zahang and Yoshito Ikada, eds. Proc 1st Far-Eastern Symp on
Biomedical Materials. Kyoto, Japan: Kobunshi
Kankakai, 1993: 153-154.
Lin FH, Hon MH. A study on synthesized hydroxyapa- tite bioceramics. Ceram Int 1989; 5: 53-536.
Nordberg GF. Efpects and Dose-Relationships of Toxic Metak. Amsterdam: Elsevier, 1976: 48-50.
Lin FH, Liu HC, Hon MH, Wang CY. Preparation and in
vivo evaluation of a newly developed bioglass ceramic. I BiomedEng 1993;15:481-486.
Meyers MA, Chawla KK. Mechanical Metallurgy- Principles and Applications. Englewood Cliffs, NJ:
Prentice-Hall, 1984.
Sheehan DC, Hrapchak BB. Theory and Practice of
Histotechnology. London: C.V. Mosby, 1980.
Lin FH, Hon MI-I, Huang YY. A study on the sintered j?- TCP bioceramics as bone substitute. J Biomed Eng Appl
Basis Commun (ROCJ 1991; 2: 98-106.
Lenel FV. Sintering in presence of liquid phase. Trans
AIME1984; 175:878-891.
Shackelford JF. Introduction to Materials Sciences and
Engineering, 2nd edn. New York, USA: Macmillan, 1990.
Yoon DN, Huppmann WJ. Grain growth and densifica- tion during liquid phase sintering. Acta Metal1 1979; 27:693-698.
Davidge RW. Mechanical Behavior of Ceramics,
Cambridge Solid State Science Series. Cambridge University Press, 1979.
Rolfe ST, Barson JM. Fracture and Fatigue Control in
Structures. Englewood Cliffs, NJ: Prentice-Hall, 1977.
Holmes RE. Porous hydroxyapatite as a bone graft substitute in metaphyseal defects. 1 Bone Surg 1986;
88A: 904-910.
802 Sintered b-DCP with Na4P207.10H20 addition: F.-H. Lin et al.
22 Klawitter JJ, Hulbert SF. Application of porous ceramics
for the attachment of load bearing internal orthopedic applications. J Biomed Mater Res, Symp 1972; 161.
23 de Groot K. Bioceramics consisting of calcium phosphate salts. Biomaterials 1980; 1: 47-58.
24 Tomas KA, Kay JF, Cook SD, Jarcho M. The effect of surface macrotexture and hydroxyapatite coating on
the mechanical strength and histological profiles of titanium implant materials. J Mater Res 1987; 21:
1395-1401.
25 Burt HM, Jackson JK, Rowe11 J. Calcium pyrophosphate and monosodium urate crystal interactions with neutro- phils: the effect of crystal size and lipoprotein binding to crystals. J Rheumatoll989; 16: 809-817.