Clincal Oral Implants Research
Initial stability and bone strain evaluation of the immediately loaded dental implant: an in vitro model study
Journal: Clinical Oral Implants Research Manuscript ID: COIR-Jan-10-OR-1426.R2 Manuscript Type: Original Research
Date Submitted by the Author: n/a
Complete List of Authors: Huang, Heng-Li; China Medical University, School of Dentistry Chang, Yin-Yu; Ming Dao University, Department of Materials Science and Engineering
Lin, Dan-Jae; China Medical University, Department of Dental Hygiene
Li, Yu-Fen; China Medical University, Institute of Environmental Health
Chen, Kuan-Ting; China Medical University, Biostatistics Center Hsu, Jui-Ting; China Medical University, School of Dentistry Keywords: Bone implant interactions, Statistics, Material sciences
Clincal Oral Implants Research
Initial stability and bone strain evaluation of the immediately loaded dental implant: an in vitro model study
Heng-Li Huang1, Yin-Yu Chang2, Dan-Jae Lin3, Yu-Fen Li4,5, Kuan-Ting Chen4, Jui-Ting Hsu1,*
1
School of Dentistry, China Medical University, 91 Hsueh-Shih Road, Taichung 404, Taiwan.
2
Department of Materials Science and Engineering, Ming Dao University, 369 Wen-Hua Road, Peetow Township, ChangHua 523, Taiwan.
3
Department of Dental Hygiene, China Medical University, 91 Hsueh-Shih Road, Taichung 404, Taiwan.
4
Biostatistics Center, China Medical University, 91 Hsueh-Shih Road, Taichung 404, Taiwan.
5
Institute of Environmental Health, China Medical University, 91 Hsueh-Shih Road, Taichung 404, Taiwan.
Running title: Initial stability and bone strain of immediate-load implants Keywords: primary implant stability, bone strain, insertion torque, Periotest,
resonance frequency analysis, cortical–bone thickness, elastic modulus of trabecular bone.
Corresponding author:
Jui-Ting Hsu, Assistant Professor; Address: School of Dentistry, College of Medicine, China Medical University, 91 Hsueh-Shih Road, Taichung 404, Taiwan; Tel: 886-4-22053366 ext. 2308; Fax: 886-4-22014043; E-mail address: jthsu@mail.cmu.edu.tw 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28 29 30 31 32 33 34 35 36 37 38 39 40 41 42 43 44 45 46 47 48 49 50 51 52 53 54 55 56 57 58 59 60
Clincal Oral Implants Research
Abstract
Objectives: The aim of this study was to evaluate the effects of cortical bone thickness and trabecular bone elastic modulus on the strain in the bone surrounding an immediately loaded implant. We also examined the correlations between bone structure and the following indices of primary implant stability: insertion torque value (ITV), Periotest value (PTV), and implant stability quotient (ISQ). Material and Methods: The ITV, PTV, and ISQ were measured in 24 artificial jaw bone models representing cortical bone with 4 thicknesses (0, 1, 2, and 3 mm) and trabecular bone with 4 elastic moduli (137, 47.5, 23, and 12.4 MPa). Two loading conditions were applied (force of 130 N applied vertically and at 45 degrees laterally), and the strains in the crestal region were measured by rosette strain gauges with a data acquisition system. Results: When the cortical–bone thickness and the elastic modulus of trabecular bone decreased, the bone strains increased by 10.3–52.1% and 39–73.1%, respectively, for vertical loading and by 35.0–62.0% and 42.4–56.2% for lateral loading. The cortical–bone thickness has a stronger correlation (R2 = 0.95–0.71) with ITV, PTV, and ISQ than the elastic modulus of trabecular bone (R2 = 0.89–0.59). Conclusions: The initial stability at the time of implant placement is influenced by both the cortical–bone thickness and the elastic modulus of trabecular bone; however, these parameters are not totally linearly correlated with ITV, PTV, and ISQ. The placement of an immediately loaded implant in cases with thin cortical bone and/or weak trabecular bone can induce extreme bone strains and may increase the risk of implant failure. 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28 29 30 31 32 33 34 35 36 37 38 39 40 41 42 43 44 45 46 47 48 49 50 51 52 53 54 55 56 57 58 59 60
Clincal Oral Implants Research
Introduction
Immediate loading of implants has been widely used in single tooth replacement and partially edentulous restorations (Gapski et al. 2003). This treatment is associated with higher satisfaction of patients due to the immediate restoration of esthetics and chewing functions. Although high survival rates have been reported for immediately loaded implants (Attard & Zarb 2005; Nkenke & Fenner 2006), this approach is still thought to be associated with a higher risk of implant failure (Ioannidou & Doufexi 2005). Bone quality and quantity have been considered important in both conventional (delayed) and immediate loading of implants (Gapski et al. 2003; Avila et al. 2007; Ostman 2008). The rate of successful implantation is high in the mandible (Adell et al. 1990; Laney et al. 1994), but implant failures remain relatively common in other regions, such as the posterior maxilla (Glauser et al. 2003; Tolstunov 2007), which has been related to bone softness.
Characteristics of the host bone such as the cortical bone thickness and the quality of the trabecular bone significantly influence the likelihood of implant success. Lekholm and Zarb (1985) classified bone quality into types 1–4, and this classification has been applied in clinics to evaluate the patient’s bone quality prior to implant placement. Some studies found that only 3% of implants placed in bone of type 1, 2, or 3 were lost, whereas the rate increased to 35% in bone of type 4; this was attributed to the presence of a thin cortical shell and softer trabecular bone (Jaffin & Berman 1991). Both orthopedic and dental-implant investigations have found that the cortical–bone thickness and the elastic modulus of trabecular bone significantly influence the holding strength of an implant (Seebeck et al. 2004; Hsu et al. 2007) as well as the stress and strain of bone tissue (Tada et al. 2003; Koca et al. 2005; Sevimay et al. 2005). Since the stress concentration around the implant is higher in 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28 29 30 31 32 33 34 35 36 37 38 39 40 41 42 43 44 45 46 47 48 49 50 51 52 53 54 55 56 57 58 59 60
Clincal Oral Implants Research
immediate-loading treatment than in conventional (delayed) treatment (Huang et al. 2008; Hsu et al. 2009), it is worth investigating the effects of bone quality and quantity on the loading of peri-implant bone, especially for immediately loaded implants.
The likelihood of implant failure is mainly determined by the primary stability. A higher primary stability—which corresponds to less micromotion between the implant and bone—is required for osseointegration during the healing period (Brunski et al. 2000). Several noninvasive techniques—including the peak insertion torque value (ITV), Periotest value (PTV), resonance frequency analysis (RFA) (Molly 2006), and peak removal torque value (Akkocaoglu et al. 2005; Tabassum et al. 2009)—can be used in both clinical and laboratory situations to diagnose stability problems of an implant related to thin cortical bone and poor-quality trabecular bone (Miyamoto et al. 2005; Alsaadi et al. 2007). However, the clinically used Lekholm and Zarb classification only provides a rough standard with which to assess the quality and quantity of jaw bone. Therefore, the correlations of these parameters with implant stability remain to be clarified.
This study applied strain-gauge analysis to artificial bone samples to investigate how the biomechanical performance (e.g., bone strain) is related to the precise characteristics of bone quality and quantity—including the thickness of cortical bone and the elastic modulus of trabecular bone—of an immediately loaded implant. In addition, the relationships of the primary implant stability with the cortical–bone thickness and the elastic modulus of trabecular bone were examined by measuring the implant stability quotient (ISQ), ITV, and PTV.
Materials and Methods 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28 29 30 31 32 33 34 35 36 37 38 39 40 41 42 43 44 45 46 47 48 49 50 51 52 53 54 55 56 57 58 59 60
Clincal Oral Implants Research
Specimen preparationModels of trabecular bone with four elastic moduli were created by attaching closed-cell foam representing trabecular bone with elastic moduli of 137, 47.5, 23, and 12.4 MPa (models 1522-09, 1522-10, 1522-11, and 1522-12; Pacific Research Laboratories, Vashon Island, WA, USA) to 2-mm-thick commercially available synthetic cortical shell (model 3401-01; Pacific Research Laboratories) with an elastic modulus of 16.7 GPa (Fig. 1a). The range of the elastic moduli of trabecular bone used in this study was based on the work of Misch et al. (1999). Similarly, closed-cell-foam rigid trabecular bone models with an elastic modulus of 23 MPa (model 1522-11, Pacific Research Laboratories) were prepared with and without attaching cortical shells of three thicknesses (1, 2, and 3 mm for models 3401-07, 3401-01, and 3401-02, respectively; Pacific Research Laboratories) (Fig. 1b). The synthetic bone had a rectangular shape with dimensions of 38 cm × 20 cm × 42 cm. Three specimens of each combination of artificial foam bone were prepared for implant stability measurements.
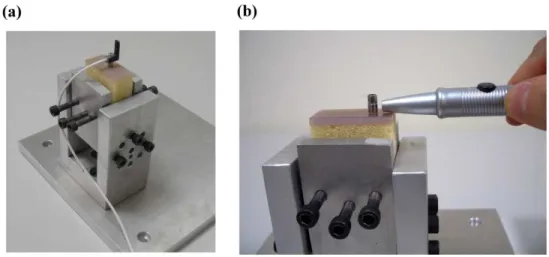
Implant stability measurement
The peak ITV was measured by inserting a 3.75 mm × 13 mm self-tapping implant (ICE®, 3i Implant Innovation, Palm Beach, FL, USA) into a 3.2-mm-diameter pilot hole into the bone block specimen by using a digital torque meter (TQ-8800, Lulton Electronic Enterprise, Taipei, Taiwan). After placing the implant, a resonance frequency analyzer (OsstellTM, Osstell, Göteborg, Sweden) was used to measure ISQ. The L-shaped transducer (Type F1 L5, Osstell) was kept perpendicular to the implant and was screwed by hand into the implant body as recommended by the manufacturer (Fig. 2a). In order to standardize the procedure, all measurements of ISQ were made 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28 29 30 31 32 33 34 35 36 37 38 39 40 41 42 43 44 45 46 47 48 49 50 51 52 53 54 55 56 57 58 59 60
Clincal Oral Implants Research
with the transducer perpendicular to the jaw. After connecting a 6-mm-long temporary abutment (hexed temporary cylinder, 3i Implant Innovation), the implant mobility was measured using the PeriotestTM device (Siemens, Bensheim, Germany) (Fig. 2b). The tip of the measurement device was positioned perpendicularly at 2 mm from the abutment, and it impacted the implant four times per second for 4 seconds (Alsaadi et al. 2007). ITV, ISQ, and PTV were all measured three times for each specimen.
Strain gauge measurement
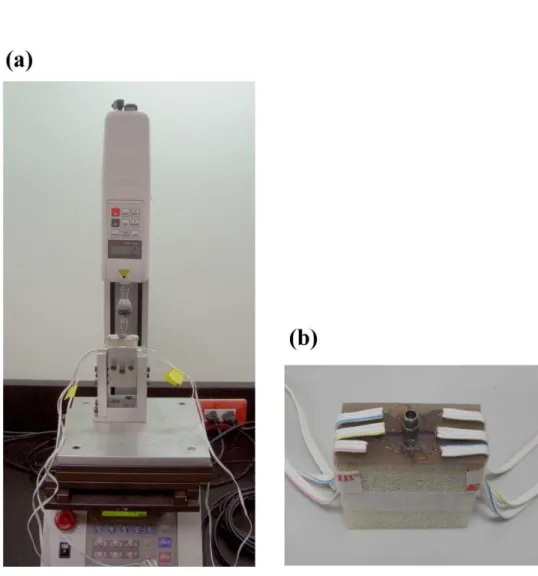
A self-developed jig was designed with an adjustable rotational screwing device so that both a vertical load and a 45-degree lingual lateral force could be applied in the experiments. Each loading procedure involved applying a force of 130 N to the cylindrical abutment using a universal testing machine (JSV-H1000, Japan Instrumentation System, Nara, Japan) with a head speed of 1 mm/min (Fig. 3a). Rectangular rosette strain gauges (KFG-1-120-D17-11L3M3S, Kyowa, Tokyo, Japan) were attached to the buccal and lingual sides of the crestal cortical region around the implant using cyanoacrylate cement (CC-33A, Kyowa) (Fig. 3b). Signals corresponding to the three independent strains εa, εb, and εc measured by the rosette strain gauge were sent to a data acquisition system (NI CompackDAQ, National Instruments, Austin, TX, USA) and analyzed by the associated software (LabVIEW SignalExpress, National Instruments). After each measurement was repeated three times for each specimen, the maximum (εmax) and minimum (εmin) principal strains were obtained (Hsu et al. 2009).
Correlation and statistical analysis 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28 29 30 31 32 33 34 35 36 37 38 39 40 41 42 43 44 45 46 47 48 49 50 51 52 53 54 55 56 57 58 59 60
Clincal Oral Implants Research
The peak minimum principal strains under vertical and lateral loading for the designed scenarios of cortical–bone thickness and elastic modulus of trabecular bone were summarized as medians and standard deviation values. Kruskal-Wallis test and Multiple comparison with Bonferroni test were used to assess differences. The same approach was applied for the measures of stability (ITV, PTV, and ISQ) in the models with various cortical–bone thicknesses and elastic moduli of trabecular bone. Quadric regression models were applied for the nonlinear relations between stability measures and both the cortical–bone thickness and the elastic modulus of trabecular bone. The goodness of fit for regression models was quantified using squared correlation coefficients (R2 values). All statistical analysis was performed with SAS software (SAS v9.1.2, SAS Institute, Cary, NC, USA) with an alpha value of 0.05.
Results
Strain gauge analysis
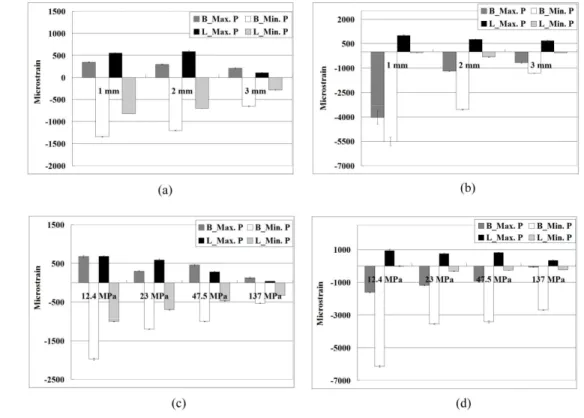
Peak strains in bone (minimum principal strains) around immediately loaded implants were at least twofold higher for lateral loading than for vertical loading (Fig. 4). The peak bone strains differed significantly between the models with cortical bone thicknesses of 1, 2, and 3 mm in both Kruskal-Wallis test and Multiple comparison with Bonferroni test (p < 0.05) (Table 1). The bone strains in the model with a 1-mm-thick cortical bone were 10.3% and 52.1% higher than those in models with 2- and 3-mm-thick cortical bone, respectively, for vertical loading (Fig. 4a), and 35.0% and 62.0% for lateral loading (Fig. 4b).
The highest strain values (minimum principal strains) differed significantly between the models with trabecular bone with elastic moduli of 12.4, 23, 47.5, and 137 MPa in Kruskal-Wallis test and Multiple comparison with Bonferroni test (p < 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28 29 30 31 32 33 34 35 36 37 38 39 40 41 42 43 44 45 46 47 48 49 50 51 52 53 54 55 56 57 58 59 60
Clincal Oral Implants Research
0.05) (Table 2). For vertical loading the bone strain was 39.0%, 49.1%, and 73.1% higher in the model with 12.4-MPa trabecular bone than those with 23-, 47.5-, and 137-MPa trabecular bone, respectively (Fig. 4c); the corresponding differences for lateral loading were 42.4%, 44.0%, and 56.2% (Fig. 4d).
Primary stability of implant
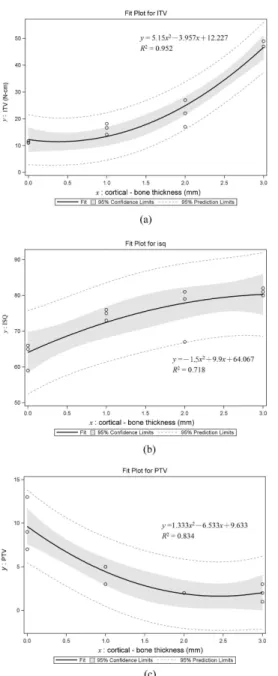
ITV, ISQ, and PTV and the correlation equations obtained by second-order quadratic regression between cortical bone thickness and implant stability are shown in Fig. 5a, 5b, and 5c, respectively. The R2 values are 0.95–0.71. In general, thinner cortical bone produced higher ITV and ISQ but a lower PTV (Fig. 5). ITV, ISQ, and PTV all varied significantly with the thickness of cortical bone in Kruskal-Wallis test (p ≦ 0.05): ITV rose increasingly while ISQ rose decreasingly when the thickness of cortical bone increased (Fig. 5a, Fig. 5b, and Table 3). In addition, PTV was reduced decreasingly when cortical bone was thicker (Fig. 5c and Table 3).
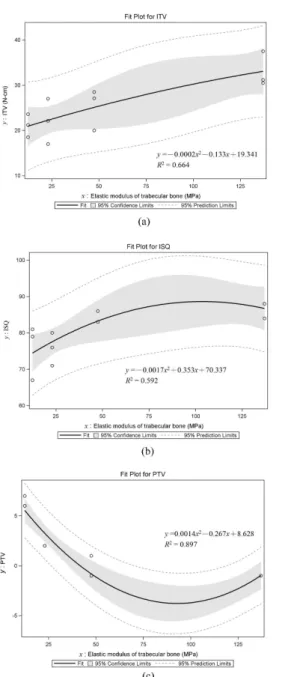
ITV, ISQ, and PTV and the correlation equations obtained by second-order regression between the elastic modulus of trabecular bone and implant stability are shown in Fig. 6a, 6b, and 6c, respectively. The R2 values are 0.89–0.59. ISQ and PTV varied significantly with the elastic modulus of trabecular bone in Kruskal-Wallis test (p < 0.05) (Table 4): ITV increased linearly (Fig. 6a) while PTV diminished decreasingly (Fig. 6b) and ISQ rose decreasingly (Fig. 6c) as the elastic modulus of trabecular bone increased (Table 4).
Discussion
The relationships of the bone strain and implant primary stability with bone quality and quantity have been investigated recently (Tada et al. 2003; Sevimay et al. 2005; 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28 29 30 31 32 33 34 35 36 37 38 39 40 41 42 43 44 45 46 47 48 49 50 51 52 53 54 55 56 57 58 59 60
Clincal Oral Implants Research
Alsaadi et al. 2007; Rozé et al. 2009). The bone quality is especially important in the planning of treatments involving immediate loading of an implant, which makes a specific classification related to implant primary stability necessary. Many studies have used the classification of Lekholm and Zarb (1985) to quantify the bone quality (Molly 2006; Alsaadi et al. 2007; Fischer et al. 2009) because it is easy and inexpensive to apply. However, this classification provides only a rough estimate of bone quality. Moreover, no controlled studies have compared precisely how the behavior of immediately loaded implants is influenced by the bone quality. The use of artificial bone is one approach to investigating how the cortical–bone thickness and the elastic modulus of trabecular bone affect the bone strain and implant primary stability. Previous studies (Song et al. 2007; Tabassum et al. 2009) have represented trabecular bone using solid rigid foam, whereas the samples of trabecular bone in the present study were all closed-cell rigid foam with an architecture similar to that of trabecular bone (Krenn et al. 2008). This approach might provide more reliable data on how the bone quality and quantity actually affect the bone strain and primary stability in implants subject to immediate loading.
Strain distributions in bone are greatly dependent on the quantity of cortical bone adjacent to an immediately loaded implant. In this study, the bone strain in immediately loaded implants increased significantly when the cortical bone was thinner. The actual thickness of the cortical bone differs with its location, condition, and age, such as mandible versus maxilla, anterior region versus posterior region, and even dentate versus edentulous jaws. Therefore, clinical evaluations of possible implantation sites (e.g., using dental CT) might be necessary for precise measurements of the cortical bone thickness. There are also other critical components to consider in the planning of dental implant therapy (Katranji et al. 2007). In the 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28 29 30 31 32 33 34 35 36 37 38 39 40 41 42 43 44 45 46 47 48 49 50 51 52 53 54 55 56 57 58 59 60
Clincal Oral Implants Research
present study, thicker cortical bone was associated with a lower bone strain, which might represent a favorable clinical condition especially for patients requiring immediate-loading treatment.
The peri-implant bone strain does not decrease linearly with increasing the elastic modulus of trabecular bone. In this study, even though increasing the elastic modulus of trabecular bone significantly decreased the bone strain around the implant, the degree of the reduction differed between dense and soft trabecular bone. For example, in contrast to the situation for dense trabecular bone (e.g., elastic modulus > 23 MPa), halving the elastic modulus of softer trabecular bone (e.g., from 23 to 12.4 MPa) markedly increased the bone strain especially under a lateral occlusal load. Looking closely at the anatomy of the jaw bone reveals that trabecular bone is surrounded by a thick cortical shell; therefore, both cortical bone and trabecular bone are well designed to dissipate and transfer loads. In contrast, in aged patients the structures of both cortical bone and trabecular bone are degenerated, and poor trabecular bone is normally accompanied with thin cortical bone. This condition would greatly increase the bone strain and may be unsuitable for the treatment of immediate implant loading.
The relationships of bone quality and quantity with ISQ and PTV have been widely discussed by many authors (Morris et al. 2003; Molly 2006; Alsaadi et al. 2007; Ito et al. 2008; Fischer et al. 2009; Rozé et al. 2009; Trisi et al. 2009). RFA and PTV are noninvasive techniques that are easy to apply clinically, which makes them favorable for use by dentists wanting to determine the primary implant stability after implantation, although some factors should be considered before using RFA (Scarano et al. 2006) and PTV (Chavez et al. 1993). The application of second-order regression in the present study revealed strong correlations of PTV with cortical bone–thickness 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28 29 30 31 32 33 34 35 36 37 38 39 40 41 42 43 44 45 46 47 48 49 50 51 52 53 54 55 56 57 58 59 60
Clincal Oral Implants Research
(R2 = 0.83) and elastic modulus of trabecular bone (R2 = 0.89). Moreover, the initial implant stabilities as measured by RFA and PTV were influenced by the thickness of the cortical shell and the elastic modulus of trabecular bone (p ≦ 0.05). Even though the correlation between ISQ and cortical-bone thickness is lowest (R2 = 0.72) among 3 types of measurements, the present finding indicates that increasing the cortical–bone thickness increases ISQ is also in accordance with data obtained in studies of cadavers and animals (Miyamoto et al. 2005; Yi et al. 2005; Ito et al. 2008; Rozé et al. 2009). For example, Miyamoto et al. (2005) found a significant correlation between ISQ and the thickness of the cortex shell. Because the elastic modulus is much higher for cortical bone (16.7 MPa) than for trabecular bone, increasing the thickness of thin cortical bone (1- or 2-mm thick) can provide adequate holding for an implant at the time of installation.
ISQ rose with the increase of the elastic modulus of trabecular bone, which may improve bone anchorage in a nonosseointegrated bone–implant contact (BIC). Our results consist with Nkenke et al.’s study (2003) which found that ISQ measured in cadaver jaw bone was correlated with the nonosseointegrated BIC of the oral aspect of the specimen even though the value of correlation coefficient was low. However, care is needed when using the BIC to assess the relationship between implant stability and ISQ, since some studies have found no correlation between the progressive osseointegration of BICs and ISQs (Ito et al. 2008; Abrahamsson et al. 2009). The effectiveness of using RFA to predict the osseointegration level of an implant needs further scientific investigations. In addition, thickening the cortical bone and increasing the elastic modulus of trabecular bone decreased PTV in the present study, which is consistent with the clinical study of Alsaadi et al. (2007) finding that bone quality is related to PTV based on the Lekholm and Zarb index (Lekholm & Zarb 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28 29 30 31 32 33 34 35 36 37 38 39 40 41 42 43 44 45 46 47 48 49 50 51 52 53 54 55 56 57 58 59 60
Clincal Oral Implants Research
1985). An increased primary implant stability may promote osseointegration.
In the present study, ITV was correlated with both the cortical–bone thickness and the elastic modulus of trabecular bone. These observations agree with those of Trisi et al. (2009), who found that ITV differed significantly between hard and soft bone. An increased ITV improves the primary stability (O’Sullivan et al. 2000) and reduces micromotion between the implant and bone (Trisi et al. 2009). The decreased micromotion might help to achieve better osseointegration for the immediately loaded implant and reduce the risk of implant failure (Gapski et al. 2003; Nkenke & Fenner 2006), and hence produce a favorable clinical outcome. Therefore, the bone quality and quantity—both of which are related to the thickness of corticalbone thickness and the elastic modulus of trabecular bone—should be determined at the time of surgery so as to improve the likelihood of success of immediate-loading therapy.
Conclusions
The present study investigated how the bone strain is affected by the cortical– bone thickness and the elastic modulus of trabecular bone, and measured the correlations between these bone characteristics and the clinically used ITV, ISQ, and PTV parameters. Although this study employed bone models with an advanced closed-cell-foam structure in order to accurately simulate bone characteristics, real bone is a living tissue and hence bone remodeling and other biological factors could influence the initial stability of an implant. Therefore, further clinical studies are needed to elucidate the detailed biomechanical mechanisms underlying how primary implant stability is affected by bone quality and quantity. Moreover, although vertical and oblique loads have been suggested to accurately represent occlusal loads (Geng et al. 2001), the chewing movement represents a dynamic loading that needs to be 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28 29 30 31 32 33 34 35 36 37 38 39 40 41 42 43 44 45 46 47 48 49 50 51 52 53 54 55 56 57 58 59 60
Clincal Oral Implants Research
considered in future investigations.
Within the limitations of the current study, the following conclusions can be drawn:
1. The strains induced in bone around an immediately loaded implant are at least twofold higher for lateral loading than for vertical loading.
2. In the presence of thin cortical bone and/or weak trabecular bone, immediate implant loading induces a large bone strain. The bone strain around an immediately loaded implant can be reduced by placing the implant into thicker cortical bone and/or trabecular bone with a denser structure. In addition, there is a nonlinear relationship between the bone strain and the elastic modulus of trabecular bone—the bone strain is increased more when the elastic modulus of softer trabecular bone is decreased than when that of denser trabecular bone is decreased.
3. In second-order regression the cortical–bone thickness has a stronger correlation (R2 = 0.95–0.71) with ITV, PTV, and ISQ than the elastic modulus of trabecular bone (R2 = 0.89–0.59). The effect of cortical–bone thickness on ITV and ISQ increases but that on PTV decreases when cortical bone is thicker. Moreover, the increase in ITV becomes linear in trabecular bone with a higher elastic modulus.
Acknowledgement
This research was supported by National Science Council (NSC 98-2320-B-039-005-MY3), Taiwan. References 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28 29 30 31 32 33 34 35 36 37 38 39 40 41 42 43 44 45 46 47 48 49 50 51 52 53 54 55 56 57 58 59 60
Clincal Oral Implants Research
Abrahamsson, I., Linder, E. & Lang, N. P. (2009) Implant stability in relation to osseointegration: an experimental study in the labrador dog. Clinical Oral Implants
Research 20: 313-318.
Adell, R., Eriksson, B., Lekholm, U., Branemark, P. I. & Jemt, T. (1990) Long-term follow-up study of osseointegrated implants in the treatment of totally edentulous jaws. The International Journal of Oral and Maxillofacial Implants 5: 347-359. Akkocaoglu, M., Uysal, S., Tekdemir, I., Akca, K. & Cehreli, M. C. (2005) Implant design and intraosseous stability of immediately placed implants: a human cadaver study. Clinical Oral Implants Research 16: 202-209.
Alsaadi, G., Quirynen, M., Michiels, K., Jacobs, R. & van Steenberghe, D. (2007) A biomechanical assessment of the relation between the oral implant stability at
insertion and subjective bone quality assessment. Journal of Clinical Periodontology
34: 359-366.
Attard, N. J. & Zarb, G. A. (2005) Immediate and early implant loading protocols: a literature review of clinical studies. The Journal of Prosthetic Dentistry 94: 242-258. Avila, G., Galindo, P., Rios, H. & Wang, H. L. (2007) Immediate implant loading: current status from available literature. Implant Dentistry 16: 235-245.
Brunski, J. B., Puleo, D. A. & Nanci, A. (2000) Biomaterials and biomechanics of oral and maxillofacial implants: current status and future developments. The International
Journal of Oral and Maxillofacial Implants 15: 15-46.
Chavez, H., Ortman, L.F., DeFranco, R.L. & Medige, J. (1993) Assessment of oral implant mobility. Journal of Prosthetic Dentistry 70:421-426.
Fischer, K., Backstrom, M. & Sennerby, L. (2009) Immediate and early loading of oxidized tapered implants in the partially edentulous maxilla: a 1-year prospective clinical, radiographic, and resonance frequency analysis study. Clinical Implant 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28 29 30 31 32 33 34 35 36 37 38 39 40 41 42 43 44 45 46 47 48 49 50 51 52 53 54 55 56 57 58 59 60
Clincal Oral Implants Research
Dentistry and Related Research 11: 69-80.Gapski, R., Wang, H. L., Mascarenhas, P. & Lang, N. P. (2003) Critical review of immediate implant loading. Clinical Oral Implants Research 14: 515-527.
Geng, J.P., Tan, K.B. & Liu, G.R. (2001) Application of finite element analysis in implant dentistry: a review of the literature. Journal of Prosthetic Dentistry
85:585-598.
Glauser, R., Lundgren, A. K., Gottlow, J., Sennerby, L., Portmann, M., Ruhstaller, P. & Hammerle, C. H. F. (2003) Immediate occlusal loading of branemark tiunite(tm) implants placed predominantly in soft bone: 1-year results of a prospective clinical study. Clinical Implant Dentistry and Related Research 5: 47-56.
Hsu, J. T., Chang, C. H., Huang, H. L., Zobitz, M. E., Chen, W. P., Lai, K. A. & An, K. N. (2007) The number of screws, bone quality, and friction coefficient affect
acetabular cup stability. Medical Engineering and Physics 29: 1089-1095.
Hsu, J. T., Fuh, L. J., Lin, D. J., Shen, Y. W. & Huang, H. L. (2009) Bone strain and interfacial sliding analyses of platform switching and implant diameter on an immediately loaded implant: experimental and three-dimensional finite element analyses. Journal of Periodontology 80: 1125-1132.
Huang, H. L., Hsu, J. T., Fuh, L. J., Tu, M. G., Ko, C. C. & Shen, Y. W. (2008) Bone stress and interfacial sliding analysis of implant designs on an immediately loaded maxillary implant: a non-linear finite element study. Journal of Dentistry 36: 409-417. Ioannidou, E. & Doufexi, A. (2005) Does loading time affect implant survival? A meta-analysis of 1,266 implants. Journal of Periodontology 76: 1252-1258. Ito, Y., Sato, D., Yoneda, S., Ito, D., Kondo, H. & Kasugai, S. (2008) Relevance of resonance frequency analysis to evaluate dental implant stability: simulation and histomorphometrical animal experiments. Clinical Oral Implants Research 19: 9-14. 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28 29 30 31 32 33 34 35 36 37 38 39 40 41 42 43 44 45 46 47 48 49 50 51 52 53 54 55 56 57 58 59 60
Clincal Oral Implants Research
Jaffin, R. A. & Berman, C. L. (1991) The excessive loss of branemark fixtures in type iv bone: A 5-year analysis. Journal of Periodontology 62: 2-4.
Katranji, A., Misch, K. & Wang, H. L. (2007) Cortical bone thickness in dentate and edentulous human cadavers. Journal of Periodontology 78: 874-878.
Koca, O. L., Eskitascioglu, G. & Usumez, A. (2005) Three-dimensional finite-element analysis of functional stresses in different bone locations produced by implants placed in the maxillary posterior region of the sinus floor. The Journal of Prosthetic
Dentistry 93: 38-44.
Krenn, M.H., Piotrowski, W.P., Penzkofer, R. & Augatm P. (2008) Influence of thread design on pedicle screw fixation. Laboratory investigation. Journal of Neurosurgery.
Spine. 9:90-95.
Laney, W. R., Jemt, T., Harris, D., Henry, P. J., Krogh, P. H., Polizzi, G., Zarb, G. A. & Herrmann, I. (1994) Osseointegrated implants for single-tooth replacement: progress report from a multicenter prospective study after 3 years. The International Journal of
Oral and Maxillofacial Implants 9: 49-54.
Lekholm, U. & Zarb, G. A. (1985) Patient selection and preparation. In: Brånemark
PI, Zarb GA, Albrektsson T (eds). Tissue-integrated prostheses: osseointegration in clinical dentistry. Chicago: Quintessence: 199-209.
Misch, C.E., Qu, Z. . & Bidez, M.W. (1999) Mechanical properties of trabecular bone in the human mandible: implications for dental implant treatment planning and surgical placement. Journal of Oral and Maxillofacial Surgery 5: 700-706.
Miyamoto, I., Tsuboi, Y., Wada, E., Suwa, H. & Iizuka, T. (2005) Influence of cortical bone thickness and implant length on implant stability at the time of surgery—clinical, prospective, biomechanical, and imaging study. Bone 37: 776-780.
Molly, L. (2006) Bone density and primary stability in implant therapy. Clinical Oral 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28 29 30 31 32 33 34 35 36 37 38 39 40 41 42 43 44 45 46 47 48 49 50 51 52 53 54 55 56 57 58 59 60
Clincal Oral Implants Research
Implants Research 17(s): 124-135.Morris, H. F., Ochi, S., Crum, P., Orenstein, I. & Plezia, R. (2003) Bone density: its influence on implant stability after uncovering. Journal of Oral Implantology 29: 263-269.
Nkenke, E. & Fenner, M. (2006) Indications for immediate loading of implants and implant success. Clinical Oral Implants Research 17(s): 19-34.
Nkenke, E., Hahn, M., Weinzierl, K., Radespiel-Troger, M., Neukam, F. W. & Engelke, K. (2003) Implant stability and histomorphometry: a correlation study in human cadavers using stepped cylinder implants. Clinical Oral Implants Research 14: 601-609.
O’Sullivan, D., Sennerby, L. & Meredith, N. (2000) Measurements comparing the initial stability of five designs of dental implants: a human cadaver study. Clinical
Implant Dentistry and Related Research 2: 85-92.
Ostman, P. O. (2008) Immediate/early loading of dental implants. Clinical documentation and presentation of a treatment concept. Periodontology 2000 47: 90-112.
Rozé, J., Babu, S., Saffarzadeh, A., Gayet-Delacroix, M., Hoornaert, A. & Layrolle, P. (2009) Correlating implant stability to bone structure. Clinical Oral Implants
Research 20: 1140-1145.
Scarano, A., Degidi, M., Iezzi, G., Petrone, G. & Piattelli, A. (2006) Correlation between implant stability quotient and bone-implant contact: a retrospective
histological and histomorphometrical study of seven titanium implants retrieved from humans. Clinical Implant Dentistry and Related Research 8:218-222.
Seebeck, J., Goldhahn, J., Stadele, H., Messmer, P., Morlock, M. M. & Schneider, E. (2004) Effect of cortical thickness and cancellous bone density on the holding 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28 29 30 31 32 33 34 35 36 37 38 39 40 41 42 43 44 45 46 47 48 49 50 51 52 53 54 55 56 57 58 59 60
Clincal Oral Implants Research
strength of internal fixator screws. Journal of Orthopaedic Research 22: 1237-1242. Sevimay, M., Turhan, F., Kilicarslan, M. A. & Eskitascioglu, G. (2005)
Three-dimensional finite element analysis of the effect of different bone quality on stress distribution in an implant-supported crown. The Journal of Prosthetic Dentistry
93: 227-234.
Song, Y. Y., Cha, J. Y. & Hwang, C. J. (2007) Mechanical characteristics of various orthodontic mini-screws in relation to artificial cortical bone thickness. The Angle
Orthodontist 77: 979-985.
Tabassum, A., Meijer, G. J., Wolke, J. G. C. & Jansen, J. A. (2009) Influence of the surgical technique and surface roughness on the primary stability of an implant in artificial bone with a density equivalent to maxillary bone: a laboratory study. Clinical
Oral Implants Research 20: 327-332.
Tada, S., Stegaroiu, R., Kitamura, E., Miyakawa, O. & Kusakari, H. (2003) Influence of implant design and bone quality on stress/strain distribution in bone around
implants: a 3-dimensional finite element analysis. The International Journal of Oral
and Maxillofacial Implants 18: 357-368.
Tolstunov, L. (2007) Implant zones of the jaws: implant location and related success rate. Journal of Oral Implantology 33: 211-220.
Trisi, P., Perfetti, G., Baldoni, E., Berardi, D., Colagiovanni, M., Scogna, G. & Pellico, V. S. (2009) Implant micromotion is related to peak insertion torque and bone density.
Clinical Oral Implants Research 20: 467-471.
Yi, Y. J., Park, C. J. & Cho, L. R. (2005) An evaluation of the primary implant stability and the immediate load-bearing capacity according to the change of cortical bone thickness. The Korean Academy of Prosthodontics 43: 248-257.
3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28 29 30 31 32 33 34 35 36 37 38 39 40 41 42 43 44 45 46 47 48 49 50 51 52 53 54 55 56 57 58 59 60
Clincal Oral Implants Research
Figure Legends
Fig. 1. (a) Artificial jawbone specimens of trabecular bone with elastic moduli of 12.4, 23, 47.5, and 137 MPa (from left to right). (b) Cortical shells with various thicknesses (d=0, 1, 2, and 3 mm) were attached to trabecular bone with an elastic modulus of 23 MPa.
Fig. 2. (a) L-shaped transducer set up as recommended by the manufacturer (Osstell) after the bone model was fixed into the jig. (b) PTVs were acquired after the rod of the Periotest device touched the abutment.
Fig. 3. (a) Application of forces to the top of the implant by a loading machine. (b) Two rosette strain gauges were attached to the bone surface buccolingually near the implant.
Fig. 4. Median and standard deviation values of maximum and minimum principal microstrains of bone in the models with cortical bone thicknesses of 1, 2, and 3 mm under vertical loading (a) and lateral loading (b), and in those with trabecular bone with elastic moduli of 12.4, 23, 47.5, and 137 MPa under vertical loading (c) and lateral loading (d). B and L represent the buccal and lingual sides, respectively; Max. P and Min. P represent the maximum and minimum principal strains, respectively. Fig. 5. Second-order regressions and squared correlation coefficients of ITV (a), ISQ (b), and PTV (c) with the cortical-bone thicknesses.
Fig. 6. Second-order regressions and squared correlation coefficients of ITV (a), ISQ (b), and PTV (c) with the elastic moduli of trabecular bone.
3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28 29 30 31 32 33 34 35 36 37 38 39 40 41 42 43 44 45 46 47 48 49 50 51 52 53 54 55 56 57 58 59 60
Clincal Oral Implants Research
Table 1. Peak values of the minimum principal strain of bone around implants with four cortical-bone thicknesses (the elastic modulus of trabecular bone was 23 MPa).
Microstrain, Median‡ (IQR) Thickness of cortical
bone Vertical loading Oblique loading 1 mm -1340.7 c (8.7) -5510.7 c (482.1) 2 mm -1200.4 b (14.9) -3552.3 b (38.9) 3 mm -645.3 a (14.6) -1310.6 a (20.0) P† 0.03 0.03 † Kruskal-Wallis test. ‡
Multiple comparison with Bonferroni test; Medians with the same letter are not significantly different at the 0.05 level. Three
specimens of each combination of artificial foam bone were prepared for the measurement.
Table 2. Peak values of the minimum principal strain of bone around implants with four elastic moduli of trabecular bone (the cortex thickness was 2 mm).
Microstrain, Median‡ (IQR) Elasticities of trabecular
bone Vertical loading Oblique loading 12.4 MPa -1975.6 d (63.9) -6136.9 c (107.4) 23 MPa -1200.4 c (14.9) -3552.3 b (38.9) 47.5 MPa -1005.3 b (14.5) -3412.4 b (99.3) 137 MPa -532.1 a (15.0) -2695.0 a (19.2) P† 0.02 0.02 † Kruskal-Wallis test. ‡
Multiple comparison with Bonferroni test; Medians with the same letter are not significantly different at the 0.05 level. Three specimens of each
combination of artificial foam bone were prepared for the measurement. 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28 29 30 31 32 33 34 35 36 37 38 39 40 41 42 43 44 45 46 47 48 49 50 51 52 53 54 55 56 57 58 59 60
Clincal Oral Implants Research
Table 4. ITV, PTV and ISQ of implants with trabecular bone with four elastic moduli (the cortex thickness was 2 mm).
Evaluation Approaches, Median‡ (IQR) Elasticities of trabecular
bone ITV PTV ISQ
12.4 MPa 21.2b (5.1) 6.0a (1.0) 76.0a (9.0) 23 MPa 22.1ab (10.0) 2.0b (0.0) 79.0a (14.0) 47.5 MPa 27.1ab (8.5) -1.0c (2.0) 83.0a (3.0) 137 MPa 31.2a (7.0) -1.0c (0.0) 88.0a (4.0) P† 0.06 0.02 0.03 † Kruskal-Wallis test. ‡
Multiple comparison with Bonferroni test; Medians with the same letter are not significantly different at the 0.05 level. Three specimens of each combination of artificial foam bone were prepared for the measurement.
Table 3. ITV, PTV, and ISQ of implants with four cortical-bone thicknesses (the elastic modulus of trabecular bone was 23 MPa).
Evaluation Approaches, Median‡ (IQR) Thickness of cortical
bone ITV PTV ISQ
None 11.1c (0.7) 9.0a (6.0) 65.0c (7.0) 1 mm 16.6bc (4.1) 5.0b (2.0) 75.0ab (3.0) 2 mm 22.1b (10.0) 2.0c (0.0) 79.0abc (14.0) 3 mm 47.0a (2.0) 2.0b (2.0) 80.5ab (1.0) P† 0.02 0.02 0.05 † Kruskal-Wallis test. ‡
Multiple comparison with Bonferroni test; Medians with the same letter are not significantly different at the 0.05 level. Three specimens of each
combination of artificial foam bone were prepared for the measurement. 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28 29 30 31 32 33 34 35 36 37 38 39 40 41 42 43 44 45 46 47 48 49 50 51 52 53 54 55 56 57 58 59 60
Clincal Oral Implants Research
Fig. 1. (a) Artificial jawbone specimens of trabecular bone with elastic moduli of 12.4, 23, 47.5, and 137 MPa (from left to right). (b) Cortical shells with various thicknesses (d=0, 1, 2, and 3 mm)
were attached to trabecular bone with an elastic modulus of 23 MPa. 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28 29 30 31 32 33 34 35 36 37 38 39 40 41 42 43 44 45 46 47 48 49 50 51 52 53 54 55 56 57
Clincal Oral Implants Research
Fig. 2. (a) L-shaped transducer set up as recommended by the manufacturer (Osstell) after the bone model was fixed into the jig. (b) PTVs were acquired after the rod of the PeriotestTM device
touched the abutment. 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28 29 30 31 32 33 34 35 36 37 38 39 40 41 42 43 44 45 46 47 48 49 50 51 52 53 54 55 56 57
Clincal Oral Implants Research
Fig. 3. (a) Application of forces to the top of the implant by a loading machine. (b) Two rosette strain gauges were attached to the bone surface buccolingually near the implant.
91x89mm (300 x 300 DPI) 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28 29 30 31 32 33 34 35 36 37 38 39 40 41 42 43 44 45 46 47 48 49 50 51 52 53 54 55 56 57
Clincal Oral Implants Research
Fig. 4. Median and standard deviation values of maximum and minimum principal microstrains of bone in the models with cortical bone thicknesses of 1, 2, and 3 mm under vertical loading (a) and lateral loading (b), and in those with trabecular bone with elastic moduli of 12.4, 23, 47.5, and 137
MPa under vertical loading (c) and lateral loading (d). B and L represent the buccal and lingual sides, respectively; Max. P and Min. P represent the maximum and minimum principal strains,
respectively. 175x133mm (300 x 300 DPI) 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28 29 30 31 32 33 34 35 36 37 38 39 40 41 42 43 44 45 46 47 48 49 50 51 52 53 54 55 56 57
Clincal Oral Implants Research
Fig. 5. Second-order regressions and squared correlation coefficients of ITV (a), ISQ (b), and PTV (c) with the cortical-bone thicknesses.
116x239mm (300 x 300 DPI) 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28 29 30 31 32 33 34 35 36 37 38 39 40 41 42 43 44 45 46 47 48 49 50 51 52 53 54 55 56 57
Clincal Oral Implants Research
Fig. 6. Second-order regressions and squared correlation coefficients of ITV (a), ISQ (b), and PTV (c) with the elastic moduli of trabecular bone.
116x239mm (300 x 300 DPI) 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28 29 30 31 32 33 34 35 36 37 38 39 40 41 42 43 44 45 46 47 48 49 50 51 52 53 54 55 56 57