Role of marine larval duration and growth rate of glass eels in determining the distribution of Anguilla reinhardtii and A. australis on Australian eastern coasts
全文
(2) Mar. Freshwater Res., 2002, 53, 687–695. Role of marine larval duration and growth rate of glass eels in determining the distribution of Anguilla reinhardtii and A. australis on Australian eastern coasts Jen-Chieh ShiaoA, Wann-Nian TzengA,D, Adrian CollinsB and Yoshiyuki IizukaC A. Department of Zoology, College of Science, National Taiwan University, Taipei, Taiwan 106, ROC. Bribie Island Aquaculture Research Centre, 144 North Street Woorim, Bribie Island, QLD 4057, Australia. C Institute of Earth Sciences, Academia Sinica, Nankang, Taipei, Taiwan 106, ROC. D To whom correspondence should be addressed. Email: wnt@ccms.ntu.edu.tw. B. Abstract. The differences in geographical distribution between Anguilla reinhardtii and A. australis on the eastern coast of Australia can be understood by comparing otolith growth increments and microchemistry, the ages between species of the eels at metamorphosis from leptocephalus to glass eels and the ages of glass eels at estuarine arrival. The ages at metamorphosis were determined from where the increment width dramatically increased and the Sr/Ca ratio dropped. The mean age (± s.d.) of A. reinhardtii (n = 176) at metamorphosis was 144.5 ± 12.2 days and at estuarine arrival was182.7 ± 16.3 days. For A. australis (n = 150) it was 173.7 ± 20.5 days and 229.2 ± 29.4 days, respectively. The differences in age between species were significantly larger than the annual and seasonal variations within species. Australian eels are believed to spawn in the tropical oceans and larval eels drift in the South Equatorial Current to eastern Australia. The younger ages at estuarine arrival of A. reinhardtii suggest that the spawning grounds of this species lie closer to Australia than those of A. australis. In addition, the mean total length at recruitment of A. reinhardtii (49.9 ± 2.0 mm) was significantly smaller than for A. australis (54.6 ± 5.4 mm) (t = 3.8, P < 0.01). However, the growth rates of A. reinhardtii (0.25 ± 0.02 mm/d) were significantly faster than for A. australis (0.23 ± 0.022 mm/d)(t = 7.6, P < 0.01). The smaller sizes of A. reinhardtii at recruitment were likely due to the shorter marine larval period and faster growth rate compared with A. australis. The duration of the marine larval period and growth rate may be the principal factors in determining the geographical distribution of both A. reinhardtii, which tend to occur in tropical-subtropical waters, and A. australis, which predominate in more temperate waters. Extra keywords: otolith, early life history. MF01037 .eJD-Ctna.elSrmChiaenohi,gSWh.-gialNo.sTeznlgdi,sAtr.CiboulitonsbyandlYrv.aIlizduakrtionand growth. Introduction Anguillid eels are catadromous fishes. Their leaf-like larvae, leptocephali, are transported by oceanic currents and metamorphose into glass eels near the continental shelf. After invading coastal waters, glass eels develop into pigmented elvers within estuaries (Tesch 1977). Along the eastern coasts of Australia and Tasmania, the longfinned eels (Anguilla reinhardtii) and the shortfinned eels (A. australis) are found in a wide variety of wetland habitats (Beumer 1996). The adult A. reinhardtii ranges from 10–43°S but is most abundant between latitudes 20–34°S. In contrast, the adult A. australis is distributed from 27–44°S, yet it is most abundant between latitudes 35–44°S (Beumer and Sloane 1990; Beumer 1996). The invasions of A. reinhardtii glass eels occur on a year round basis in tropical and subtropical regions, peaking in the summer and autumn months from © CSIRO 2002. January to March. In contrast, the annual invasion of A. australis glass eels into temperate waters occurs during a shorter period, and peaks in the winter–spring months from July through to September (Beumer and Sloane 1990). Recently, a few leptocephali of both species were caught in the vicinity of New Caledonia (Aoyama et al. 1999). Their presence in this region supports the hypothesis that Australian eels spawn in tropical oceans and larval eels drift in the South Equatorial Current (SEC) to eastern Australia (Jellyman 1987). However, since the larvae of both species are transported in the same current, the question of why A. reinhardtii predominate over tropical and subtropical waters whereas A. australis are predominant in temperate waters arises. The duration of the marine larval period and growth rate are proposed to be the principal factors affecting the geographical distributions in A. japonica (Cheng and Tzeng 1996) and the larval segregative migration in 10.1071/MF01037. 1323-1650/02/030687.
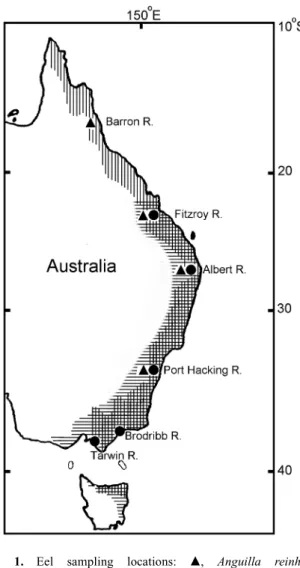
(3) 688. Jen-Chieh Shiao et al.. A. anguilla and A. rostrata (Wang and Tzeng 2000; Arai et al. 2000b). Anguilla australis was consistently larger and heavier than A. reinhardtii at arrival within the same estuaries (Sloane 1984; Beumer and Sloane 1990), perhaps because of differences in larval duration or growth rates between the two species (Sloane 1984). The objective of this study is to compare the ontogenetic duration and the growth rate of glass eels of A. australis and A. reinhardtii. By examining growth increments and Sr/Ca ratios in the eel otolith, it is possible to reconstruct growth and environmental history (Tzeng and Tsai 1994; Arai et al. 1997). We use this method to estimate the age of the eels at metamorphosis from leptocephalus to glass eel, and the age of the glass eels at estuarine arrival along the eastern Australian coast. The results advance the understanding of hatching dates and the geographical distribution of these eels. Materials and methods Sampling design Glass eels of A. australis and A. reinhardtii were collected from the estuaries of the Fitzroy, Albert and Port Hacking Rivers. Only A. reinhardtii glass eels were collected from the Barron River and only A. australis were collected from the Brodribb and Tarwin Rivers (Fig. 1). The specimens collected were immediately preserved in 95% alcohol. The total length of the eels was measured and their pigmentation stage assessed according to the pigment distribution on the body surface (Strubberg 1913). To understand the ontogenetic differences between species, we compared ages of the eels from the same estuary and year. Anguilla reinhardtii glass eels collected from the Albert River between 1997 and 1999 were used to evaluate the interannual variations in ontogenetic development. Microchemistry analysis The sagittal otoliths of glass eels were extracted for microchemistry analysis and age determination. The otoliths were embedded in epofix resin then ground and polished until the core was exposed. For electron probe microanalysis, the polished otoliths were carbon coated under a high vacuum evaporator. Strontium (Sr) and calcium (Ca) concentrations were measured from the core to the edge of otolith at 10 µm intervals with an electron beam of 10 µm in diameter, using an electron probe microanalyzer (JEOL JXA-8900R). The accelerating voltage was set at 15 kV and probe current at 5 nA. The peak concentration of Sr was counted for 90 s with background measurements for 20 s on each side. The peak concentration of Ca was counted for 20 s and each background for 10 s. SrCO3 (USNMR10065) and CaCO3 (USNM-36321) from the Department of Mineral Sciences, National Museum of Natural History, Smithsonian Institution, Washington DC, USA were used as calibration standards for Sr and Ca respectively.. Fig. 1. Eel sampling locations: 䉱, Anguilla reinhardtii; 䊉, A. australis. Vertical lines indicate the distribution of A. reinhardtii; horizontal lines indicate the distribution of A. australis.. and Tzeng 1998). If freshwater checks (Kawakami et al. 1998) appeared near the edge of the otolith, the age at estuarine arrival was counted from the first increment to the innermost check, otherwise it was counted to the edge of the otolith. Thus, the age at capture (Tt), estuarine arrival (Tr) and metamorphosis of leptocephalus (Tm) were estimated from the counts of daily growth increments. The hatching date of individual glass eel was back-calculated from the age at capture and the sampling date. The growth rate of the glass eel was estimated from the total length divided by the age at capture. Statistical methods. Age determination and growth rate After microchemistry analysis, the otolith was polished to remove the carbon layer, then etched with 0.05 M HCl for 13–15 s, dried in an oven and coated with gold for SEM observation. SEM photographs were taken of the otoliths at a magnification of 2000× and used to count their daily growth increments. The age at metamorphosis from leptocephalus to glass eel was determined from the growth increments between the core and where the increment width drastically increased and Sr/Ca ratios abruptly dropped (Tzeng and Tsai 1994; Arai et al. 1997; Wang. Total lengths, daily ages and growth rates among rivers were tested for homogeneity. Statistical differences between groups (rivers) were analysed using one-way ANOVA. Tukey’s pairwise comparison was used to isolate the groups that differ from the others if the data were normally distributed with equal variance. Otherwise, the Kruskal–Wallis test on ranks and Dunn’s pairwise comparison (Dunn 1964) were used to isolate the groups that differ from the others. Daily ages and growth rates between A. australis and A. reinhardtii from the same river were tested with Student’s t-test..
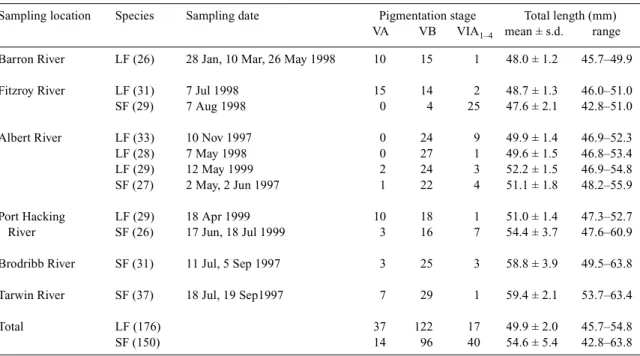
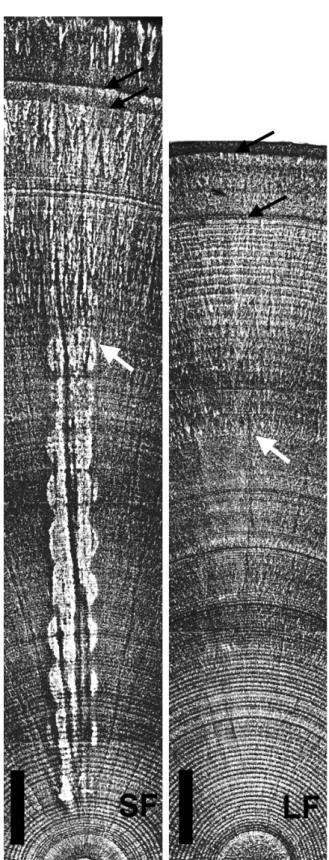
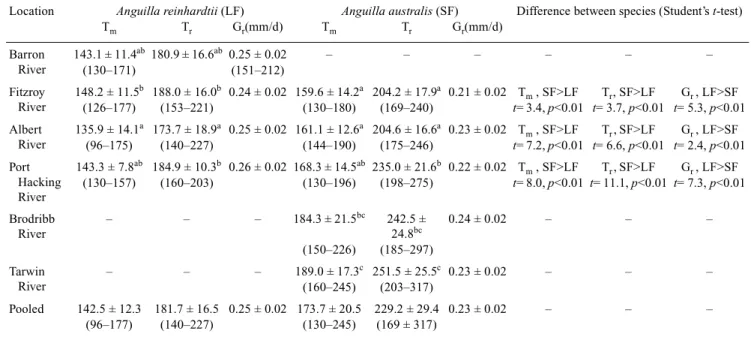
(4) Determining glass eel distribution by larval duration and growth. Results Pigmentation stage and total length In most rivers, the glass eels were predominantly of pigmentation Stage VA and VB, with few individuals at Stages VIAI through to VIAIV. However, most A. australis from the Fitzroy River were at Stage VIAI to VIAIV with only four individuals at Stage VB (Table 1). The eels of Stages VA and VB were the new recruits, but the eels of VIAI to VIAIV may have resided in the estuary for a period of time. Anguilla reinhardtii ranged from 45.7–54.8 mm in total length and A. australis from 42.8–63.8 mm (Table 1). The mean total lengths of A. reinhardtii were significantly smaller than for A. australis in the Albert (t = 2.8, P < 0.01,) and Port Hacking Rivers (t = 4.5, P < 0.01) (Table 1). In the Fitzroy River, the mean total lengths of A. reinhardtii were significantly larger than A. australis (t = 2.4, P = 0.01) (Table 1). Changes in increment width and Sr/Ca ratios The core of the otolith represents the embryo stage. Beyond the core are the conspicuously concentric growth increments (Fig. 2). The first growth increment is assumed to deposit at the beginning of leptocephalus stage. The increment width increases with growth to 0.8–1.0 µm around 30 days from the beginning of the leptocephalus stage, then declines gradually to about 0.3 µm after approximately 170 days in A. australis and 150 days in A. reinhardtii (Fig. 3). The Sr/Ca ratio at the core is relatively lower, approximately 0.009, then the value gradually increases and peaks at approximately 0.018–0.02 around 170 days in A. australis and 150 days in. Table 1.. 689. A. reinhardtii. Beyond the peak, the Sr/Ca ratio dramatically decreases to 0.006 at the edge of the otolith (Fig. 3). The decrease in Sr/Ca is accompanied by an abrupt increase in increment width to 1.5 µm in A. australis and 2 µm in A. reinhardtii. The decrease in Sr/Ca ratios from peak levels, in conjunction with an increase in increment width, indicates the metamorphosis from leptocephalus to glass eel (Tzeng and Tsai 1994; Arai et al. 1997). The oceanic glass eel stage follows the leptocephalus stage. Freshwater checks were found in most otoliths (83% in A. reinhardtii, and 75% in A. australis) (Fig. 2). The estuarine glass eel stage is considered to be between the innermost check and the edge of the otolith and daily growth increments usually become vague. Daily age and growth rate For A. reinhardtii collected from the four estuaries, the mean ages at metamorphosis from leptocephalus to glass eel (Tm) ranged from 136–148 days and the mean ages of glass eels at estuarine arrival (Tr) ranged from 174–188 days. The mean Tm of eels from the Albert River was significantly smaller than for the Fitzroy River (P < 0.05, Dunn’s pairwise comparison). Furthermore, the mean Tr of glass eels from the Albert River was significantly lower than for the Fitzroy and Port Hacking rivers (P < 0.05, Dunn’s pairwise comparison). No significant differences among other rivers were found (Table 2). The mean Tm of A. australis ranged from 160–189 days and the mean Tr ranged from 204–252 days. The mean Tm and Tr of the eels from the Brodribb and Tarwin Rivers were. The sampling locations, dates, pigmentation stages and total length of A. reinhardtii (LF) and A. australis (SF) Numerals in the parenthesis are the sample size for age determination.. Sampling location. Species. Sampling date. Pigmentation stage Total length (mm) range VA VB VIA1–4 mean ± s.d.. Barron River. LF (26). 28 Jan, 10 Mar, 26 May 1998. 10. 15. 1. 48.0 ± 1.2. 45.7–49.9. Fitzroy River. LF (31) SF (29). 7 Jul 1998 7 Aug 1998. 15 0. 14 4. 2 25. 48.7 ± 1.3 47.6 ± 2.1. 46.0–51.0 42.8–51.0. Albert River. LF (33) LF (28) LF (29) SF (27). 10 Nov 1997 7 May 1998 12 May 1999 2 May, 2 Jun 1997. 0 0 2 1. 24 27 24 22. 9 1 3 4. 49.9 ± 1.4 49.6 ± 1.5 52.2 ± 1.5 51.1 ± 1.8. 46.9–52.3 46.8–53.4 46.9–54.8 48.2–55.9. Port Hacking River. LF (29) SF (26). 18 Apr 1999 17 Jun, 18 Jul 1999. 10 3. 18 16. 1 7. 51.0 ± 1.4 54.4 ± 3.7. 47.3–52.7 47.6–60.9. Brodribb River. SF (31). 11 Jul, 5 Sep 1997. 3. 25. 3. 58.8 ± 3.9. 49.5–63.8. Tarwin River. SF (37). 18 Jul, 19 Sep1997. 7. 29. 1. 59.4 ± 2.1. 53.7–63.4. Total. LF (176) SF (150). 37 14. 122 96. 17 40. 49.9 ± 2.0 54.6 ± 5.4. 45.7–54.8 42.8–63.8.
(5) 690. Jen-Chieh Shiao et al.. 20. A. australis Age: 256 d TL: 49.6 mm Stage: VIAII. 15. 1. 10. 0.5. 5. 0. 0 0. 2.5 Mean increment width (µm). 1000×(Sr/Ca). 1.5. 20 40 60 80 100 120 140 160 180 200 220 240 260 Age (days). 20. A. reinhardtii Age: 203 d TL: 52.7mm Stage: VB. 2. 15. 1.5 10 1. 1000×(Sr/Ca). Mean increment width (µm). 2. 5. 0.5 0. 0 0. 20. 40. 60. 80. 100 120 140 160 180 200 Age (days). Fig. 3. Temporal (10 day) changes of mean growth increment width (䊉) and Sr/Ca ratios (䊊) in the otoliths of Anguilla australis and A. reinhardtii. The otoliths are shown in Fig. 2.. Fig. 2. Daily growth increments in the otolith of Anguilla australis (SF): total length 49.6 mm, Stage VIAII from the Fitzroy River. Daily growth increments in the otolith of A. reinhardtii (LF): total length 52.7 mm, Stage VB from the Port Hacking River. White arrows indicate the onset of metamorphosis from leptocephali to glass eel; black arrows indicate freshwater checks. Scale bars: 15 µm.. significantly higher than for eels from the Fitzroy and Albert Rivers, with the eels from the Port Hacking River having intermediate values (P < 0.05, Dunn’s pairwise comparison). Between northern and southern rivers, Tm differed by approximately 30 days and Tr differed by 40–50 days (Table 2). For both species, the ages at metamorphosis (Tm) were linearly related with ages at estuarine arrival (Tr) (Fig. 4). This indicates that the leptocephalus metamorphosing at a younger age will arrive at the estuary as a younger glass eel. The Tm and Tr of A. reinhardtii from each of the Fitzroy, Albert and Port Hacking Rivers were significantly lower than for A. australis from the same rivers (P < 0.01, t-test). The difference between these two species was about 10–15 days in the Fitzroy River, 20–30 days in the Albert River and 20–50 days in the Port Hacking River (Table 2). In all three rivers, A. reinhardtii grew significantly faster than A. australis (t = 7.6, P < 0.01). The mean growth rate was estimated at 0.25 mm per day for A. reinhardtii and 0.23 mm per day for A. australis (Table 2)..
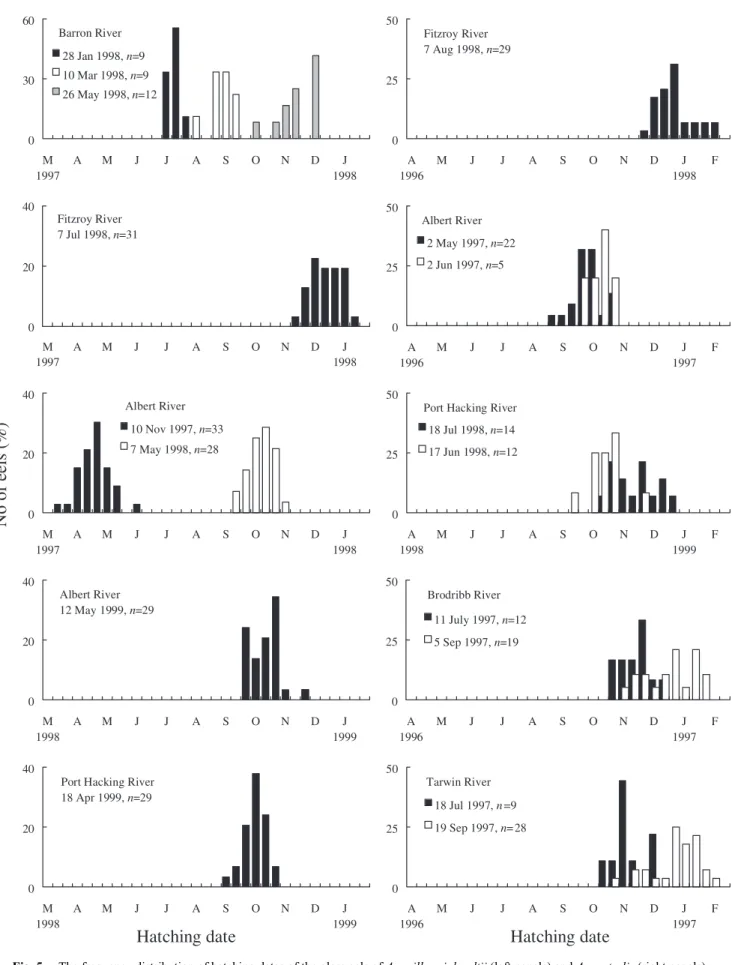
(6) Determining glass eel distribution by larval duration and growth. 691. Table 2. Comparison of ages at metamorphosis (Tm), estuarine arrival (Tr) and growth rate (Gr) of eels For comparison within species among the rivers, ages with the same letters (i.e. a, b, ab) are not significantly different. Samples of Anguilla reinhardtii in Albert River are from 10 November 1997. Another two samples of Anguilla reinhardtii from 1998 and 1999 are not used herein. Location. Anguilla reinhardtii (LF) Tr Gr(mm/d) Tm. Tm. Anguilla australis (SF) Tr Gr(mm/d). Difference between species (Student’s t-test). Barron River. 143.1 ± 11.4ab 180.9 ± 16.6ab 0.25 ± 0.02 (130–171) (151–212). Fitzroy River. 148.2 ± 11.5b 188.0 ± 16.0b 0.24 ± 0.02 159.6 ± 14.2a 204.2 ± 17.9a 0.21 ± 0.02 Tm , SF>LF Tr , SF>LF Gr , LF>SF (126–177) (153–221) (130–180) (169–240) t= 3.4, p<0.01 t= 3.7, p<0.01 t= 5.3, p<0.01. Albert River. Tr , SF>LF Gr , LF>SF 135.9 ± 14.1a 173.7 ± 18.9a 0.25 ± 0.02 161.1 ± 12.6a 204.6 ± 16.6a 0.23 ± 0.02 Tm , SF>LF (96–175) (140–227) (144–190) (175–246) t= 7.2, p<0.01 t= 6.6, p<0.01 t= 2.4, p<0.01. Port Hacking River. 143.3 ± 7.8ab 184.9 ± 10.3b 0.26 ± 0.02 168.3 ± 14.5ab 235.0 ± 21.6b 0.22 ± 0.02 Tm , SF>LF Tr , SF>LF Gr , LF>SF (130–157) (160–203) (130–196) (198–275) t= 8.0, p<0.01 t= 11.1, p<0.01 t= 7.3, p<0.01. Brodribb River. –. –. –. –. 184.3 ± 21.5bc. –. Tarwin River. –. –. Pooled. 142.5 ± 12.3 (96–177). –. –. –. –. –. –. 189.0 ± 17.3c 251.5 ± 25.5c 0.23 ± 0.02 (160–245) (203–317). –. –. –. –. –. –. 181.7 ± 16.5 0.25 ± 0.02 173.7 ± 20.5 (140–227) (130–245). 229.2 ± 29.4 0.23 ± 0.02 (169 ± 317). Table 3.. Seasonal and annual variability in ages and total length (TL) of A. reinhardtii collected from Albert River Ages with the same letters (i.e. a, b) are not significantly different. Tm, age at metamorphosis, Tr, age at estuarine arrival.. 350. Age at estuarine arrival (day). –. 0.24 ± 0.02. (150–226). 242.5 ± 24.8bc (185–297). –. A. reinhardtii Y = 1.10X + 23.07, n= 176 2. R = 0.68, p < 0.01. 300. A. australis. Y = 1.24X + 14.12, n =150 2. R = 0.74, p < 0.01. 250. Sampling date. 200. 10-Nov-97 7-May-98 12-May-99. 150. Tm. Tr. TL. 135.9 ± 14.2 a 151.0 ± 11.5 b 146.4 ± 9.80 b. 173.7 ± 18.9 a 188.0 ± 17.7 b 181.4 ± 13.6 ab. 49.93 ± 1.44 49.62 ± 1.54 52.17 ± 1.49. 100 90. 130. 170. 210. 250. Age at metamorphosis (day). Fig. 4. The relationship between the age at metamorphosis from leptocephali to glass eel and the age of glass eels at estuarine arrival for Anguilla reinhardtii and A. australis.. (P < 0.05, Tukey’s pairwise comparison ) (Table 3). The small difference (~3 mm) in length was most likely due to shrinkage during preservation for different periods. No significant differences in growth rates were found among species or rivers during the three years.. Seasonal and annual variation of Tm and Tr. Hatching date. Anguilla reinhardtii from the Albert River showed a seasonal and annual variation in Tm and Tr during the three years in November (late spring) of 1997, and May (late autumn) of 1998 and 1999. The Tm of the eels ranged from 135 ± 14.2 to 151 ± 11.5 days, with the spring group in 1997 significantly younger than the autumn groups in 1998 and 1999 (P < 0.05, Tukey’s pairwise comparison) (Table 3). The Tr of the eels ranged from 173.7 ± 18.9 to 188 ± 17.7 days, with the spring group in 1997 significantly younger than the autumn group in 1998 (P < 0.05, Tukey’s pairwise comparison ) (Table 3). On the other hand, the total length ranged from 49.6 ± 1.54 to 52.17 ± 1.49 mm, with the autumn group in 1999 significantly larger than the other groups in 1997 and 1998. The back-calculated hatching dates indicate that A. reinhardtii has an extended hatching period. The hatching dates last from March through to January of the next year (Fig. 5). The glass eels collected from individual rivers in different months were from separate spawning events. For example, A. reinhardtii collected from the Barron River in January, March and May 1998 hatched in July, August–September and October–December 1997 respectively. In the Albert River, A. reinhardtii collected in November 1997 and May 1998 hatched from March–May and September–November 1997 respectively. For A. australis, the hatching dates ranged from late August to early February and overlapped with those of A. reinhardtii. Unlike.
(7) 692. Jen-Chieh Shiao et al.. 60. 50. Barron River. Fitzroy River 7 Aug 1998, n=29. 28 Jan 1998, n=9 10 Mar 1998, n=9. 30. 25. 26 May 1998, n=12. 0. 0. M 1997. A. M. J. J. A. S. O. N. D. J 1998. 40. A 1996. J. J. A. S. O. N. D. J 1998. F. A. S. O. N. D. J 1997. F. A. S. O. N. D. J 1999. F. A. S. O. N. D. J 1997. F. A. S. O. N. D. J 1997. F. 50. Fitzroy River 7 Jul 1998, n=31. Albert River 2 May 1997, n=22. 20. 25. 0. 0 M 1997. A. M. J. J. A. S. O. N. D. J 1998. 40. 2 Jun 1997, n=5. A 1996. M. J. J. 50 Albert River. No of eels (%). M. Port Hacking River. 10 Nov 1997, n=33. 18 Jul 1998, n=14. 7 May 1998, n=28. 20. 17 Jun 1998, n=12. 25. 0. 0 M 1997. A. M. J. J. A. S. O. N. D. J 1998. A 1998. M. J. J. 50. 40 Albert River 12 May 1999, n=29. Brodribb River 11 July 1997, n=12 25. 20. 5 Sep 1997, n=19. 0. 0 M 1998. A. M. J. J. A. S. O. N. D. A 1996. J 1999. M. J. J. 50. 40 Port Hacking River 18 Apr 1999, n=29. Tarwin River 18 Jul 1997, n =9 19 Sep 1997, n= 28. 25. 20. 0. 0 M 1998. Fig. 5.. A. M. J. J. A. S. Hatching date. O. N. D. J 1999. A 1996. M. J. J. Hatching date. The frequency distribution of hatching dates of the glass eels of Anguilla reinhardtii (left panels) and A. australis (right panels)..
(8) Determining glass eel distribution by larval duration and growth. A. reinhardtii, the hatching dates of individual A. australis samples collected from different months overlapped (Fig. 5). Discussion The age determination of glass eels depends on the daily growth increments in otoliths. The formation of daily growth increments in otoliths has been validated in A. japonica at the early leptocephalus stage (Umezawa et al. 1989) and in A. rostrata (Martin 1995) and A. celebesensis (Arai et al. 2000a) at the glass eel stage. Whether the growth increment during metamorphosis is deposited daily is still controversial. Cieri and McCleave (2000) speculate that otolith growth of A. rostrata may stop and a portion of the periphery of the otolith may be resorbed during metamorphosis from leptocephalus to glass eels. However, this speculation remains to be validated because otolith resorption has not previously been reported in any fish caught in the wild. If otolith resorption does occur, the calcium content of the otolith should change. However, this study and others (Tzeng and Tsai 1994; Arai et al. 1997; Arai et al. 1999) do not find any obvious change in the calcium content of glass eel otoliths during metamorphosis. The peak spawning season of A. japonica estimated from elver otoliths coincided with that from leptocephali otoliths (Tsukamoto 1990). This indicates that the ageing of A. japonica glass eels is reliable. In this study, we assume that otolith growth increments are deposited daily in A. reinhardtii and A. australis, although possible errors cannot be ignored until the reality of the daily growth increment during metamorphosis is validated. Furthermore, Cieri and McCleave (2001) argued that the check near the otolith edge is not linked to freshwater entry and the deposition of the check cannot be explained by a reduction in salinity. They found that 61 of 126 glass eels that were not exposed to fresh or brackish water still deposited the checks. This indicated that their results provide evidence that the check can be deposited when the glass eels are in a seawater environment. However, the glass eels in their study were collected in the lower estuary and had thus experienced a habitat shift from coastal waters. Their results cannot exclude the possibility that the habitat shift from seawater to the estuary may trigger the deposition of this otolith check. Kawakami et al. (1998) found that the percentage of the eels with a freshwater check increases from 0% to 100% as glass eels migrate upstream. We collected the glass eels for this study in the inner estuary, 4.6 to 53 km upstream. The freshwater check may reflect the physiological adjustment and adaptability of eels to the distinct environment of the estuary. The hypothesized drifting routes of larval eels via the South Equatorial Current (SEC) to eastern Australia are widely accepted (Tesch 1977; Jellyman 1987; Aoyama et al. 1999). However, the locations of spawning grounds for A. reinhardtii and A. australis have yet to be identified. On. 693. average, A. reinhardtii take approximately six months (182.7 ± 16.3 days) to migrate from the spawning grounds to Australia’s eastern coasts (17–34°S), with 4–5 months in the leptocephalus stage. For A. australis to arrive within this area (23–34°S), it takes 7–8 months (average 204.2–235.0 days), and even up to nine months to reach southern Australia (37–38°S). During this period, 5–6 months are spent in the marine larval stage (Table 2). The estimated larval ages and current speed are used to deduce the possible spawning grounds of the eels. In the Albert and Fitzroy Rivers, the mean leptocephalus stages of A. australis are about 160 days (Arai et al. 1999; this study). The mean SEC speed between the coast of South America and the mid-Pacific is about 0.5–0.6 ms–1 (Tchernia 1980). This current speed was applied to the calculation of larval transport in the western Pacific (Jellyman 1987; Arai et al. 1999; Shiao et al. 2001), regardless of the spatial heterogeneity in the SEC. According to the recent surveys in the western Pacific, the SEC in this area was about 0.2–0.3 ms–1 (Gouriou and Toole 1993, fig. 14; Sprintall and McPhaden 1994, fig. 7; Reverdin et al. 1994, fig. 8; Lagerloef et al. 1999, fig. 10). Based on the SEC speed of 0.2–0.3 ms–1 and 160 days drift, the larval eels would be transported about 2500–4000 km and the spawning ground of A. australis may thus lie in the areas between Fiji and Samoa. The A. reinhardtii leptocephali were consistently younger (11–26 days) than A. australis leptocephali. The difference in age between the two species indicates that their spawning grounds must be different. Based on the age differences, the spawning site of A. reinhardtii may lie about 500 km west of the spawning site of A. australis. This distance puts the spawning ground of A. reinhardtii near or west of Fiji, which is further east than Jespersen’s (1942) presumption of the Coral Sea. However, this simplified calculation ignores the temporal and spatial variability of oceanic currents and assumes a smooth ocean circulation. Furthermore, the active swimming ability of leptocephali is not considered. Adults of A. reinhardtii were recently identified in northern New Zealand waters (Jellyman et al. 1996). How A. reinhardtii arrived in New Zealand is still unknown, since knowledge of its early life history is very scarce. McDowall et al. (1998) believed that the only reasonable explanation for this is transoceanic dispersal through the Tasman Sea to New Zealand. If the conclusion that the spawning ground of A. reinhardtii lies around Fiji is correct, some migrating larvae of A. reinhardtii may entrain within the anticyclonic gyre prevailing in south-western Pacific and arrive in New Zealand from the north west by the East Auckland Current. The actual migration route needs further investigation. The westward SEC is directly wind driven, it responds quickly to variations in the wind field and may, as a result of its link with El Niño and the Southern Oscillation (ENSO), vary dramatically from one year to the next (Tomczak and.
(9) 694. Godfrey 1994). Since the current plays a vital role in the transport and distribution of larval eels, any changes that occur in the dynamics of the SEC are likely to influence the dispersal of Australian glass eels. A slight but significant difference was observed in the ages of A. reinhardtii glass eels collected over three successive years from the Albert River. Individuals collected in late spring (November 1997), which had undergone the marine phase of their migration during the 1997 El Niño event, were significantly younger (8–15 days) at estuarine arrival than glass eels collected in late autumn (May 1998 and 1999) (Table 3). In addition, the age at metamorphosis of the late spring group was also significantly younger (11–15 days) than the late autumn group (Table 3). These results indicate that seasonal variation in the ages of glass eels is greater than annual change. The 1997–1998 El Niño event started in early 1997 and weakened in early 1998. During the onset of the 1997 El Niño, the equatorial surface flow reversed from westward to eastward movement in the western Pacific. In the southwestern Pacific where the larval eels migrated, the speed of westward surface currents (SEC) evidently increased (Lagerloef et al. 1999, fig. 10). The faster flow may have shortened the migrating time of A. reinhardtii from the spawning ground to Australian coasts. The variation of 10–15 days in Tm and Tr within species is smaller than the difference between species. This result indirectly supports the validity of differences in ages between A. reinhardtii and A. australis. The back-calculated hatching dates for A. reinhardtii do not show a particular spawning period but suggest a yearround spawning pattern (Fig. 5). Therefore, based on age determination and the timing of peak glass eel abundances, the main spawning season for this species appears to be during the winter–spring months of June to September. In contrast, the dominant spawning season for A. australis occurs in summer–autumn from January to March (Fig. 5) and the recruitment of glass eels is more restricted. Thus, the main spawning seasons of both species are separate. Anguilla australis has more variable larval and glass eel stages compared with A. reinhardtii because the hatching dates of A. australis, collected over several months, consistently overlap each other. The total lengths of A. australis glass eels were longer than A. reinhardtii glass eels at recruitment (Sloane 1984; Beumer and Sloane 1990). We found the same result in the Albert River and Port Hacking River, but the opposite situation in the Fitzroy River (Table 1). The Fitzroy River data were not directly comparable to the previous results of Sloane (1984) and Beumer and Sloane (1990) since the comparison was not based on the same development stage. The size of glass eels varies with age and stage. Most glass eels of A. australis from the Fitzroy River were in Stage VIAI to VIAIV whereas those of A. reinhardtii were in Stage VA and VB. Stages VA and VB indicate freshly invading glass. Jen-Chieh Shiao et al.. eels but Stages VIAI to VIAIV indicate a longer estuarine residence (Jellyman 1977, 1979). Anguilla australis declined in length from Stage VB to Stage VIAIII (Sloane 1984). A reduction in length with advancing pigmentation stage was also found in A. anguilla (Strubberg 1913; Tesch 1977), A. japonica (Tzeng 1990) and A. rostrata (Jessop 1998). Consequently, the shortest total lengths of A. australis found in the Fitzroy River were likely to be the result of longer residence in the estuary. Anguilla australis glass eels are longer than A. reinhardtii at the same developmental stage. Our results support Sloane’s (1984) conclusion that A. reinhardtii has a shorter marine larval duration and younger age at estuarine arrival than A. australis (Fig. 4). Anguilla reinhardtii also grows faster than A. australis. Most A. reinhardtii leptocephali are probably able to metamorphose when approaching northern Australia. Close to or over the continental shelf, most A. reinhardtii leptocephali metamorphose to glass eels and then recruit to tropical and subtropical areas. On the other hand, A. australis has a longer marine larval stage and slower growth rate suitable for a longer migration from more distant spawning grounds to subtropical and temperate areas. Anguilla australis glass eels in the Brodribb and Tarwin Rivers (southern Australia) were about one month older at metamorphosis and at estuarine arrival than the northern group (Table 2). Shiao et al. (2001) recently found that the growth rate of glass eels is slower in southern Australia than in northern Australia. The A. reinhardtii glass eels inhabiting southern Australia and Tasmania may also have a longer marine larval stage and slower growth rate than their northern counterparts. Marine larval duration and growth rate are evidently important factors affecting the distribution of Australian eels and may also account for the segregation of the American eel (A. rostrata) and European eel (A. anguilla) (Wang and Tzeng 2000; Arai et al. 2000b). Acknowledgments The National Science Council, Republic of China (NSC892311-B002-007) financially supported this study. The authors thank Dr L. McKinnon for providing specimens from Victoria, Miss C. Y. Lin for assistance in taking SEM photographs and Dr D. J. Jellyman and B. M. Jessop for many comments on the manuscript. References Aoyama, J., Mochioka, N., Otake, T., Ishikawa, S., Kawakami, Y., Castle, P., Nishida, M., and Tsukamoto, K. (1999). Distribution and dispersal of anguillid leptocephali in the western Pacific Ocean revealed by molecular analysis. Marine Ecology Progress Series 188, 193–200. Arai, T., Otake, T., and Tsukamoto, K. (1997). Drastic changes in otolith microstructure and microchemistry accompanying the onset of metamorphosis in the Japanese eel Anguilla japonica. Marine Ecology Progress Series 161, 17–22..
(10) Determining glass eel distribution by larval duration and growth. Arai T., Otake T., Jellyman D. J., and Tsukamoto K. (1999). Differences in the early life history of the Australasian shortfinned eel Anguilla australis from Australia and New Zealand, as revealed by otolith microstructure and microchemistry. Marine Biology 135, 381–9. Arai, T., Limbong, D., Otake, T., and Tsukamoto, K. (2000a). Validation of otolith daily increments in the tropical eel Anguilla celebesensis. Canadian Journal of Zoology 78, 1078–84. Arai, T., Otake, T., and Tsukamoto, K. (2000b). Timing of metamorphosis and larval segregation of the Atlantic eels Anguilla rostrata and A. anguilla, as revealed by otolith microstructure and microchemistry. Marine Biology 137, 39–45. Beumer, J. P. (1996). Freshwater eels. In ‘Freshwater Fishes of Southeastern Australia’. (Ed. R. M. McDowall.) pp. 247. (Reed Books: Sydney.) Beumer, J. P., and Sloane, R. (1990). Distribution and abundance of glass-eels Anguilla spp. in East Australian waters. Internationale Revue der Gesamten Hydrobiologie 75, 721–36. Cheng, P. W., and Tzeng, W. N. (1996). Timing of metamorphosis and estuarine arrival across the dispersal range of the Japanese eel Anguilla japonica. Marine Ecology Progress Series 131, 87–96. Cieri, M. D., and McCleave, J. D. (2000). Discrepancies between otoliths of larvae and juveniles of the American eel: is something fishy happening at metamorphosis? Journal of Fish Biology 57, 1189–98. Cieri, M. D., and McCleave, J. D. (2001). Validation of daily otolith increments in glass-phase American eels Anguilla rostrata (Lesueur) during estuarine residency. Journal of Experimental Marine Biology and Ecology 257, 219–27. Gouriou, Y., and Toole, J. (1993). Mean circulation of the upper layers of the western equatorial Pacific Ocean. Journal of Geophysical Research 98(C12), 22495–520. Jellyman, D. J. (1977). Invasion of a New Zealand freshwater stream by glass-eels of two Anguilla spp. New Zealand Journal of Marine and Freshwater Research 11, 193–209. Jellyman, D. J. (1979). Upstream migration of glass-eels (Anguilla spp.) in the Waikato River. New Zealand Journal of Marine and Freshwater Research 13, 13–22. Jellyman, D. J. (1987). Review of the marine life history of Australian temperate species of Anguilla. In ‘Symposium of Common Strategies of Anadromous and Catadromous Fishes’. (Eds M. J. Dadswell, R. J. Klauda, C. M. Moffitt, R. L. Saunders, R. A. Rulifson and J. E. Cooper.) pp. 276–85. (American Fish Society.) Jellyman, D. J., Chisnall, B. L. Dijkstra, L. H., and Boubee, J. A. T. (1996). First record of the Australian longfinned eel, Anguilla reinhardtii, in New Zealand. Marine and Freshwater Research 47, 1037–40. Jespersen, P. (1942). Indo-Pacific leptocephalids of the genus Anguilla. Dana Report 22, 1–128. Jessop, B. M. (1998). Geographic and seasonal variation in biological characteristics of American eel elvers in the Bay of Fundy area an on the Atlantic coast of Nova Scotia. Canadian Journal of Zoology 76, 2172–85. Kawakami, Y., Mochioka, N., Morishita, K., Toh, H., and Nakazono, A. (1998). Determination of the freshwater mark in otoliths of Japanese eel elvers using microstructure and Sr/Ca ratios. Environmental Biology of Fishes 53, 421–7.. 695. Lagerloef, G. S. E., Mitchum, G. T., Lukas, R. B., and Niiler, P. P. (1999). Tropical Pacific near-surface currents estimated from altimeter, wind, and drifter data. Journal of Geophysical Research 104(C10), 23313–26. Martin, M. H. (1995). Validation of daily growth increments in otoliths of Anguilla rostrata (Lesueur) elvers. Canadian Journal of Zoology 73, 208–11. McDowall, R. M., Jellyman, D. J., and Dijkstra, L. H. (1998). Arrival of an Australian anguillid eel in New Zealand: an example of transoceanic dispersal. Environmental Biology of Fishes 51, 1–6. Reverdin, G., Frankignoul, C., Kestenare, E., and McPhaden, M. J. (1994). Seasonal variability in the surface currents of the equatorial Pacific. Journal of Geophysical Research 99(C10), 20323–44. Shiao, J. C., Tzeng, W. N., Collins, A., and Jellyman, D. J. (2001). Dispersal pattern of glass eel stage of Anguilla australis revealed by otolith growth increments. Marine Ecology Progress Series 219, 241–50. Sloane, R. D. (1984). Invasion and upstream migration by glass-eels of Anguilla australis australis Richardson and A. reinhardtii Steindachner in Tasmanian freshwater streams. Australian Journal of Marine and Freshwater Research 35, 47–59. Sprintall, J., and McPhaden, M. J. (1994). Surface layer variations observed in multiyear time series measurements from the western equatorial Pacific. Journal of Geophysical Research 99(C1), 963–79. Strubberg, A. C. (1913). The metamorphosis of elvers as influenced by outward conditions. Meddelelser fra Kommissionen for Havundersøgelser, Series Fiskeri 4(3), 1–11. Tchernia, P. (1980). ‘Descriptive Regional Oceanography.’ Pergamon Marine Series 3. (Pergamon Press: Oxford, England.) Tesch, F. W. (1977). ‘The Eel: Biology and Management of Anguillid Eels.’ 1st Edition. (Chapman and Hall: London.) Tomczak, M., and Godfrey, J. S. (1994). ‘Regional Oceanography. An Introduction.’ (Pergamon Press: Oxford, England.) Tsukamoto, K. (1990). Recruitment mechanism of the eel, Anguilla japonica, to the Japanese coast. Journal of Fish Biology 36, 659–71. Tzeng, W. N. (1990). Relationship between growth rate and age at recruitment of Anguilla japonica elvers in a Taiwan estuary as inferred from otolith growth increments. Marine Biology 107, 75–81. Tzeng, W. N., and Tsai, Y. C. (1994). Changes in otolith microchemistry of the Japanese eel, Anguilla japonica, during its migration from the ocean to the rivers of Taiwan. Journal of Fish Biology 45, 671–83. Umezawa, A., Tsukamoto, K., Tabeta, O., and Yamakawa, H. (1989). Daily growth increments in the larval otolith of the Japanese eel Anguilla japonica. Japanese Journal of Ichthyology 35, 440–4. Wang, C. H., and Tzeng, W. N. (2000). The timing of metamorphosis and growth rates of American and European eel leptocephali: A mechanism of larval segregative migration. Fisheries Research 46, 191–205.. Manuscript received 13 March 2001; revised and accepted 30 October 2001. http://www.publish.csiro.au/journals/mfr.
(11)
數據
相關文件
In particular, we present a linear-time algorithm for the k-tuple total domination problem for graphs in which each block is a clique, a cycle or a complete bipartite graph,
You are given the wavelength and total energy of a light pulse and asked to find the number of photons it
Xianggang zaji (miscellaneous notes on Hong Kong) was written by an English and translated into Chinese by a local Chinese literati.. Doubts can therefore be cast as to whether
Wang, Solving pseudomonotone variational inequalities and pseudocon- vex optimization problems using the projection neural network, IEEE Transactions on Neural Networks 17
Define instead the imaginary.. potential, magnetic field, lattice…) Dirac-BdG Hamiltonian:. with small, and matrix
incapable to extract any quantities from QCD, nor to tackle the most interesting physics, namely, the spontaneously chiral symmetry breaking and the color confinement..
• Formation of massive primordial stars as origin of objects in the early universe. • Supernova explosions might be visible to the most
This paper deals with Zhu Shixing and the sūtras emphasized by him, the prajñā thought of Zhi Qian of Eastern Wu Dynasty, the prajñā and dhyāna thoughts of Saṃghavarman of