Amiodarone use is associated with increased risk of stroke in patients
with non-valvular atrial fibrillation: A nationwide population-based
cohort study
Wei-Chun Chen1,2*, Wei-Cheng Chen1,2*Chih-Yu Chen1,Biing-Ru Wu1, Wen-Chien
Cheng1 , Kuo-Hung Lin4,6, Te-Chun Hsia1,2,3, Wei Chen7, Chia-Hung Chen1,3,6,
Chih-Hsin Muo8, Wei-Chih Liao1,2,6#,Chia-Hsiang Li1,2#
1Division of Pulmonary and Critical Care Medicine, Department of Internal Medicine,
China Medical University Hospital, Taichung, Taiwan
2Hyperbaric Oxygen Therapy Center, China Medical University, Taiwan,
3Department of Respiratory Therapy, China Medical University, Taichung, Taiwan 4Division of Cardiology, Department of Internal Medicine, 5Department of Life
Science, National Chung Hsing University
6Graduate Institute of Clinical Medical Science, China Medical University
7Division of Pulmonary and Critical Care Medicine, Chiayi Christian Hospital,
Taiwan
8Management Office for Health Data, China Medical University Hospital; China
Medical University
*The first two authors contributed equally to this work #Dr. Li CH and Liao WC are both co-corresponding authors.
Running head: Amiodarone and stroke risk in AF patients Corresponding authors:
Division of Pulmonary and Critical Care Medicine, Department of Internal Medicine, China Medical University Hospital, No. 2, Yude Road, Taichung, Taiwan
Telephone: +886-4-22052121 EXT 3485 Fax: +886-4-22038883
E-mail address: u701105@yahoo.com.tw
Chia-Hsiang Li
Division of Pulmonary and Critical Care Medicine, Department of Internal Medicine, China Medical University Hospital, No. 2, Yude Road, Taichung, Taiwan
Telephone: +886-4-22052121 EXT 3485 Fax: +886-4-22038883
ABSTRACT
Purpose: Atrial fibrillation (AF), the most common sustained arrhythmia requiring
treatment worldwide, is one the major causes of ischemic stroke. Although amiodarone is commonly used for rhythm control in AF, its relationship with stroke has rarely been addressed.
Methods: We evaluated 16,091 patients who were diagnosed with AF (ICD-9-CM
427.31 and 427.32) between 1998 and 2011; The date of AF diagnosis was set as the index date. Patients with a history of stroke (ICD-9-CM 430-438) who received amiodarone before the index date or during the following 30 days, or who experienced stroke within 30 days of receiving amiodarone were excluded. Finally, 7,548 AF patients were included in this study and divided into two groups according to whether or not they received amiodarone (ATC code: C01BD01) during the study period.
Results: The risk of ischemic stroke in AF patients receiving amiodarone was 1.81
(95% CI 1.52–2.16), 1.79 (95% CI 1.50–2.14), and 1.78 (95% CI 1.49–2.13) fold higher than in those who did not receive amiodarone, according to crude, Model 1, and Model 2 Cox proportional hazard regression models, respectively. In a demographically stratified analysis, the risk of ischemic stroke was significantly higher in patients aged <65 years, with no comorbidities, who were also taking
digoxin, or had a low CHA2DS2VASc score.
Conclusion: Amiodarone treatment is associated with an increased risk of stroke in
patients with AF, especially in those who have an initial low risk of stroke. Antiplatelet drugs and warfarin could reduce the stroke risk in AF patients receiving amiodarone. However, as the combination of digoxin and amiodarone increases the risk of stroke in these patients, the combination of these two drugs should be avoided.
Introduction
Atrial fibrillation (AF) is the most common sustained arrhythmia requiring treatment and one the major causes of ischemic stroke worldwide.1 The incidence of
ischemic stroke among patients with non-valvular AF is approximately 5% per year and increases with age, resulting in higher morbidity and mortality,2, 3 as AF-induced
ischemic stroke is more disabling and fatal than other types of ischemic stroke. The treatment of AF includes rhythm correction, rate control, and anticoagulant therapy, and aims to improve the symptoms and reduce the complications. Although warfarin has been commonly used in the past few decades to reduce stroke risk in patients with AF, recent Phase III clinical trials have shown that new oral anticoagulants are superior or non-inferior to warfarin, with respect to their efficacy in preventing ischemic stroke and systemic embolism.3
A cohort study showed that digoxin, a rate control agent, was associated with an increased risk of stroke in patients with non-valvular AF.4 Increased expression of
CD62P in platelets and platelet-leukocyte conjugates, and endothelial activation markers, was proposed as a possible mechanism to explain the higher risk of stroke in these patients.5 Furthermore, while the new rhythm control agent dronedarone,
restores sinus rhythm and reduces hospitalization or death in AF patients,6 it also
of stroke from cardiovascular causes in patients with permanent AF.
Despite these adverse effects, maintenance of sinus rhythm is considered an important goal in AF patients, as it improves their prognosis by enhancing cardiac function and relieving symptoms. Rhythm management in patients with AF involves electrical and pharmacological cardioversion.8 While electrical cardioversion shows a
superior success rate (~88%), it entails a risk of thromboembolism (up to 5.6%) when it is performed without anticoagulation.8,9 The efficacy of pharmacological
cardioversion to maintain sinus rhythm using amiodarone is around 50–60% and better than other agents, including dronedarone.7 However, although neurological
effects have been reported,10 large studies investigating the relationship between
amiodarone and ischemic stroke among patients with non-valvular AF are lacking. Therefore, in this study we aimed to investigate the association between amiodarone and the risk of stroke among a nationwide population-based cohort of 7,548 patients with non-valvular AF.
Data source
For this study, we used data from the Longitudinal Health Insurance Database (LHID), which is a part of the Taiwan National Insurance Research Database (NHIRD). The NHIRD was set up on March 1, 1995 by the Bureau of National Health Insurance of Taiwan. The LHID includes all medical claims reported between 1996 and 2011 from one million beneficiaries randomly selected from among all insurants. Disease definition was based on the International Classification of Diseases, 9th Revision, Clinical Modification (ICD-9-CM), as recorded in the NHIRD. Medication definitions were based on the Anatomical Therapeutic Chemical (ATC) classification system. In accordance with the Personal Information Protection Act, the identities of all beneficiaries were recorded by computer. This study was approved by the Institutional Review Board of the China Medical University Hospital, Taiwan.
Study subjects, outcomes and covariates
We evaluated 16,091 patients who were diagnosed with AF (ICD-9-CM 427.31 and 427.32) between 1998 and 2011 with the date of AF diagnosis set as the index date. Patients with a history of stroke (ICD-9-CM 430-438) who received amiodarone before the index date or during the following 30 days, or who experienced stroke within 30 days of receiving amiodarone were excluded. Finally, 7,548 patients with
AF were included in this study and divided into two groups according to whether or not they received amiodarone (ATC code: C01BD01) during the study period.
Hospitalized ischemic stroke (ICD-9-CM 433 and 434) was defined as the outcome of interest and cases due to accident were excluded. The first event of hospitalized ischemic stroke was defined as the endpoint event in our study population. Coding of ischemic stroke in the NHIRD was based on the neurologist’s diagnosis, brain computer tomography or brain magnetic resonance imaging findings. All study subjects were followed from the index date to the date on which the outcome was recorded, to the time at which they withdrew from this insurance program, or to the end of 2011, whichever occurred first.
Covariates in this study included age (<65, 65–74, and 75+ years), gender, comorbidities, and medications. Comorbidities included ischemic heart disease, diabetes, hypertension, heart failure, and hyperlipidemia with ICD-9-CM codes 410– 414, 250, 401–405, 428, and 272, respectively. The CHA2DS2VASc score was also
considered as a covariate in this study. Medications used included the antiplatelet agents aspirin, clopidogrel, and dipyridamole; warfarin; and digoxin, with ATC codes B01AC06, B01AC04, B01AC07, B01AA03, and C01AA05, respectively. Cardiac conversion (procedure code 18028B) and transcatheter radiofrequency ablation (procedure code 33091A) were also analyzed.
Statistical analysis
The chi-square and Student’s t-tests were used to examine differences in categorical and continuous variables respectively, between the two groups. As not all patients received amiodarone during the study period, we used a Cox proportional hazard model with time-dependent exposure covariates to reduce the bias resulting from overestimation of the effect of amiodarone on ischemic stroke risk. Model 1 controlled for age, gender, ischemic heart disease, diabetes, hypertension, heart failure, hyperlipidemia, antiplatelet agent, warfarin, and digoxin covariates. Model 2 controlled for CHA2DS2VASc score, hyperlipidemia, antiplatelet agent, warfarin, and
digoxin covariates. The association between ischemic stroke and amiodarone dosage was also assessed. The age-, gender-, comorbidity-, and CHA2DS2VASc
score-specific risks of ischemic stroke in patients who received amiodarone was compared with those who did not receive amiodarone. The joint effect of amiodarone and AF-associated medications on ischemic stroke was also analyzed. All statistical analyses were performed using SAS software version 9.3 (SAS Institute Inc., Carey, NC).
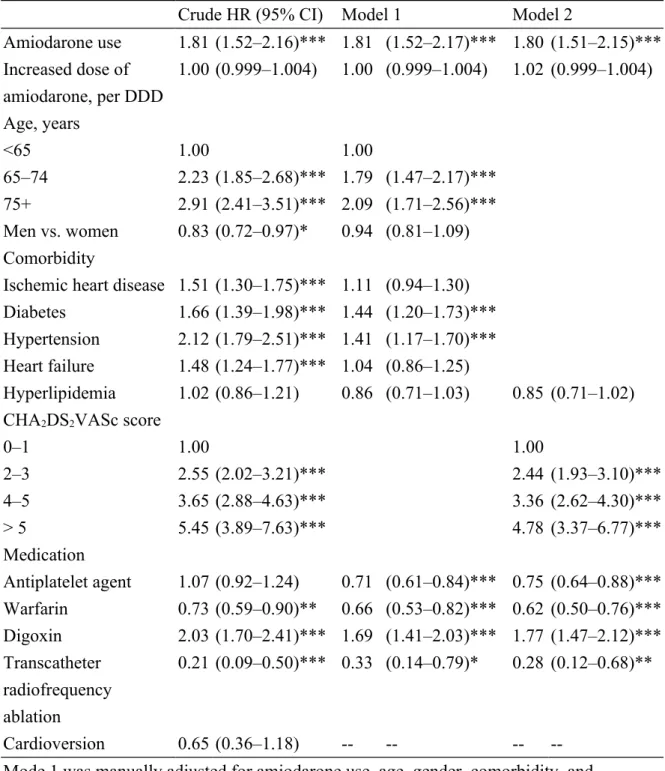
During the study period, 2,587 (34.3%) patients with AF received amiodarone and 4,961 (65.7%) did not. The mean age in both groups was comparable (66.0 vs. 65.8 years, respectively) (Table 1). Patients who received amiodarone had more comorbidities than those who did not receive the drug, including ischemic heart disease (50.7% vs. 41.9%), hypertension (66.3% vs. 58.4%), heart failure (66.3% vs. 17.6%), and hyperlipidemia (27.2% vs. 23.4%). Moreover, amiodarone-treated patients received more medications, including antiplatelet agents (67.3% vs. 45.9%), warfarin (20.2% vs. 12.0%), and digoxin (67.4% vs. 56.7%), and underwent more cardiac conversions (2.63% vs. 1.21%) and transcatheter radiofrequency ablations (4.56% vs. 1.65%) than non-amiodarone-treated patients.
Furthermore, the results of the crude, Model 1, and Model 2 Cox proportional hazard regression analyses showed that the risk of ischemic stroke was 1.81 (95% confidence intervals [CI]= 1.52–2.16), 1.81 (95% CI= 1.50–2.17), and 1.80 (95% CI =1.51–2.15) fold higher, respectively, in patients who received amiodarone (P<0.001 for all models; Table 2). In model 1, AF patients aged ≥75 and between 65–74 years had an increased risk of stroke compared with those aged <65 years (age ≥ 75 years, hazard ratio [HR]= 2.09. CI= 1.71–2.56, P<0.001; age 64–75 years, HR= 1.79, CI= 1.47-2.17, P<0.001). In addition, AF patients with diabetes or hypertension had a higher risk of ischemic stroke (HR= 1.44 and 1.41, 95% CI= 1.20–1.73 and 1.17–
1.70, P<0.001). Patients who also received digoxin had a 1.69 fold increase in the risk of stroke (95% CI= 1.41–2.03), whereas those who received an antiplatelet agent or warfarin had a lower risk (HR= 0.71 and 0.66, 95% CI= 0.61–0.84 and 0.53–0.82, P<0.001). Moreover, cardiac conversion had no effect but transcatheter radiofrequency ablation reduced stroke risk (HR 0.33, 95% CI 0.09–0.50). The results of Model 2 show that the risk of stroke increased with increasing CHA2DS2VASc scores (scores 2–3, HR= 2.44, CI= 1.93-2.10, P<0.001; scores 4–5,
HR=3.36, CI=2.62–4.30, P<0.001; scores >5, HR= 4.78, CI= 3.37-6.77, P<0.001) compared to patients with CHA2DS2VASc scores of 0–1.
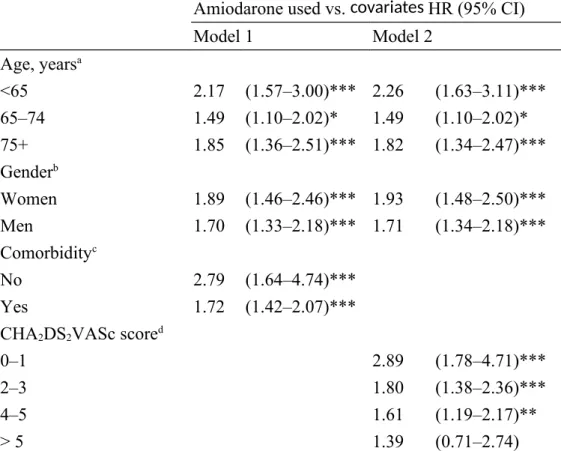
Analysis of the risk of stroke stratified by age, sex, or comorbidity showed that patients who took received amiodarone had a significantly higher risk than those who did not (Table 3). Moreover, significant differences were observed between the two cohorts when patients were stratified by CHA2DS2VASc score, except in those with a
CHA2DS2VASc score >5.
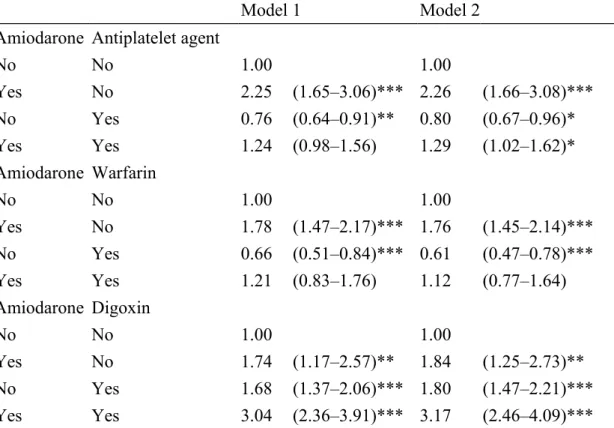
Compared with patients who did not receive amiodarone and an antiplatelet agent, patients receiving amiodarone alone had a higher risk of stroke than those taking amiodarone and antiplatelet agents, but this difference was not significant in Model 1. A similar trend was observed in patients who received amiodarone and warfarin, compared to those who received neither. Furthermore, patients who received
amiodarone and digoxin had the highest risk of ischemic stroke, followed by those who only received amiodarone, and those who only received digoxin, an effect seen in both models.
Discussion
Amiodarone displays multiple effects, including sodium, potassium, and calcium channel blocking, and noncompetitive beta-blocking.2 It is the most commonly used
drug for rhythm control with superior effects in restoring and maintaining sinus rhythm, reducing the AF recurrence rate, and improving patient quality of life.2, 11-13
However, cardiac and non-cardiac adverse events have been reported in patients receiving amiodarone therapy.10
The European Society of Cardiology recommends the use of the CHA2DS2VASc
score to guide the administration of antithrombotic therapy to patients with AF.14 This
scoring system includes two risk factor categories: “major” and “clinically relevant non-major” risk factors for stroke. The two major risk factors are age ≥75 years; and prior stroke, transient ischemic attack, or thromboembolism, whereas clinically relevant non-major risk factors include congestive heart failure, hypertension, diabetes mellitus, vascular disease (myocardial infarction, complex aortic plaque, and peripheral arterial disease), age 65–74 years, and female sex. Similarly, the American
College of Cardiology Foundation (ACCF) and American Heart Association (AHA) guidelines include diabetes mellitus, hypertension, dyslipidemia, smoking, obesity, and a family history of premature coronary artery disease (CAD) as classical risk factors for CAD.15 In our population-based cohort of 7,548 patients with non-valvular
AF, we found that amiodarone and digoxin use, age, diabetes, hypertension, and CHA2DS2VASc score were independent risk factors for stroke, but the use of
antiplatelet agents or warfarin had a protective effect. Although amiodarone use was associated with a 1.81-fold increase in the risk of stroke. However, a higher daily dose of the drug did not increase this risk. AF patients who were taking amiodarone and had no relative comorbidities had a higher risk of stroke than those who had comorbidities (HR= 2.79, 95% CI= 1.64–4.74, P<0.001, vs. HR= 1.72, 95% CI= 1.42–2.07, P<0.001; Table 3).
Based on the CHA2DS2VASc score, age ≥ 75 and 65–74 years are considered a
major and a classical risk factor, respectively.14 Accordingly, in our study we found
that the risk of stroke increased with age (Table 2). Moreover, after adjustment for gender, comorbidities, and medications used, patients who were aged ≥ 75 years and received amiodarone treatment had a higher risk of stroke than those aged 65–74 years (HR=1.85, 95% CI= 1.36–2.51, P<0.001, vs. HR= 1.49, 95% CI= 1.10–2.02, P<0.05; Table 3). However, compared with older patients, those below 65 years who
received amiodarone had a greater risk of stroke (HR= 2.17, 95% CI = 1.57–3.00, P<0.001). The stroke risk remained similar after adjustment for CHA2DS2VASc
score, hyperlipidemia, and medications used. These results suggest that compared with older patients, amiodarone treatment in patients less than 65 years of age could be associated with a higher stroke risk. In addition, gender is considered another classical risk factor, with women with AF considered to be at a higher risk of stroke. Our results reflected this effect (Table 2). Furthermore, amiodarone use was associated with a higher risk of stroke in women with AF (Table 3).
According to the CHA2DS2VASc score, oral anticoagulation should be used in AF
patients with a CHA2DS2VASc score ≥2.14 As shown in Table 2, we also found that
the stroke risk in AF patients increased significantly with the CHA2DS2VASc score.
However, focusing on the interaction between amiodarone use and CHA2DS2VASc
score, amiodarone use in AF patients with a CHA2DS2VASc score of 0–1 was
associated with a higher risk of stroke (HR= 2.89, 95% CI= 1.78–4.71, P<0.001; Table 3). Furthermore, although the stroke risk associated with amiodarone use in AF patients decreased with increasing CHA2DS2VASc scores, the stroke risk was not
significant in patients with a CHA2DS2VASc score >5 (HR= 1.39, 95% CI= 0.71–
2.74; Table 3). One possible explanation may be that the effect of amiodarone use on stroke risk is weaker in AF patients who are already at a higher risk, including those
with more comorbidities, aged ≥75 years, and a CHA2DS2VASc score >5. These
findings, i.e. the interaction of amiodarone with age, gender, comorbidities, and CHA2DS2VASc score, suggest that amiodarone should be used with caution in AF
patients who have a low risk of stroke, because of the higher stroke risk associated with amiodarone use.
Chang et al. observed that digoxin use increased the risk of stroke in patients with AF, suspecting that the increase in intracellular calcium levels may contribute to digoxin-mediated platelet activation.4 Moreover, Chirinos et al. observed increased
levels of CD62P expression in platelets and platelet-leukocyte conjugates, and endothelial activation markers in patients receiving digoxin.5 In this study, further
examination of the interaction between amiodarone and digoxin revealed that digoxin use was an independent risk factor for stroke in AF patients, whether adjusted for amiodarone use, age, gender, comorbidity, and medication used, or for amiodarone use, hyperlipidemia, CHA2DS2VASc score and medication used. (HR= 1.69, 95% CI
= 1.41–2.03, P<0.001, and HR= 1.77 95% CI= 1.47–2.12, P<0.001, Table 2). As shown in Table 4, we found that the combined use of digoxin and amiodarone had a cumulative effect on stroke risk in AF patients. However, although the mechanisms underlying this effect are not yet clear, a possible explanation may be that, due to the multiple effects of amiodarone, a crossover effect with digoxin may enhance
intracellular calcium levels, thus contributing to digoxin-mediated platelet activation.5
As prevention of thromboembolism is an important measure to reduce in AF patients, several guidelines for the administration of antithrombotic therapy have been published to address this issue, including the CHA2DS2VASc score published by the
European Society of Cardiology. In addition, according to the Canadian Cardiovascular Society Guidelines for the Management of Atrial Fibrillation,16 oral
anticoagulant therapy is indicated in AF patients with prior stroke, hypertension, heart failure and diabetes mellitus, while acetylsalicylic acid (aspirin) was recommended to AF patients with coronary artery disease but without a history of prior stroke, hypertension, heart failure and diabetes mellitus. In our study, 44.89% patients had ischemic heart disease (or coronary artery disease), and antiplatelet agent use among all patients was around 53.23% (4018/7548). Moreover, we found that the ratio of antiplatelet agent use was proportional to the ratio of patients with ischemic heart disease, a finding that resulted in antiplatelet agents and warfarin having a similar protective effect in decreasing the stroke risk of amiodarone. Furthermore, our results show that when administering amiodarone, AF patients should also receive oral anticoagulation therapy with warfarin or antiplatelet agents according to their CHA2DS2VASc score to decrease stroke risk (Table 4).
ablation status, we found that the ratio of patients receiving cardiac conversion and transcatheter radiofrequency ablation was low in our study population. From our results, it appeared that undergoing cardiac conversion had no effect on stroke risk among AF patients, but transcatheter radiofrequency ablation had a protective effect.
Our study had some limitations associated with retrospective cohorts. For example, the AFFIRM study17 concluded that the majority of strokes in rhythm
correction and rate control groups occurred in patients who had stopped taking warfarin or whose international normalization ratio (INR) was subtherapeutic at the time of the stroke. However, although a limitation of our study was that warfarin use status and actual INR level could not be evaluated, even though warfarin was not used by the majority of patients (14.82%, 1119/7548) (Table 1), it had a protective effect in stroke risk among AF patients (Table 2). A second limitation is that smoking status and family history of stroke could not be analyzed, as this information was not available in the NHIRD. Third, we could not classify hyperlipidemia based on high density lipoprotein, low density lipoprotein, and triglyceride levels, as this information was also not available in the NHIRD.
Amiodarone shows the highest rates of conversion to sinus rhythm, and the Cardioversion of Atrial Fibrillation (RHYTHM-AF) study in the International Registry of Poland, reported a success rate of up to 75%. Moreover, maintenance of
sinus rhythm leads to a superior prognosis by improving cardiac function and relieving symptoms in AF patients. Although in our study, patients with or without any comorbidity treated with amiodarone had a significantly higher risk of stroke (HR= 1.79 and 1.78, 95% CI= 1.50–2.14 and 1.49–2.13, P<0.001), the addition of an antiplatelet agent or warfarin appeared to reduce this risk. This finding suggests that the high sinus rhythm conversion rates of amiodarone may not reduce the risk of stroke in AF patients. However, we are not able to fully explain the mechanism by which amiodarone increases stroke risk in AF patients, and further basic science research and randomized controlled studies are needed to understand the underlying mechanisms behind this association.
In conclusion, our results suggest that AF patients receiving amiodarone treatment are at an increased risk of stroke, especially those with an initially low risk, but this risk may be reduced with the addition of antiplatelet drugs and warfarin. However, as the combination of digoxin and amiodarone further increases the risk of stroke, its administration to AF patients should be avoided.
Acknowledgements
This study is was supported in part by the Taiwan Ministry of Health and Welfare Clinical Trial and Research Center of Excellence (MOHW103-TDU-B-212-113002), China Medical University Hospital, Academia Sinica Taiwan Biobank, Stroke
Biosignature Project (BM104010092), NRPB Stroke Clinical Trial Consortium (MOST 103-2325-B-039-006), Tseng-Lien Lin Foundation, Taichung, Taiwan, Taiwan Brain Disease Foundation, Taipei, Taiwan, and Katsuzo and Kiyo Aoshima Memorial Funds, Japan.
References
1. Fuster V, Ryden LE, Asinger RW, et al. ACC/AHA/ESC Guidelines for the management of patients with atrial fibrillation: Executive summary A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the European Society of Cardiology Committee for Practice Guidelines and Policy Conferences (Committee to Develop Guidelines for the management of patients with atrial gibrillation) developed in collaboration with the North American Society of Pacing and Electrophysiology. Circulation. Oct 23 2001;104(17):2118-2150.
2. Anter E, Callans DJ, Wyse DG. Pharmacological and electrical conversion of atrial fibrillation to sinus rhythm is worth the effort. Circulation. Oct 6 2009;120(14):1436-1443.
3. Bang OY, Hong KS, Heo JH, et al. New oral anticoagulants may be particularly useful for Asian stroke patients. J Stroke. May 2014;16(2):73-80.
4. Chang SS, Chang KC, Wang YC, et al. Digoxin use is associated with increased risk of stroke in patients with non-valvular atrial fibrillation--a nationwide population-based cohort study. Int J Cardiol. Oct 30 2013;169(2):e26-27.
5. Chirinos JA, Castrellon A, Zambrano JP, et al. Digoxin use is associated with increased platelet and endothelial cell activation in patients with nonvalvular atrial fibrillation. Heart Rhythm. May 2005;2(5):525-529.
6. Hohnloser SH, Crijns HJ, van Eickels M, et al. Effect of dronedarone on cardiovascular events in atrial fibrillation. N Engl J Med. Feb 12 2009;360(7):668-678.
7. Gillis AM, Verma A, Talajic M, Nattel S, Dorian P. Canadian Cardiovascular Society atrial fibrillation guidelines 2010: rate and rhythm management. Can J Cardiol. Jan-Feb 2011;27(1):47-59.
8. Kiliszek M, Opolski G, Wlodarczyk P, Dabrowski R, Ponikowski P. Cardioversion of atrial fibrillation (RHYTHM-AF) international registry in Poland. Cardiol J. Mar 27 2014.
9. Klein AL, Murray RD, Grimm RA. Role of transesophageal echocardiography-guided cardioversion of patients with atrial fibrillation. J Am Coll Cardiol. Mar 1 2001;37(3):691-704.
10. Yapa RS, Green GJ. Embolic stroke following cardioversion of atrial fibrillation to sinus rhythm with oral amiodarone therapy. Postgrad Med J. May 1990;66(775):410.
11. Singh BN, Singh SN, Reda DJ, et al. Amiodarone versus sotalol for atrial fibrillation. N Engl J Med. May 5 2005;352(18):1861-1872.
12. Roy D, Talajic M, Dorian P, et al. Amiodarone to prevent recurrence of atrial fibrillation. Canadian Trial of Atrial Fibrillation Investigators. N Engl J Med. Mar 30 2000;342(13):913-920.
13. Al-Khatib SM, Allen LaPointe NM, Chatterjee R, et al. Rate- and rhythm-control therapies in patients with atrial fibrillation: a systematic review. Ann Intern Med. Jun 3 2014;160(11):760-773.
14. Camm AJ, Kirchhof P, Lip GY, et al. Guidelines for the management of atrial fibrillation: the Task Force for the Management of Atrial Fibrillation of the European Society of Cardiology (ESC). Eur Heart J. Oct 2010;31(19):2369-2429.
15. Greenland P, Alpert JS, Beller GA, et al. 2010 ACCF/AHA guideline for assessment of cardiovascular risk in asymptomatic adults: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. Dec 14 2010;56(25):e50-103.
16. Verma A, Cairns JA, Mitchell LB, et al. 2014 focused update of the Canadian Cardiovascular Society Guidelines for the management of atrial fibrillation. Can J Cardiol. Oct 2014;30(10):1114-1130.
17. Wyse DG, Waldo AL, DiMarco JP, et al. A comparison of rate control and rhythm control in patients with atrial fibrillation. N Engl J Med. Dec 5 2002;347(23):1825-1833.
Amiodarone use Yes (N = 2587) No (N = 4961) P-value Age, years n % n % <0.0001 <65 1091 42.2 2036 41.0 65–74 746 28.8 1293 26.1 75+ 750 29.0 1632 32.9 Mean (SD) 66.0 (13.9) 65.8 (16.3) 0.59 Men 1530 59.1 2851 57.5 0.16 Comorbidity
Ischemic heart disease 1311 50.7 2078 41.9 <0.0001
Diabetes 475 18.4 871 17.6 0.39
Hypertension 1716 66.3 2898 58.4 <0.0001
Heart failure 562 21.7 871 17.6 <0.0001
Hyperlipidemia 704 27.2 1161 23.4 0.0003
CHA2DS2VASc score 0.003
0–1 621 24.0 1422 28.7 2–3 1024 39.6 1855 37.4 4–5 794 30.7 1422 28.7 > 5 148 5.72 262 5.28 Mean (SD) 2.85 (1.69) 2.70 (1.71) 0.0003 Medication Antiplatelet agent 1742 67.3 2276 45.9 <0.0001 Warfarin 522 20.2 597 12.0 <0.0001 Digoxin 1744 67.4 2815 56.7 <0.0001 Transcatheter radiofrequency ablation 118 4.56 82 1.65 <0.0001 Cardioversion 68 2.63 60 1.21 <0.0001
Table 2. Hazard ratios (HR) and 95% confidence intervals (CI) for stroke in time-depended models
Crude HR (95% CI) Model 1 Model 2
Amiodarone use 1.81 (1.52–2.16)*** 1.81 (1.52–2.17)*** 1.80 (1.51–2.15)*** Increased dose of amiodarone, per DDD 1.00 (0.999–1.004) 1.00 (0.999–1.004) 1.02 (0.999–1.004) Age, years <65 1.00 1.00 65–74 2.23 (1.85–2.68)*** 1.79 (1.47–2.17)*** 75+ 2.91 (2.41–3.51)*** 2.09 (1.71–2.56)*** Men vs. women 0.83 (0.72–0.97)* 0.94 (0.81–1.09) Comorbidity
Ischemic heart disease 1.51 (1.30–1.75)*** 1.11 (0.94–1.30) Diabetes 1.66 (1.39–1.98)*** 1.44 (1.20–1.73)*** Hypertension 2.12 (1.79–2.51)*** 1.41 (1.17–1.70)*** Heart failure 1.48 (1.24–1.77)*** 1.04 (0.86–1.25)
Hyperlipidemia 1.02 (0.86–1.21) 0.86 (0.71–1.03) 0.85 (0.71–1.02) CHA2DS2VASc score
0–1 1.00 1.00 2–3 2.55 (2.02–3.21)*** 2.44 (1.93–3.10)*** 4–5 3.65 (2.88–4.63)*** 3.36 (2.62–4.30)*** > 5 5.45 (3.89–7.63)*** 4.78 (3.37–6.77)*** Medication Antiplatelet agent 1.07 (0.92–1.24) 0.71 (0.61–0.84)*** 0.75 (0.64–0.88)*** Warfarin 0.73 (0.59–0.90)** 0.66 (0.53–0.82)*** 0.62 (0.50–0.76)*** Digoxin 2.03 (1.70–2.41)*** 1.69 (1.41–2.03)*** 1.77 (1.47–2.12)*** Transcatheter radiofrequency ablation 0.21 (0.09–0.50)*** 0.33 (0.14–0.79)* 0.28 (0.12–0.68)** Cardioversion 0.65 (0.36–1.18) -- -- --
--Mode 1 was manually adjusted for amiodarone use, age, gender, comorbidity, and medication used.
Model 2 was manually adjusted for amiodarone use, hyperlipidemia, CHA2DS2VASc
score, and medication used. DDD: defined daily dose ** P<0.01, *** P<0.001
Table 3. Hazard ratios (HR) and 95% confidence intervals (CI) for stroke stratified by demographic and clinical covariates in time-dependent models
Amiodarone used vs. covariates HR (95% CI)
Model 1 Model 2 Age, yearsa <65 2.17 (1.57–3.00)*** 2.26 (1.63–3.11)*** 65–74 1.49 (1.10–2.02)* 1.49 (1.10–2.02)* 75+ 1.85 (1.36–2.51)*** 1.82 (1.34–2.47)*** Genderb Women 1.89 (1.46–2.46)*** 1.93 (1.48–2.50)*** Men 1.70 (1.33–2.18)*** 1.71 (1.34–2.18)*** Comorbidityc No 2.79 (1.64–4.74)*** Yes 1.72 (1.42–2.07)***
CHA2DS2VASc scored
0–1 2.89 (1.78–4.71)***
2–3 1.80 (1.38–2.36)***
4–5 1.61 (1.19–2.17)**
> 5 1.39 (0.71–2.74)
Mode 1: a adjusted for gender, comorbidity, and medication used b adjusted for age, comorbidity, and medication used
c adjusted for age, gender, and medication used
Model 2: a adjusted for CHA
2DS2VASc score, hyperlipidemia, and medication
used
b adjusted for CHA
2DS2VASc score, hyperlipidemia, and medication used d adjusted for hyperlipidemia, and medication used
Table 4. Hazard ratios (HR) and 95% confidence intervals (CI) for stroke and stroke-associated medications used in time-dependent models
Model 1 Model 2
Amiodarone Antiplatelet agent
No No 1.00 1.00 Yes No 2.25 (1.65–3.06)*** 2.26 (1.66–3.08)*** No Yes 0.76 (0.64–0.91)** 0.80 (0.67–0.96)* Yes Yes 1.24 (0.98–1.56) 1.29 (1.02–1.62)* Amiodarone Warfarin No No 1.00 1.00 Yes No 1.78 (1.47–2.17)*** 1.76 (1.45–2.14)*** No Yes 0.66 (0.51–0.84)*** 0.61 (0.47–0.78)*** Yes Yes 1.21 (0.83–1.76) 1.12 (0.77–1.64) Amiodarone Digoxin No No 1.00 1.00 Yes No 1.74 (1.17–2.57)** 1.84 (1.25–2.73)** No Yes 1.68 (1.37–2.06)*** 1.80 (1.47–2.21)*** Yes Yes 3.04 (2.36–3.91)*** 3.17 (2.46–4.09)*** Mode 1, manually adjusted for age, gender, comorbidity, and medication used Model 2, manually adjusted for hyperlipidemia, CHA2DS2VASc score, and
medication used.