行政院國家科學委員會專題研究計畫成果報告
N
-烷基二苯胺光化學反應
Photochemistr y of
N
-Alkyldiphenylamines
計畫編號:NSC 90-2113-M-002-031
執行期限:90 年 8 月 1 日至 91 年 7 月 31 日
主持人:何東英 台灣大學化學系
中文摘要 研究二苯胺衍生物及類似物在酸催化 下之光化學反應。化合物 N-烷基(p-甲氧 基苯基)芳香環基胺(1a-1f)在酸催化下 會進行一種新型態的光重排反應,而產生 一系列 1,2,4-三氫(4aH)-卡唑-3-酮之衍生物 及類似物(2a-2f)。反應機構是經由中間 體二氫卡唑質子化後,再相繼經過形式上 的[1,5]、[1,3]氫轉移和甲基醚水解而完成。 關鍵詞:雜環化合物、質子化、光化學、 重排反應。 Abstr actA novel photochemical transformation from N-alkyl(p-methoxyphenyl)arylamines
(1a-1f) to 1,2,4-trihydro(4aH)-carbazol-3-ones (2a-2f) is reported with the assistance of protonation at the dihydrocarbazole inter-mediate followed by sequential formal [1,5]hydrogen, [1,3]hydrogen shifts and proton assisted hydrolysis.
Keywor ds: heterocycles, protonation, photochemistry, rearrangement.
Intr oduction
Aliphatic as well as aromatic amines are photolytically sensitive, for example,
N-methyldiphenylamines can be effectively photocyclized to N-methyl-carbazole.1-5 The reactive intermediates studied by laser flash photolysis are believed to involve triplet state of N-methyldiphenylamine and zwitterionic
dihydrocarbazole.6
Diphenylamines can undergo many photochemical reactions which are similar to the stilbene-type system, for example, photo-cyclization and photoinduced electron transfer. In our previous work, we have found a novel acid-catalyzed photorearrangement of stilbenes and we would like to report here a novel photochemical transformation of 1a-1f with the assistance of an external protic acids. OMe O hv 0.5MHCl CH3CN
Results
We have prepared various
N-methyl-(p-methoxyphenyl)arylamines 1a-1f (Scheme
1) by using the coupling reaction with tris(dibenzylideneacetone)dipalladium(0) Pd2(dba)3 and imidazolium salt IprHCl (Ipr =
1,3-bis(2,6-diisopropylphenyl)-imidazol-2-yli dene) as catalysts.7
Scheme 1 Reactants of the various diaryamines
N OCH3 R2 N OCH3 CH3 N OCH3 CH3 R1 1a: R1= H, 1b: R1= H, 1c: R1= CH3, 1d: R1= OCH3, 1e 1f R2= CH3 R2= C2H5 R2= CH3 R2= CH3
A degassed acetonitrile solution containing
N-methyl-(p-methoxyphenyl)-phenylamine 1a (5 × 10–3 M) and 2.5 × 10–4 M aqueous hydrochloric acid8 was irradiated with a Rayonet photolysis apparatus (16 × 12 W) at 300nm for 20 minutes. The hydrochloric acid was removed and the solvent was evaporated to afford only two products. The major one after column chromatography (silica gel and n-hexane : ethyl acetate = 3:1 as eluent) is 9-methyl-1,2,4-trihydro(4aH)-carbazol-3-one 2a in 85% yields.9 The minor product is
N-methyl-3-methoxycarbazole (15% yield).
Photolysis without aqueous HCl or with higher concentration of aqueous HCl (0.5M) will not lead to the isolation of the major product 2a, because of complete protonation
occurs at the amine nitrogen atom when higher acid concentration is used.
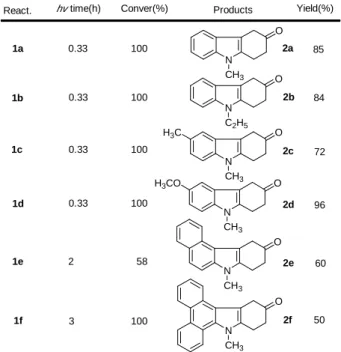
The conversions and yields for the series of
N-alkyl(p-methoxyphenyl)aryl-amines 1a-1f are summarized in Table 1 along with the structure of the products. All products (2a-2f) are supported with spectroscopic data and compound 2a is a known compound.10 Compound 1d with two methoxy groups has the highest yield (96%). Compound 1e shows highly regioselectivity that the isolated product is 2e only.
Table 1 Conversions and yields of the
photo-reactions of 1a-1f. N O CH3 N O C2H5 N O CH3 N O CH3 N O CH3 N O CH3 H3C H3CO 1a 1b 1c 1d 1e 1f 2a 2b 2c 2d 2e 2f 0.33 0.33 0.33 0.33 2 3 100 100 100 100 58 100 85 84 72 96 60 50 React. hv time(h) Conver(%) Products Yield(%)
When 1a is irradiated in the presence of 2.5 × 10-4 M DCl in CH3OD at 300nm for 20
minutes the isolated product 2g show that the content of deuterium labeling at 1(50%), 2(73%), 4(64%) and 8(43%) positions [Eq.(1)].11 This experiment shows the positions of protonation (position 1,4 in
DHC) and proton exchange (position 2,8 in DHC)in the photolysis media.
N CH3 OCH3 N CH3 O D D D D DCl/CH3OD 8 1 2 1a 2g 3 4 5 6 7 hv (1) Discussion A conceivable mechanism is summarized in Scheme 2 based on the experimental results.
Scheme 2 Mechanism for the photoreactions of
the N-methyl-(p-methoxyphenyl)phenylamines
1a to 2a (Deuterium exchange occurs at 1,2,4
and 8 positions) N OCH3 CH3 N OCH3 CH3 DHCa N CH3 OCH3 N CH3 OCH3 H H H N CH3 OCH3 DHCd H+ H+ H+ N CH3 O DHCb DHCc 2a 1a formal [1,5]H formal [1,3]H hydrolysis hv 1 2 8 3 4 5 6 7
The zwitterionic dihydrocarbazole intermediate (DHCa) derives from a six electron cyclization in the triplet state.6 The dihydrocarbazole intermediate (DHCb) is from DHCa.12 Then follows a formal [1,5] hydrogen shift to afford the dihydrocarbazole (DHCc) and a formal [1,3] hydrogen shift to another dihydrocarbazole (DHCd). Then the acid assisted hydrolysis on DHCd leads to the product.
In conclusion, a novel and efficient photochemical reaction is reported for a series of
N-alkyl(p-methoxyphenyl)aryl-amines through the protonation of the zwitterionic dihydrocarbazole intermediate followed by a series of formal [1,n]hydrogen shifts. Four dihydrocarbazole intermediates (DHCa-DHCd) are involved in the reaction.
This photochemical reaction can afford new trihydro-carbazol-3-one in a clean and efficient way.
In the presence of two ortho methyl substituents in the p-methoxyphenyl ring,
compound 1g and 1h, shows different reaction products (Equation 3 and Scheme 3) as compared with the 1,2,4-trihydro(4aH)-carbazol-3-one reaction pathway.
N H O N O N H O N H O CH3CN hv H+ in CDCl3 1g dark in CDCl3 dark (3)
Thus the mechanism is quite different. A possible reaction mechanism for this photochemical transformation is described in Scheme 3.
The formation of dihydrocarbazole intermediates III-a and III-b is adapted from the previous studies.6 Competitive demethane reaction leading to the formation of oxidative cyclized product and [1,5] hydrogen shift leading to the formation of intermediate III-c is the course of the product distribution. Then
a six-electron photocycloreversion has occurred to obtain the butadienyl indole intermediate III-d. Followed by the acid catalyzed hydrolysis to obtaine the product 2h. The more stable trans-product is derived
from the cis-product by photochemical
isomerization.
Scheme 3 Mechanism for the photoreactions of
the N -methyl-(4-methoxy-2,6-dimethylphenyl)-phenylamines 1h to 2h. N OCH3 N OCH3 [1,4]H N OCH3 [1,5]H formal N OCH3 N OCH3 N O hv 1h III-a III-b III-c III-d 2h Refer ences
1 G. Kaupp, Angew. Chem. 1980, 92,
245-277.
2 Lewis, F. D. In Advances in Electron Transfer Chemistry, Mariano, P. S., Ed.;
JAI Press: Greenwich, Conn., 1996; Vol. 5, pp 1-39.
3 Parker, C. A.; Barnes, W. J. Analyst (London) 1957, 82, 606-618.
4 Bowen, E. J.; Eland, J. H. D. Proc. Chem. Soc., London 1963, 202.
5 Terry, G. C.; Uffindel, V. E.; Willets, F. W. Nature (London) 1969, 223,
1050-1051.
6 G. Fischer, E. Fischer, K. H. Grellmann, H. Linschitz, A. Temizer, J. Am. Chem. Soc.
1974, 96, 6267-6269.
7 J. Huang, G. Grasa, S. P. Nolan, Org. Lett.
1999, 1, 1307-1309.
8 The aqueous HCl were prepared by dilution of the concentrated aqueous HCl in acetonitrile solution.
9 The spectral data for compound 2a: 1H NMR (300 MHz, CDCl3): = 7.43 (d, J = 8.0 Hz, 1H), 7.29 (d, J = 8.0 Hz, 1H), 7.22 (td, J = 7.4, 1.1 Hz, 1H), 7.11 (td, J = 7.4, 1.3 Hz, 1H), 3.68 (s, 3H), 3.63 (s, 2H), 3.16 (t, J = 6.8 Hz, 2H), 2.82 (t, J = 6.8 Hz, 2H); 13C NMR (75 MHz, CDCl3): = 209.53, 137.59, 133.19, 126.22, 121.59, 119.33, 117.76, 108.97, 106.09, 38.55, 36.50, 29.37, 21.62; MS (EI, 70eV) m/e (%): 199 (M+, 92), 170 (100), 157 (8), 144 (17), 128 (10), 115 (9); HRMS (C13H13NO)
estimated: 199.0997, calculated: 199.0991.
10 A. Urrutia, J. G. Rodriguez, Tetrahedron
1999,55, 11095-11108.
11 The exchange occurs only in the presence of light. The solvent can also be D2O
containing acetonitrile. There is no deuterium exchange found in position 6 due to the low electron density.
12 K. H. Grellmann, U. Schmitt, J. Am. Chem. Soc. 1982, 104, 6267-6272.
5