World Congress on
Fertility Preservation
President of the Congress:
Professor Jacques Donnez
Brussels
, Be
lgi
um
- D
ec
em
b
e
r
1
0
-1
GENERAL INFORMATION
VENUE
The Congress will take place at the:
SHERATON BRUSSELS HOTEL
Place Rogier, 3 - Brussels 1210 - Belgium Phone:+39-2-224-31-11 www.starwoodhotels.com
LANGUAGE
The official language of this Congress will be English.
TRAVEL INFORMATION
Brussels, officially the Brussels Capital-Region, is the de facto capital city of the European Union (EU) and the largest urban area in Belgium. Brussels has grown from a 10th
century fortress town founded by Charlemagne's grandson into a metropolis of more than one million inhabitants. Since the end of World War II, Brussels has been an important centre for international politics. It hosts the main institutions of the European Union, and the headquarters of the North Atlantic Treaty Organization (NATO). Thus, Brussels is the polyglot home of many international organisations, diplomats and civil servants.
Brussels, Belgium - December 10-12, 2009
AIM OF THE CONGRESS
The increasing successes of oncologic treatments make implementation of procedures aimed at preserving fertility even more crucial. These procedures must not be limited to preserving and restoring fertility in patients undergoing therapies, but also be applied to any cases where the reproductive function is threatened. The aim of this congress is to offer an updated review of fertility preservation techniques that are currently available as well as those under evaluation. This Congress will involve members of the International Society for Fertility Preservation (ISFP) and world experts in the objective of developing a network among health care professionals interested in this challenging field.
LEARNING OBJECTIVES
After attending this Congress, the participants will have an updated knowledge on:
•How to approach fertility preservation in adult and children.
•Impact of cancer treatment on gonads.
•Cryopreservation techniques.
•Other most recent laboratory procedures used in fertility preservation.
•Advantages and disadvantages of fertility preservation strategies using gonadic cells, tissues and whole organs.
TARGET AUDIENCE
The Congress is addressed to clinicians and scientists involved in the fertility field, fertility preservation practice, oncologic patient management and endocrinologists interested in this topic.
ACCREDITATION
Serono Symposia International Foundation (www.seronosymposia.org) submitted this program “World Congress on
Fertility Preservation” (Brussels, December 10-12, 2009) for accreditation by the European Accreditation Council for
Continuing Medical Education (EACCME), the Royal College of Physicians and the Italian Ministry of Health.
All Serono Symposia International Foundation programs are organized solely to promote the exchange and dissemination of scientific and medical information. No forms of promotional activities are permitted. There may be presentations discussing investigational uses of various products. These views are the responsibility of the named speakers, and do not represent an endorsement or recommendation on the part of Serono Symposia International Foundation. This program is made possible thanks to the unrestricted Educational grant received from: ComtecMed, Congrex Sweden, Congrex Switzerland, Cryo-Save, Datanalysis, ISFP International Society for Fertility Preservation, ISMH International Society of Men’s Health, JDB consulting, K.I.T.E., Merck Serono, Meridiano Viaggi e Turismo, Università degli Studi di Catania.
SCIENTIFIC SECRETARIAT
Serono Symposia International Foundation Salita di San Nicola da Tolentino, 1/b 00187 Rome, Italy
Project Manager: Francesca Caputo Tel.: +39-06-420 413 568
Fax: +39-06-420 413 677 E-mail: info@seronosymposia.org
Serono Symposia International Foundation is a Swiss Foundation with headquarters in 14, rue du Rhône, 1204 Genève, Switzerland
ORGANIZING SECRETARIAT
Meridiano Congress International Via Mentana, 2/B - 00185 Rome - Italy Congress Coordinator: Federica Russetti Phone: +39-06-88595 209 Fax: +39-06-88595 234 E-mail: f.russetti@meridiano.it
SCIENTIFIC ORGANIZER
Jacques Donnez Department of Gynecology Saint-Luc University Hospital Catholic University of Louvain Brussels, BelgiumUniversité Libre de Bruxelles Brussels, Belgium Robert Fischer Fertility Center Hamburg, Germany Debra Gook Reproductive Services Royal Women’s Hospital Parkville and Melbourne IVF East Melbourne, Victoria, Australia
Stephan Gordts
Life - Leuven Institute for Fertility and Embryology
Leuven, Belgium
Roger G. Gosden
Cornell University Weill Medical College
Centre for Reproductive Medicine and Infertility New York, USA
Outi Hovatta
Karolinska Institute
Karolinska University Hospital Stockholm, Sweden
Dror Meirow
Division of Obstetrics and Gynecology IVF Unit
Sheba Medical Center Tel Hashomer, Israel
Philippe Morice
Gynecologic Surgery Institut Gustave Roussy Villejuif, France
Claus Yding Andersen
Laboratory of Reproductive Biology University Hospital of Copenhagen University of Copenhagen
Copenhagen, Denmark
Pedro N. Barri
Service of Reproductive Medicine Department of Obstetrics, Gynecology and Reproduction Institut Universitari Dexeus Barcelona, Spain
Andrea Borini
Centre for Reproductive Health Tecnobios Procreazione Bologna, Italy
Isabelle Demeestere
Laboratory for
Human Reproduction Research Faculty of Medicine
Université Libre de Bruxelles Brussels, Belgium
Marie-Madeleine Dolmans
Department of Gynecology Saint-Luc University Hospital Catholic University of Louvain Brussels, Belgium
Jacques Donnez
Department of Gynecology Saint-Luc University Hospital Catholic University of Louvain Brussels, Belgium
Herman Tournaye
Centre for Reproductive Medicine Universitair Ziekenhuis Brussels Brussels, Belgium
Chii-Ruey Tzeng
Department of Obstetrics and Gynecology Taipei Medical University Hospital Taipei, Taiwan
Etienne Van den Abbeel
Centre for Reproductive Medicine Academic Hospital, Dutch-speaking Brussels Free University
Laarbeeklaan Brussels, Belgium
Anna Veiga
Centre of Reproductive Medicine Institut Universitari Dexeus Barcelona, Spain
W. Hamish Wallace
Department of Haematology/Oncology Royal Hospital for Sick Children Edinburgh, UK
Teresa K. Woodruff
Department of Obstetrics and Gynecology Northwestern University
Feinberg School of Medicine Chicago, USA
Christine Wyns
Department of Gynecology Saint-Luc University Hospital Catholic University of Louvain Brussels, Belgium
S. Samuel Kim
Reproductive Endocrinology & Infertility University of Kansas
Kansas City, USA
Pasquale Patrizio
Yale University Fertility Center New Haven, USA
Antonio Pellicer
Department of Obstetrics and Gynecology Valencia University
Instituto Valenciano de Infertilidad (IVI) Valencia Valencia, Spain
Guido Pennings
Ghent Bioethics Institute
Department of Philosophy and Moral Science Ghent University
Gent, Belgium
Mikkel Rosendahl
Laboratory of Reproductive Biology Copenhagen University Hospital Rigshospitalet
Copenhagen, Denmark
Bruno Salle
Reproductive Medicine Service Hospices Civils de Lyon
University Claude Bernard Lyon I
Unité INSERM 846, Cellules Souches & Cerveau Lyon, France
Sherman J. Silber
Infertility Center of St. Louis St. Luke's Hospital
St. Louis, USA
Johan Smitz
Follicle Biology Laboratory UZbrussel
Brussels, Belgium
Seang Lin Tan
Department of Obstetrics and Gynecology McGill University
Montreal, Canada
Evelyn E. Telfer
Institute of Cell Biology and Centre for Integrative Physiology The University of Edinburgh Edinburgh, Scotland, UK
THURSDAY - DECEMBER 10, 2009
08.00 Registration
08.15 Welcome on behalf of Serono Symposia International Foundation (SSIF)
Robert Fischer, Germany
08.30 Welcome on behalf of International Society for Fertility Preservation (ISFP)
Jacques Donnez, Belgium
CANCER THERAPY AND FERTILITY PRESERVATION
Chairmen: Jacques Donnez, Belgium - Roger G. Gosden, USA08.45 L1: Opening Lecture: Fertility preservation in children.
Effect of radiotherapy on the uterus
W. Hamish Wallace, UK
09.25 L2: Effect of cytotoxic therapies on the ovary
Dror Meirow, Israel
09.50 L3: Effect of cytotoxic therapies on the testis
Herman Tournaye, Belgium
10.15 L4: Evaluation of ovarian function before and after chemotherapy - an approach to determine the cytotoxicity of different chemotherapy regimens?
Mikkel Rosendahl, Denmark
10.40 Coffee Break
Chairmen: S. Samuel Kim, USA - W. Hamish Wallace, UK
11.10 L5: GnRH agonists, is there evidence for a protective effect on the ovary?
Yvon Englert, Belgium
11.35 L6: Effect of chemotherapy and fertility preservation in women with breast cancer
Jehoshua Dor, Israel
12.00 L7: The real risk of conservative surgery in women with borderline and malignant ovarian tumors
Philippe Morice, France
CRYOPRESERVATION AND TRANSPLANTATION OF OVARIAN TISSUE
FROM OVARIAN TISSUE TO ISOLATED FOLLICLES
AND WHOLE OVARY PRESERVATION
Chairmen: Dror Meirow, Israel - Isabelle Demeestere, Belgium - Pasquale Patrizio, USA
14.00 L8: Heterotopic autotransplantation of cryobanked human ovarian tissue: eight year clinical experience in cancer patients
S. Samuel Kim, USA
14.25 L9: Orthotopic transplantation of ovarian cortex: review of the world experience
Jacques Donnez, Belgium
14.50 L10: Transport of ovarian tissue prior to cryopreservation - Experience from Denmark
Claus Yding Andersen, Denmark
15.05 Selected oral communications on cryopreservation and transplantation
15.05 OC1: Oncologists’ practice and attitudes regarding fertility preservation in female cancer patients: a pilot study in the Netherlands
L.A. Louwé, The Netherlands
15.15 OC2: Autotransplantation of cryopreserved ovarian tissue in a cohort of 12 Danish women
K. Tryde Schmidt, Denmark
15.25 OC3: Ovarian cortex cryopreservation in girls under 16 years of age
P. Jadoul, Belgium
15.35 OC4: Cryopreservation of ovarian tissue for fertility preservation in girls
K. Tryde Schmidt, Denmark
15.45 OC5: How to provide a good vascularisation on ovarian tissue grafting and pregnancies
P. Piver, France
15.55 Coffee break
Chairmen: Claus Yding Andersen, Denmark - Marie-Madeleine Dolmans, Belgium
16.30 L11: Risk of transplanting ovarian tissue in breast cancer
Antonio Pellicer, Spain
16.55 L12: Risk of transplanting ovarian tissue in leukemia
17.20 Selected oral communications on cryopreservation and transplantation
17.20 OC6: Systematic follow-up of young patients undergoing chemotherapy in order to assess the dynamics of follicular depletion: what should we learn? The experience of the Lille University Hospital
C. Decanter, France
17.30 OC7: Microarray approach to investigate gene expression in human ovarian tissue after xenografting
L. Romeu, Belgium
17.40 OC8: In vitro maturation and vitrification of immature oocytes combined with ovarian tissue cryopreservation: a new strategy of fertility preservation
G. Fasano, Belgium
17.50 OC9: Localization of c-kit/kit ligand and anti-Müllerian hormone in ovarian follicles following 28 weeks of human ovarian tissue xenotransplantation
D. Anu, Belgium
18.00 OC10: Successful propagation of human spermatogonial stem cells in vitro H. Sadri-Ardekani, the Netherlands
18.10 OC11: Morphological and ultra-structural features of cryopreserved ovine ovarian tissue: deleterious
effect of PROH applying different thawing protocols
I.C. Oskam, Norway
18.20 OC12: Minimal residual disease in cryopreserved ovarian cortex from patients with leukaemia M. Rosendahl, Denmark
18.30 OC13: From the analysis on 5571 autopsy findings of females under the age of 40 in Japan K. Kyono, Japan
WHOLE OVARY TRANSPLANTATION OR ARTIFICIAL OVARY:
FROM ANIMAL STUDIES TO HUMAN
Chairmen: Stephan Gordts, Belgium - Marie-Madeleine Dolmans, Belgium - Chii-Ruey Tzeng, Taiwan
08.00 L13: Lessons from experimental ovarian transplantation
Bruno Salle, France
08.25 L14: The preservation of the whole ovary in humans: where we are
Pasquale Patrizio, USA
08.50 L15: Transplantation of fresh ovarian tissue: cortical graft versus whole ovary?
Sherman J. Silber, USA
09.15 Coffee break
09.15 Selected oral communications on ovarian cortex and whole ovary transplantation
09.45 OC14: Glucose/lactate metabolism as a general marker for ovarian tissue survival after
cryopreservation of an intact ovary
H.R. Westphal, The Netherlands
09.55 OC15: Which are the ideal donor and recipient vessels for a whole ovarian transplantation? S. Ploteau, Belgium
10.05 OC16: A decade of experience with fertility preservation at Karolinska University Hospital K.A. Rodriguez-Wallberg, Sweden
10.15 OC17: Heterotopic autotransplantation of ovarian cortex in cynomolgus monkeys N. Suzuki, Japan
10.25 OC18: In vitro growth of isolated follicles in three dimensional alginate-collagen matrix R. Talevi, Italy
10.35 OC19: Mixed origin of neovessels and host-graft vascular link-up in human ovarian xenografts A.S. Van Eyck, Belgium
10.45 OC20: Effects of ionizing radiation on ovulation rate and oocyte morphology in mouse M. Sapmaz Metin, Turkey
TESTICULAR TISSUE PRESERVATION
Chairmen: Sherman J. Silber, USA - Christine Wyns, Belgium11.30 L16: Testicular tissue preservation: a review
Christine Wyns, Belgium
11.40 Selected oral communications on male fertility preservation
11.55 OC21: Reconstitution of spermatogenesis from testis failure after transplantation of germinal cells
for male fertility preservation - a transgenic mouse model
C.H. Chen, Taiwan
12.05 OC22: Effective cryopreservation of prepubertal mouse testicular tissue by vitrification M. Curaba, Belgium
12.15 OC23: Amifostine-doxorubicin association effects on prepubertal rat testes: long term damage on
sperm DNA integrity and early embryo developmental delay
V. Vendramini, Brasil
ISOLATED FOLLICLES TRANSPLANTATION OR IN VITRO MATURATION
Chairmen: Andrea Borini, Italy - Antonio Pellicer, Spain14.00 L17: Folliculogenesis in humans: in vivo and in vitro
Johan Smitz, Belgium
14.25 L18: In vitro maturation: factors affecting oocyte quality
David F. Albertini, USA
14:50 L19: Isolation and grafting of human isolated follicles: why and how? A place for an artificial ovary?
Marie-Madeleine Dolmans, Belgium
15.15 L20: Bioengineering the ovary
Christiani Andrade Amorim, Belgium
15.40 L21: Oncofertility: the preservation of fertility options for young people with cancer
Teresa K. Woodruff, USA
16.05 Coffee break
Chairmen: Pedro N. Barri, Spain - Johan Smitz, Belgium - Teresa K. Woodruff, USA
16.30 L22: Closing the gap between in vitro growth (IVG) and IVM using fresh and cryopreserved tissue
Evelyn E. Telfer, UK
16.55 L23: Fresh human ovarian tissue: from primordial to antral follicle in culture
Outi Hovatta, Sweden
17.20 L24: Impact of cryopreservation and grafting on folliculogenesis
Debra Gook, Australia
17.45 L25: In-vitro maturation and oocyte vitrification for the preservation of fertility in cancer patients
Seang Lin Tan, Canada
Chairmen: S. Samuel Kim, USA - Jacques Donnez, Belgium
18.15 Key note lecture: Potential for stem cell renewal of function in sterile ovaries and testes Roger G. Gosden, USA
SATURDAY - DECEMBER 12, 2009
Chairmen: Christiani Andrade Amorim, Belgium - W. Hamish Wallace, UK
08.00 Selected oral communications on cryopreservation and transplantation
08.00 OC24: Human meiotic spindle alterations following slow-cool cryopreservation J.J. Bromfield, USA
08.10 OC25: Ultra-rapid vitrificaton supported follicle morphologies of cynomolgus monkeys after freezing
compared to conventional vitrification and slow freezing
S. Hashimoto, Japan
08.20 OC26: Efficiency of oocyte cryopreservation E. Borghi, Italy
08.30 OC27: Clinical grade vitrification of human ovarian tissue M. Sheikhi, Sweden
08.40 OC28: Vitrified human ovaries harbor less primordial follicles and produce less antimullerian
hormone (AMH) than slow frozen ovaries
O. Oktem, Turkey
VITRIFICATION VERSUS SLOW FREEZING
Chairman: Roger G. Gosden, USACo-chairmen and discussants:
Anna Veiga, Spain - Pedro N. Barri, Spain - Outi Hovatta, Sweden
Oocytes
09.00 L26: Slow freezing versus vitrification - What is more efficient?
Andrea Borini, Italy
09.20 L27: Pros and cons
Anna Veiga, Spain
09.30 Discussion
Embryos
10.15 L28: Slow freezing versus vitrification - What is more efficient?
Etienne Van Den Abbeel, Belgium
10.35 L29: Pros and cons
Pedro N. Barri, Spain
10.45 Discussion Ovarian tissue
11.00 L30: Slow freezing versus vitrification - What is more efficient?
Bruno Salle, France
11.20 L31: Pros and cons
Outi Hovatta, Sweden
11.30 Discussion
ETHICS: FINAL LECTURE
Chairmen: S. Samuel Kim, USA - Dror Meirow,Israel - Pasquale Patrizio, USA
11.45 Introduction: Jacques Donnez, Belgium
L32: The next debate: Future, is there a place for ovarian tissue and oocyte cryopreservation for social reasons?
Guido Pennings, Belgium
12.30 Prize for oral presentation and poster - Closing Ceremony
DISCLOSURE OF FACULTY RELATIONSHIPS
Serono Symposia International Foundation adheres to guidelines of the European Accreditation Council for Continuing Medical Education (EACCME) and all other professional organizations, as applicable, which state that programs awarding continuing education credits must be balanced, independent, objective, and scientifically rigorous. Investigative and other uses for pharmaceutical agents, medical devices, and other products (other than those uses indicated in approved product labeling/package insert for the product) may be presented in the program (which may reflect clinical experience, the professional literature or other clinical sources known to the presenter). We ask all presenters to provide participants with information about relationships with pharmaceutical or medical equipment companies that may have relevance to their lectures. This policy is not intended to exclude faculty who have relationships with such companies; it is only intended to inform participants of any potential conflicts so participants may form their own judgments, based on full disclosure of the facts. Further, all opinions and recommendations presented during the program and all program-related materials neither imply an endorsement, nor a recommendation, on the part of Serono Symposia International Foundation. All presentations solely represent the independent views of the presenters/authors.The following faculty provided information regarding significant commercial relationships and/or discussions of investigational or non-EMEA/FDA approved (off-label) uses of drugs:
David F. Albertini Declared receipt of honoraria or consultation fees from Editor in Chief/J. Assisted Reproductive Genetics / Springer.
Christiani Andrade Amorim Declared no potential conflict of interest.
Claus Yding Andersen Declared no potential conflict of interest.
Pedro N. Barri Declared no potential conflict of interest.
Andrea Borini Declared no potential conflict of interest.
Isabelle Demeestere Declared no potential conflict of interest.
Marie-Madeleine Dolmans Declared no potential conflict of interest.
Jacques Donnez Declared no potential conflict of interest.
Yvon Englert Declared no potential conflict of interest.
Robert Fischer Declared receipt of honoraria or consultation fees from Serono Symposia International Foundation and to be a member of Serono Symposia International Foundation Scientific Committee..
Debra Gook Declared no potential conflict of interest.
Stephan Gordts Declared no potential conflict of interest.
Roger G. Gosden Declared receipt of grant and contracts from Serono Research Institute, Ferring Pharmaceuticals and declared receipt of honoraria or consultation fees from Schering-Plough.
Outi Hovatta Declared no potential conflict of interest.
S. Samuel Kim Declared no potential conflict of interest.
Pasquale Patrizio Declared receipt of grant from Duramed Research and the participation in a EMD Serono sponsored speaker’s bureau.
Antonio Pellicer Declared no potential conflict of interest.
Guido Pennings Declared no potential conflict of interest.
Mikkel Rosendahl Declared no potential conflict of interest.
Sherman J. Silber Declared no potential conflict of interest.
Johan Smitz Declared no potential conflict of interest.
Seang Lin Tan Declared receipt of honoraria or consultation fees from Schering-Plough (Organoon), Ferring Canada and EMD Serono Canada.
Dror Meirow Philippe Morice Bruno Salle Evelyn E. Telfer Anna Veiga
All Serono Symposia International Foundation programs are organized solely to promote the exchange and dissemination of scientific and medical information. No forms of promotional activities are permitted. There may be presentations discussing investigational uses of various products. These views are the responsibility of the named speakers, and do not represent an endorsement or recommendation on the part of Serono Symposia International Foundation. This program is made possible thanks to the unrestricted Educational grant received from: ComtecMed, Congrex Sweden, Congrex Switzerland, Cryo-Save, Datanalysis, ISFP International Society for Fertility Preservation, ISMH International Society of Men’s Health, JDB consulting, K.I.T.E., Merck Serono, Meridiano Viaggi e Turismo, Università degli Studi di Catania.
ABSTRACTS
(L1 - L32)
Childhood cancer is rare, with an incidence of around 110 cases per million children per year. Although survival from these malignancies was very poor in the 1960’s, major advances, both in treatment, thanks to the use of multi-agent chemotherapy, and in supportive care, achivied markedly improved rates of cure over recent decades. Current data suggest that around 80% of children with cancer will stay alive five years from diagnosis. As a result, the number of long-term survivors is increasing, and fertility preservation has become a major issue for the young patient about to start treatment for cancer.
Currently the only established option to preserve fertility for males is cryopreservation of spermatozoa before commencing treatment. Patients for whom this procedure is suitable must be peri- or post-pubertal and sexually mature. In addition they must be able to give consent for the storage of the specimen. There is currently no established option to preserve fertility for the pre-pubertal young boy. Female fertility preservation provides significantly different challenges to that for the male. Embryo freezing is now an accepted and well-established procedure in many centres, but is not available for children who do not have a partner. Cryopreservation of mature oocytes has become increasingly successful. However, for the young patient, cryopreservation of ovarian cortical tissue is extremely promising with around 30 cases of auto-transplanted frozen-thawed ovarian tissue reported leading to the birth of seven live infants so far. Ovarian tissue cryopreservation has the potential advantages of preserving a large number of oocytes within primordial follicles, it does not require hormonal stimulation when time is short, and is appropriate for the pre-pubertal girl. Disadvantages include the need for an invasive procedure, and the uncertain risk of ovarian contamination in haematological and other malignancies.
Radiotherapy causes both ovarian and uterine damage. There are no reports of uterine damage after chemotherapy. Uterine damage manifest by impaired growth and blood flow is a likely consequence of radiation to a field that includes the pelvis. Exposure of the pelvis to radiation is associated with: an increased risk of miscarriage, mid-trimester pregnancy loss, preterm birth and low birth weight. The optimal dose and delivery route of oestrogen replacement required to facilitate uterine growth in adolescent women treated with total body irradiation, needs to be established.
In this lecture I will discuss our experience with 36 women, highlighting issues of patient selection especially in the young, and uncertainties over the effects of cancer treatments on subsequent fertility. Of these 36 women, 11 have died, but 5 have had spontaneous pregnancies, with to date none having requested re-implantation of their stored ovarian tissue. Ovarian cryopreservation appears to be a potentially valuable method for fertility preservation, but the indications and approaches best used remain unclear.
In addition to the many scientific and technical issues to be overcome before clinical application of these techniques, a number of ethical and legal issues must also be addressed to ensure a safe and realistic prospect for future fertility in these patients.
L2
EFFECT OF CYTOTOXIC THERAPIES ON THE OVARY
Dror Meirow
Division of Obstetrics and Gynecology, IVF Unit, Sheba Medical Center, Tel Hashomer, Israel
XXXX of non-disjunction during meiosis is a rare event in most organisms, with the exception of the human species where approximately 30% of oocytes carry a chromosomal imbalance. This condition has severe clinical consequences, as approximately one-third of spontaneous abortions are aneuploid.
The most obvious oocytes recovered are well-known risk factors. The following measures can be considered in these cases. 1. Adapting the ovarian stimulation protocol according to the patient’s individual profile and the experience from her previous
stimulation cycles.
2. Reducing FSH and compensating with LH in the stimulation protocol to selectively stimulate the greatest follicles and prevent the growth of smaller ones.
Learning Objectives
By the end of the programme participants should appreciate: • International Variation in Assisted Reproduction Practice • Need to collect data to reflect practice
• Value of e-Learning to facilitate best practice
Introduction The Methods The Results The Conclusions These results References:
1 - Chappel SC, Howles C 1991 Reevaluation of the roles of luteinizing hormone and follicle-stimulating hormone in the ovulatory process. Human Reproduction 6 1206-1212.
2 - Filicori M, Cognigni GE, Pocognoli P et al. 2003 Current concepts and novel applications of LH activity in ovarian stimulation. Trends in Endocrinology and Metabolism 14, 267-273.
Herman Tournaye
Centre for Reproductive Medicine, Universitair Ziekenhuis Brussels, Brussels, Belgium
The testis has both an endocrine and exocrine function. The endocrine output depends on an adequate Leydig cell function. For the exocrine function, i.e; spermatogenesis, both a functional Sertoli cell and spermatogonial stem cell pool are necessary. Spermatogonial stem cells are undifferentiated cells, that give rise to the spermatogenic cells and, finally, the spermatozoa. Even though there is a continual loss of differentiated cells, the spermatogenic cell lineage maintains its cell number thanks to the adult spermatogonial stem cells, which produce both new stem cells (self-renewal) and differentiating cells.
Both chemo- and radiotherapy can cause long-term or even permanent gonadal failure.
Leydig cells are rather resistant to chemo- and radiotherapy. While elevated LH indicates Leydig cell dysfunction, most men will show normal testosterone levels and will retain a normal bone density. While routine testosterone supplementation after gonadotoxic treatment is not indicated, men should have endocrine monitoring after cancer treament and in selected cases testosterone substitution mat be required. Leydig cells seem more vulnerable to chemo- and radiotherapy in pre-pubertal life.
Also Sertoli cells show a good resistance to chemo- and radiotherapy, except in the postnatal and pubertal period when their numbers are increasing through mitosis. Increases in FSH are due to indirect effects through loss of the germ cell pool.
The extent of the gonadotoxicity on this germ cell is strongly related to the nature of the specific agents that are used and their dose or to the intensity of the radiation and the place of the body where it was administered. Transient reductions of sperm count can occur even after mild forms of chemotherapy or low doses of gonadal radiation, due to the destruction of the sensitive differentiating spermatogonia. Stronger chemotherapeutic regimes or higher doses of gonadal irradiation, however, lead to prolonged reduction in sperm count or complete azoospermia. Whether sperm production will eventually recover depends on the survival of the spermatogonial stem cells and the integrity of their ability to differentiate. High doses of radiation to the testis (>2.5 Gy) cause DNA damage and cell death. The most damaging chemotherapeutic agents in the adult man are the alkylating agents. Many therapeutic agents will only lead to a temporary reduction in sperm counts or have no effect at all on sperm production. These include other antimetabolites, topoisomerase inhibitors, corticosteroids, interferons …
Even though the gonadotoxic effect of most anti-cancer treatments is known, it is difficult to predict the final effect on the fertility potential of the patient. There remain important interindividual differences in response to the treatment and even if a regimen with low gonadotoxicity is started, it is possible that eventually a more gonadotoxic treatment has to be administered because of earlier treatment failure
Even if the anti-cancer treatment does not lead to infertility, single gene and chromosomal mutations might have been induced in the surviving germ cells and will be passed on to the offspring where they may lead to genetic diseases.
Since spermatogenesis starts only at puberty, the only spermatogenic cells that are present in the seminiferous tubules before the onset of puberty are the spermatogonia. Although it has been reported that the prepubertal testis, assumed to be in a quiescent stage, is less sensitive to the gonadotoxic effects of chemo- and radiotherapy, recent findings have show that even in prepubertal life, the seminiferous tubules are mitotically active. Therefore even in prepubertal boys, fertility preservation is an important issue. Fertility preservation in prepubertal boys lies within the preservation of their spermatogonial stem cells. Three options for fertility preservation through the application of spermatogonial stem cells are currently under research: the spermatogonial stem cell transplantation, the grafting of testicular tissue pieces and the in vitro proliferation and/or maturation of spermatogonia. Each technique has of course its (dis)advantages, but each one of them might eventually find its way to the clinic.
References:
- Tournaye H, Goossens E, Verheyen G, et al. Preserving the reproductive potential of men and boys with cancer: current concepts and future prospects. Hum Reprod Update 2004;10:525-532.
- Geens M, Goossens E, De Block G, Ning L, Van Saen D, Tournaye H. Autologous spermatogonial stem cell transplantation in man: current obstacles for a future clinical application. Hum Reprod Update. 2008;14(2):121-30.
L4
EVALUATION OF OVARIAN FUNCTION BEFORE AND AFTER CHEMOTHERAPY
-AN APPROACH TO DETERMINE THE CYTOTOXICITY OF DIFFERENT
CHEMOTHERAPY REGIMENS?
Mikkel Rosendahl
Laboratory of Reproductive Biology, Copenhagen University Hospital, Rigshospitalet, Copenhagen, Denmark
Introduction
The three main factors to consider when evaluating a woman’s risk of chemotherapy induced amenorrhoea and infertility are: the age of the woman, the type and dose of chemotherapy. Nevertheless, inter-individual variations may result in a different outcome in two women of equal age receiving the same type and dose of chemotherapy.
This presentation focuses on two such possible inter-individual variations:
1. Do the pre-treatment levels of ovarian markers – and do their alterations during treatment – predict the outcome with regards to
ovarian function?
2. Is the risk of chemotherapy induced amenorrhoea in breast cancer patients related to the degree of chemotherapy induced bone
marrow suppression and hence possibly related to inter-individual variations in the bioavailability of the cytotoxic substances?
Materials and methods
To answer Question 1, 17 women with cancer had ovarian markers (i.e. AMH, FSH, antral follicles) recorded before, during and after
combination chemotherapy.
To answer Question 2, 1016 premenopausal women received 7 series of CEF chemotherapy for breast cancer. Those patients with
sub-optimal bone marrow suppression after the first series of chemotherapy were randomised to either increased or unchanged doses of FEC. Those with initial optimal bone marrow suppression continued with unaltered dosage. Bone marrow suppression and menstrual regularity was monitored.
Results
In study 1, we discovered a significant and immediate chemotherapy-induced drop in AMH and Inhibin B. These were, however, not predictive of the outcome. Irrespective of age, the pre-treatment AMH level was predictive of the AMH level one year after the end of chemotherapy.
In study 2, for younger patients, a more pronounced bone marrow suppression was – irrespective of the dose – significantly related to a higher risk of amenorrhoea.
Conclusions
The three most important factors of development of chemotherapy induced amenorrhoea remain the age of the patient and the type and dose of chemotherapy. However, individual counselling may be guided by the pre-treatment AMH level and for younger patients it seems that a higher bioavailability of the chemotherapy increases the patient’s risk of amenorrhoea.
OVARY?
Y. Englert
1, P. Brice P.
2, F. Peccatori
3, B. Dominique
4, S. Tsepelidis
1and I. Demeestere
2;
1Fertility Clinic, Department of Obstetrics and Gynecology, Hopital Erasme and Laboratory for research on human reproduction,
Medicine Faculty, Campus Erasme, Université Libre de Bruxelles, Belgium;
2Haematological Department, St Louis Hospital, APHP, Paris, France; 3Haematological Department, Istituto Europeo di Oncologia, Milano, Italy; 4Haematological Department, J. Bordet Institute, Brussels, Belgium.
Since the first publication of Ataya et al. (1985) describing a protective effect of GnRH against chemotherapy-induced gonadal damage in rats, various papers have confirmed the protective effect on ovarian function (and sometime on fertility) in animal models, including non human primates, against ovariotoxic chemotherapy, such as alkylant agents. Human literature is much weaker; especially because nearly all publications describe comparative studies between groups of patients and historical retrospective controls, in case control settings. Nevertheless, more than 300 patients received GnRH agonists in these studies and performed better than controls regarding to ovarian function. Much fewer prospective randomized studies were published and will be examined in details, including an ongoing European multicentric study with more than 100 included patients coordinated by the authors and conducted in 15 centers. In this study patients with hematologic cancer, that provide the informed consent, are randomized either to GnRH agonist or to a control group. An intermediary analysis at 18 months follow up will be presented.
All these studies, randomized as well as case controls, are focused up to now on ovarian function and it will be stressed that menstruating is a necessary but not sufficient criterion for fecundity, the ultimate goal of protection of the ovarian function in young women and children. GnRH agonist strategy to protect ovarian function is still today a matter of debate, not only because of lack of definitive clinical evidence, but also because of the FSH independence of primordial follicles recruitment, target to be protected against toxic effect of anti mitotic drugs. It has been however hypothesized no less than 5 possible explanations to understand why GnRH analogs may decrease toxic effect of alkylant drugs on the ovarian primordial follicles. The recent surprising publications on the description of undifferentiated germ line stem cells in the adult ovary as well as the unfavorable outcome reported for the use of antagonists in animal model will be put in perspective with these hypotheses to revisit them, one by one. Safety of the use of GnRH analogs during chemotherapy will be also examined.
L6
EFFECT OF CHEMOTHERAPY AND FERTILITY PRESERVATION IN WOMEN WITH
BREAST CANCER
Jehoshua Dor
Infertility and IVF unit, Sheba Medical Center, Ramat Gan, Sackler School of Medicine, Tel Aviv University, Tel Aviv, Israel
XXXX of non-disjunction during meiosis is a rare event in most organisms, with the exception of the human species where approximately 30% of oocytes carry a chromosomal imbalance. This condition has severe clinical consequences, as approximately one-third of spontaneous abortions are aneuploid.
The most obvious oocytes recovered are well-known risk factors. The following measures can be considered in these cases. 1. Adapting the ovarian stimulation protocol according to the patient’s individual profile and the experience from her previous
stimulation cycles.
2. Reducing FSH and compensating with LH in the stimulation protocol to selectively stimulate the greatest follicles and prevent the growth of smaller ones.
Learning Objectives
By the end of the programme participants should appreciate: • International Variation in Assisted Reproduction Practice • Need to collect data to reflect practice
• Value of e-Learning to facilitate best practice
Introduction The Methods The Results The Conclusions These results References:
1 - Chappel SC, Howles C 1991 Reevaluation of the roles of luteinizing hormone and follicle-stimulating hormone in the ovulatory process. Human Reproduction 6 1206-1212.
2 - Filicori M, Cognigni GE, Pocognoli P et al. 2003 Current concepts and novel applications of LH activity in ovarian stimulation. Trends in Endocrinology and Metabolism 14, 267-273.
AND MALIGNANT OVARIAN TUMORS
Philippe Morice
Gynecologic Surgery, Institut Gustave Roussy, Villejuif, France
Conservative treatment of borderline ovarian tumors (BOT) increases recurrence rate (compared to radical treatment). This risk is estimated, in literature, between 0 and 25%. It is also elevated after cystectomy (between 12 to 58%). But data in literature seem to confirm that, even though the risk of relapse is majored after conservative treatment in BOT, survival of patients is not altered with conservative management. So, conservative surgery could be safely performed in young patients treated for early stage BOT and carefully followed-up. Such conservative treatment should be considered in patients with serous BOT and non-invasive peritoneal implants. Pregnancy rates varied in literature between 30% and 80%. In Vitro Fertilization (IVF) procedures could be considered in patients treated for early stage borderline disease and having a persistent infertility after the surgical treatment.
Conservative treatment has a large place in the management of a majority of non epithelial cancer (even in the case of advanced stage disease). But the indication and modalities of such treatment depends on the tumor histological subtype.
Conservative surgery for patients with epithelial ovarian cancer could be considered in patients with stage IA grade 1 or 2 disease but should not be proposed in stage IA grade 3 disease. Recent data seems to suggest that such treatment could be safely proposed in selected cases of patients with stage IC grade 1 disease.
This treatment should not performed in patients with FIGO stage > I. Patients are selected on careful histological analysis of the tumor specimen (in order to precise the histological subtypes and tumor grade), absence of extra-ovarian spread determined after (re)staging surgery and absence of inherited syndrome predisposition to ovarian cancer. In Vitro Fertilization (IVF) procedures are contraindicated in patients treated for epithelial cancer and having a persistent infertility after the surgical treatment.
L8
HETEROTOPIC AUTOTRANSPLANTATION OF CRYOBANKED HUMAN OVARIAN
TISSUE: EIGHT YEAR CLINICAL EXPERIENCE IN CANCER PATIENTS
S. Samuel Kim
Reproductive Endocrinology & Infertility, University of Kansas, Kansas City, USA
Ovarian tissue cryobanking followed by autotransplantation has been proven to be a valuable strategy to restore ovarian function and fertility for cancer patients. There are still many unknowns including its efficacy, since our experience with human ovarian transplantation is limited. Frozen-thawed ovarian tissue can be transplanted either to an orthotopic site or heterotopic site. To date, orthotopic autotransplanation of frozen-thawed ovarian tissue resulted in seven live births worldwide. On the other hand, no baby has been born after heterotopic autotransplantation. It is however worthy to continue investigating this technology even with skepticism, as several studies showed the return of ovarian function and potential fertility after heterotopic transplantation. Besides, heterotopic transplantation is easy, convenient, less invasive, and can be cost effective if repeated transplantation is required.
We transplanted frozen-thawed ovarian tissue heterotopically in cancer patients during the past 8 years to assess the long term ovarian function and restoration of fertility. Four study patients with the age range between 28 and 35 (three with cervical cancer, one with breast cancer) were identified and consented for heterotopic transplantation. All ovarian tissue had been cryopreserved using a slow freezing method before cancer treatment. Heterotopic ovarian transplantation (to the space between rectus muscle and fascia in the abdomen) was performed between 2002 and 2005. The restoration and maintenance of ovarian function was confirmed by a serial blood test (FSH, LH, estradiol, progesterone, testosterone) and ultrasound monitoring. To investigate the restoration of fertility, three patients were stimulated with gonadotropin and oocyte retrievals were attempted, once restoration of normal ovarian function was established. The retrieved oocytes were matured in vitro and fertilized with partner’s sperm.
The hormonal profile of all four patients was consistent with the postmenopausal level before transplantation. The return of the ovarian function was evidenced by the elevation of serum estradiol levels and by the decrease of FSH levels below 20 mIU/ml between 12-20 weeks after transplantation in all patients. However, restored ovarian function lasted only 3-5 months, and all three patients (except one with relapsed disease) agreed to undergo second transplantation. The return of ovarian function after second transplantation was faster in all three patients (between 1-3 months).
In contrast to first transplantation, we observed the establishment of long term ovarian function (lasting for 9-50 months) after second transplantation. All three patients maintained the FSH levels below 15 mIU/ml during this period. We were able to retrieve 7 oocytes (two GV, four MI, one MII) from ovarian grafts in 2 patients between August 2003 and November 2006. Three of four MI oocytes were developed to full maturity in vitro. All four MII oocytes were fertilized and cultured in vitro for 2 or 3 days before cryopreservation. Currently, 4 embryos (at 6-cell, 3-cell, 2-cell, PN stage) are stored in liquid nitrogen.
Although our results are encouraging, it is a real challenge to make it a clinically practicable technology since the environment of heterotopic sites may not be as optimal for follicle development.
WORLD EXPERIENCE
Jacques Donnez
Department of Gynecology, Saint-Luc University Hospital, Catholic University of Louvain, Brussels, Belgium
It is our duty to evaluate the effects of specific cancer therapies on fertility and discuss fertility preservation options with young women requiring such treatment prior to its initiation.
The different cryopreservation options available for fertility preservation in cancer patients are: embryo cryopreservation, oocyte cryopreservation and ovarian tissue cryopreservation.
The only established method of fertility preservation is embryo cryopreservation, but this requires the patient to be of pubertal age, have a partner, and be able to undergo a cycle of ovarian stimulation.
Cryopreservation of ovarian tissue is the only option available for prepubertal girls, and for woman who cannot delay the start of chemotherapy.
Thirty cases of orthotopic reimplantation of cryopreserved ovarian tissue have so far been reported and seven live births have been achieved, yielding a pregnancy rate of almost 25%. In our department, six women have undergone orthotopic reimplantation of cryopreserved tissue either once or twice. Restoration of ovarian function, proved by follicular development and estradiol secretion, occurred in all cases. A time interval of 3.5 to 5 months was observed between reimplantation and the first signs of ovarian graft activity in all cases.
Two ongoing pregnancies were naturally obtained from this series of 6 patients. Graft activity was found to persist for 2.5 to 4 years. In non-pregnant patients, IVF was performed, but the quality of oocytes and embryos was not optimal (see Dolmans et al., Hum Reprod 2009). Prognostic factors (age, previous chemotherapy) are therefore discussed.
With all the advances in ovarian tissue, such as cryobanking and reproductive technology, fertility preservation is now a real possibility for patients whose gonadal function is threatened by radiotherapy or chemotherapy. For this reason, it should be a medicolegal obligation for gynecologists, oncologists and pediatricians to systematically propose cryopreservation before initiating cancer therapy that could impair future fertility
L10
TRANSPORT OF OVARIAN TISSUE PRIOR TO CRYOPRESERVATION - EXPERIENCE
FROM DENMARK
Claus Yding Andersen
Laboratory of Reproductive Biology, University Hospital of Copenhagen, University of Copenhagen, Copenhagen, Denmark
Survival rates of patients suffering from a malignant disease have steadily increased during this decade. This is largely, a result of the use of more aggressive and effective chemo- and radiotherapies. However, as a side effect, these therapies also exert a toxic effect on the pool of ovarian follicles. If the ovaries become depleted of follicles the woman will become sterile, loose menstrual cycles and many women experience profound effects on the physical and psychological status. For young girls it may further imply that pubertal development fails. A number of methods are now under development in order to preserve fertility in patients receiving potential gonadotoxic treatment. Cryopreservation of ovarian tissue is a new method that can be employed on a short notice and is now gaining ground as valid alternative to embryo and oocyte cryopreservation.
Cryopreservation of ovarian tissue involves removal of one ovary or parts of one ovary prior to treatment. When the women have been cured and are considered fit, the thawed ovarian tissue, with a viable pool of follicles, can be transplanted to women who entered menopause. The pool of surviving primordial follicles will be reactivated and the patients will regain fertility and will experience cyclic variation in sex hormone levels.
Laboratory of Reproductive Biology at University Hospital of Copenhagen is the only center in Denmark offering cryopreservation of ovarian tissue as a treatment in close collaboration with three fertility clinics round the country. The ovarian tissue is extracted at the local hospital and transported on ice to our laboratory, where cryopreservation and storage is performed. This transport model has now been used for more than 250 cases and totally in Denmark almost 400 girls and women have had ovarian tissue cryopreserved.
In Denmark a total of fifteen women (10 having their tissue transported prior to cryopreservation) have experienced transplantation of frozen/thawed ovarian tissue. Three women recently received transplantation and the outcome is currently unknown but the remaining 12 women all regained ovarian function. Over a period of 20 - 25 weeks, FSH gradually return to pre-menopausal levels and menstrual cycles are regained. The longevity of the tissue depends on the age of the woman at tissue retrieval and the amount of tissue transplanted. Most women experience return of ovarian function for some years with just a fraction of tissue from one ovary being replaced.
Four of the women have been pregnant; in most cases as a result of assisted reproduction. Two women have delivered three healthy babies as a result of transplanted frozen/thawed ovarian tissue. In the latter two cases the tissue was transported 4-5 hours prior cryopreservation. The presentation will review our experiences and recent results.
Antonio Pellicer, Maria Sánchez and Juana Crespo
Instituto Valenciano de Infertilidad (IVI) and Hospital Universitario Dr Peset, University of Valencia, Valencia, Spain
Ovarian tissue preservation and transplantation are intended for women undergoing aggressive regimens of chemical and/or radiological therapy, bone marrow transplantation or stem cell transplantation. Main indications for the procedure are neoplastic diseases and autoimmune disorders, breast cancer being the most frequently addressed among them. We established a program of fertility preservation (FP) in 2005 and today >300 women, with a mean age of 28.25yrs (11-39), have been treated and ovarian cortical tissue cryopreserved. Approximately 55% are breast cancer patients, 25% Hodgkin disease, and 20% other non malignant or malignant diseases. The procedure is based on the removal of the right ovarian cortex by laparoscopy and cryopreservation, before cancer treatment begins. Pieces of ~2 x 3 cm are frozen, while additional tissue from the right, as well the left ovary is sent to Pathology to search for the presence of malignant cells in the ovaries. Once the patient is free of disease, the right ovarian cortex is thawed and implanted onto left ovarian medulla (after extraction at the same surgical time of left ovarian cortex).
The main concern of this procedure is the possibility of reintroducing metastatic cells within the implant, an issue that has not been addressed systematically. Thus, a study was designed to analyse the presence of ovarian metastases in breast cancer patients undergoing ovarian tissue cryopreservation. Morphological and immunohistochemical studies following the concept of the sentinel lymph node (SLN) were performed on 100 cortical ovarian biopsies and on six frozen-thawed entire cortex from patients with the diagnosis of infiltrating ductal breast carcinoma undergoing ovarian cortex extraction and cryopreservation. The antibody panel included Cytokeratin CAM 5.2, Gross Cystic Disease Fluid Protein-15 (GCDFP15), Wilms tumour antigen-1 (WT1) and Mammaglobin 1 (MGB1). Employing only morphologic criteria, suspicious neoplastic cells were detected in the biopsies of 5 cases, but in none of the 6 entire cortex analysed. These 5 cases were reclassified as hyperplasic surface epithelium-inclusion cysts (CAM 5.2+, WT1+) or apoptotic granulosa cells (CAM 5.2-, GCDFP15+, WT1-).
To date, a total of 8 reimplantations have been performed, two of them very recently. The remaining patients have a normal endocrine function. One of them is a 36 yrs old patient diagnosed of atypical medullar breast cancer, negative for estrogen, progesterone and HER2 receptors. She underwent ovarian tissue cryopreservation before chemo and radiotherapy. Menses occurred sixty-three days after transplantation, but because she was infertile already before treatment and because she was 39 yrs old after treatment, ART was performed using vitrification because the life span of the transplanted tissue was uncertain. A total of 16 mature oocytes were obtained out of four stimulations. All vitrified oocytes survived after warming and 77.7% fertilized; two day-3 embryos were replaced and two healthy boys were born at 34 weeks. In conclusion, using the methodology of the SLN our data suggest the absence of tumour cells in biopsies obtained from patients undergoing ovarian cortex cryopreservation to preserve their fertility potential, although future methods of cancer screening may change our perception of this procedure. Ovarian tissue cryopreservation and grafting preserves fertility and oocyte vitrification can be simultaneously employed to increase the success of ART in poor prognosis patients and to avoid the consequences of a short lifespan of the transplanted tissue.
L12
RISK OF TRANSPLANTING OVARIAN TISSUE IN LEUKEMIA
Dror Meirow
Division of Obstetrics and Gynecology, IVF Unit, Sheba Medical Center, Tel Hashomer, Israel
XXXX of non-disjunction during meiosis is a rare event in most organisms, with the exception of the human species where approximately 30% of oocytes carry a chromosomal imbalance. This condition has severe clinical consequences, as approximately one-third of spontaneous abortions are aneuploid.
The most obvious oocytes recovered are well-known risk factors. The following measures can be considered in these cases. 1. Adapting the ovarian stimulation protocol according to the patient’s individual profile and the experience from her previous
stimulation cycles.
2. Reducing FSH and compensating with LH in the stimulation protocol to selectively stimulate the greatest follicles and prevent the growth of smaller ones.
Learning Objectives
By the end of the programme participants should appreciate: • International Variation in Assisted Reproduction Practice • Need to collect data to reflect practice
• Value of e-Learning to facilitate best practice
Introduction The Methods The Results The Conclusions These results References:
1 - Chappel SC, Howles C 1991 Reevaluation of the roles of luteinizing hormone and follicle-stimulating hormone in the ovulatory process. Human Reproduction 6 1206-1212.
2 - Filicori M, Cognigni GE, Pocognoli P et al. 2003 Current concepts and novel applications of LH activity in ovarian stimulation. Trends in Endocrinology and Metabolism 14, 267-273.
Bruno Salle
Reproductive Medicine Service, Hospices Civils de Lyon, University Claude Bernard Lyon I, Unité INSERM 846, Cellules Souches & Cerveau, Lyon, France
XXXX uffa
L14
THE PRESERVATION OF THE WHOLE OVARY IN HUMANS: WHERE WE ARE
P. Patrizio
1and A. Arav
2;
1 Yale University Fertility Center, New Haven, USA 2 CoreDynamics, Ness Ziona, Israel
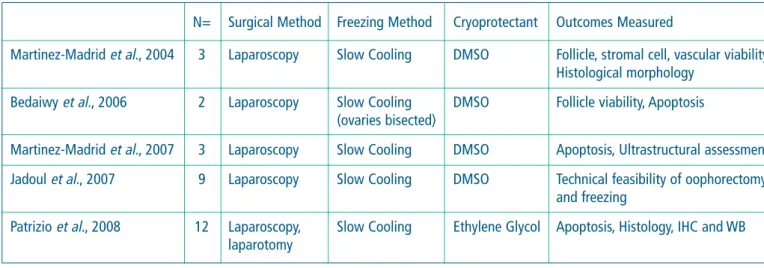
Cryopreservation and re-transplantation of ovarian cortical strips have been successful, to some extent, by documenting pregnancies and births. However, the lifespan of the transplanted avascular grafts is limited by the ischemia and the consequent loss of primordial follicles that occurs during the re-establishment of a blood supply. Thus, to overcome this problem for patients that desire long term resumption of endocrine function, we began to perform (in vitro) studies on the cryopreservation of the whole ovary with their intact vascular pedicle. This paper will review the recent advances in whole ovary cryopreservation mostly on human specimens, with a focus on surgical technique for removal, choice of cryoprotectants, freezing protocols, and histological and immunochemistry results post-organ thawing (see summary table). Two main issues in organ cryopreservation and re-transplantation had to be solved before progressing into whole ovary cryopreservation: the first, the creation of an efficient and reproducible cryopreservation protocol; the second, the demonstration that it is feasible to re-establish the vascular anastomosis of the organ to re-transplant. The first issue has been solved by utilizing the same Multi-Thermal-Gradient device and slow cooling-rapid thawing protocol described earlier (Arav et al. 2005) for whole sheep ovaries. With this method, ovaries from premenopausal women have been successfully cryopreserved for times ranging from 48 to 96 hours following either laparotomy, laparoscopic, or robotic assisted laparoscopic oophorectomy. The ovarian artery has a very narrow diameter (between 0.4- 0.5 mm) therefore it is important to leave a long pedicle (at least 4 cm long) for a successful organ perfusion with cryoprotectant (ethylene glycol). Compared to the fresh contra lateral ovary used as control, the frozen/thawed ovary was histologically indistinguishable. Immunohistochemistry and Western Blot assays did show some increase in anti-caspase 3 and p53 phospho-serine expression, suggesting some increased level of apoptosis in the frozen/thawed specimens. The functional meaning of these changes can only be assessed with re-transplant experiments.
Concerning the second issue, feasibility of whole organ re-transplant, a recent paper (Silber et al. 2008) has shown that with a whole fresh ovary the re-transplant is technically doable. However, there are no data yet of human cases of whole ovary re-transplantation after cryopreservation/thawing.
Table: Summary of whole ovary cryopreservation experiments in humans.
N= Surgical Method Freezing Method Cryoprotectant Outcomes Measured
Martinez-Madrid et al., 2004 3 Laparoscopy Slow Cooling DMSO Follicle, stromal cell, vascular viability, Histological morphology
Bedaiwy et al., 2006 2 Laparoscopy Slow Cooling DMSO Follicle viability, Apoptosis (ovaries bisected)
Martinez-Madrid et al., 2007 3 Laparoscopy Slow Cooling DMSO Apoptosis, Ultrastructural assessment Jadoul et al., 2007 9 Laparoscopy Slow Cooling DMSO Technical feasibility of oophorectomy
and freezing
Patrizio et al., 2008 12 Laparoscopy, Slow Cooling Ethylene Glycol Apoptosis, Histology, IHC and WB laparotomy
WHOLE OVARY?
Sherman J. Silber
Infertility Center of St. Louis, St. Luke's Hospital, St. Louis, USA
A series of monozygotic (MZ) twin pairs discordant for premature ovarian failure presented an unusual opportunity to study ovarian transplantation. Ten MZ twin pairs requested ovarian transplantation and nine have undergone transplantation with cryopreservation of spare tissue. Eight had a fresh cortical tissue transplant, one of whom received a second frozen-thawed transplant after the first ceased functioning at three years. One had a fresh microvascular transplant. All recipients reinitiated ovulatory menstrual cycles and normal Day 3 serum FSH levels by 77-142 days. Seven patients have already conceived naturally (three twice). Currently, seven healthy babies have been delivered out of ten pregnancies. The oldest transplant ceased functioning by three years, but then she conceived again after a frozen-twawed secondary transplant. There was no apparent difference in return of ovarian function between the nine fresh ovarian grafts and the one frozen graft. Ovarian transplantation appears to restore ovulatory function robustly. Ten pregnancies, leading to seven healthy babies, including one after cryopreservation, bode well for application to fertility preservation.
L16
TESTICULAR TISSUE PRESERVATION: A REVIEW
Christine Wyns
Department of Gynecology, Saint-Luc University Hospital, Catholic University of Louvain, Brussels, Belgium
Fertility in adult life may be severely impaired by gonadotoxic therapies. For young boys who do not yet produce spermatozoa, cryopreservation of immature testicular tissue is an option to preserve their fertility, albeit still experimental. Existing approaches to immature testicular tissue cryopreservation and fertility restoration in the literature are extensively reviewed and current knowledge on in vivo spermatogonial stem cell (SSC) protection is updated. Different cryopreservation protocols and fertility restoration options from frozen tissue, i.e. cell suspension transplantation, cell aggregate grafting, tissue grafting and in vitro maturation, are presented. Results obtained in humans are discussed in the light of lessons learned from animal studies.
Advances in reproductive technology have made fertility preservation a real possibility in young patients whose gonadal function is threatened by gonadotoxic therapies. The putative indications for such techniques, as well as their limitations according to disease, are outlined.
Johan Smitz
Follicle Biology Laboratory, UZbrussel, Brussels, Belgium
Although still experimental, the practice of oocyte and ovarian tissue cryopreservation has been spreading fastly, since most female cancer patients of reproductive age do not have the option of utilizing established assisted reproductive technologies as IVF and embryo cryopreservation to safeguard their fertility. Indeed, embryo preservation is not an option for prepubertal girls or single women.
Although knowledge of mammalian ovary regulation has exponentially increased through the availability of molecular techniques in the last two decades, it is still impossible today to obtain - with a high efficiency - oocytes that could be developed into a normal embryo. Initiatives, such as the Web-based Ovarian Kaleidoscope Database, offer unique opportunities with up-to-date information on the function and regulation of ovarian genes. Having a more precise knowledge on the major mechanisms driving the earliest stages of follicle growth has incited few laboratories to attempt cultures of small pieces of ovarian cortex. While we are obliged by 'numbers' to focus on cultures of primordial follicles, it is still precisely unknown how their first step of growth is regulated. Making use of defined matrices and purified growth factors, systematic attempts have been undertaken, which can bring - still with very low efficiency - primordial follicles up to early secondary follicles. Encouraging results using alginate matrix and defined media components have demonstrated that starting - off with one and a half - to two layered early preantral follicles, early antral follicles could be obtained in sheep (Muruvi et al., 2009), rhesus monkey (Xu et al.,2009) and human (Xu et al., 2009). For each species and each follicle growth stage, another culture system will probably have its advantages. A next step in the challenge will be further optimization of the culture conditions (as was also needed for the first live born mice by the Eppig Laboratory) in order to increase the number of oocytes capable of undergoing GVBD. At this point, we might apply the techniques used for In Vitro Maturation (IVM), which are - although still less effective as IVF - capable to provide healthy babies. There is evidence from research in the bovine model that, in large mammal’s oocytes, quality depends in events that occur before GVBD. By the time that chromosomes start to be condensed, the oocyte has generally accumulated the appropriate information for fertilisation and early embryonic development (Sirard et al., 2001; Dieleman et al., 2002).
The hypothesis is: in-vitro culture system could be improved to support better the final maturation stages of the oocyte. It is possible that oocytes are probably already predetermined at their retrieval. It is currently unexplored whether therapeutic interventions in this earlier part of folliculogenesis might be helpful in providing more optimal starting material for culture.
The selection of cumulus oocyte complexes (COC) for culture is a crucial process that determines outcome. New tools are QPCR analysis of cumulus cells to provide a more precise way to evaluate the progression in oocyte maturity. The availability of large numbers of oocytes from animal models are useful material, as a first approach, to select genes related to development that could be subsequently tested on precious human oocytes. However species-specific differences will have to be taken into account. Cumulus cells could perhaps reflect oocyte health and bear the markers for developmental competence. Such markers could be helpful to select the adequate culture system. Refinement of the culture technique for COC becomes accessible when there is a better control over meiosis.
The improvements in ovarian tissue-, follicle- and oocyte-cumulus culture go hand in hand with the increased knowledge generated from the molecular analyses of gametogenesis and early embryo development.
Prolonged culture systems need to be proactively tested to uncover eventual epigenetic changes due to in-vitro manipulations.
References:
- Dieleman SJ et al., Theriogenology, 2002 , 57, p 5-20. - Muruvi et al., 2009 Animal Reprod Sci 2009, p 36-50
- Sirard MA. Resumption of meiosis. Theriogenology , 2001, 55, p 1241-1254.
- Xu M, et al., (2009a) Encapsulated three-dimensional culture supports development of nonhuman primate secondary follicles Biol Reprod 81, 587-594. - Xu M, et al. (2009c) In vitro grown human ovarian follicles from cancer patients support oocyte growth Hum Reprod 24, 2531-2540.
L18
IN VITRO MATURATION: FACTORS AFFECTING OOCYTE QUALITY
David F. Albertini
The Center for Reproductive Sciences, Molecular and Integrative Physiology, University of Kansas Medical Centre, Kansas City, USA
Fertility preservation strategies place an increasing demand on proving the safety and efficacy of human ARTs including those that bear directly on the use of oocyte in vitro maturation protocols. While in some mammalian species the use of IVM often results in the production of developmentally competent ova capable of sustaining term gestation, for most species this is not the case. Two factors have emerged as primary determinants of oocyte quality when considering the deployment of IVM: (1) the history of the follicular milieu for a given oocyte, and (2) the meiotic status of oocytes at the time they are placed in culture. This presentation will develop the hypothesis that the quality of an oocyte will be a byproduct of intrinsic and extrinsic influences that exert their effects just prior to or during the resumption of meiosis. It follows then, that conditions required to optimize IVM for clinical use must emphasize the particular demands for oocytes whose legacies draw upon patient variations in age, metabolic status, or disease conditions that could in any way disturb the normal course of oogenesis in that individual.
Forces that are intrinsic to the oocyte that specify their quality status generally fall into the category of maturation competencies. Amongst these, we suggest abandoning the traditional view that cytoplasmic and nuclear maturation include distinct events that must occur in coordination with each other to satisfy acquisition of oocyte developmental competence. Rather, we view the oocyte as a semi-autonomous entity (see below) that uses complex signaling networks to discriminate and synergize “maturation” of chromatin, the cytocortex, and metabolic components that are collectively designed to address the needs of the zygote and not that of the oocyte per se. For the oocyte to assume autonomy in preparing for embryogenesis implies that the driving forces for the completion of the meiotic cell cycle must take their origin from somatic elements of the follicle especially those represented by the cumulus oophorus. Thus, extrinsic control over oocyte quality is indirectly satisfied by virtue of three properties of the granulose cell syncitium: (1) translating the stimulus from LH into the metabolic activation of the cumulus-oocyte complex, (2) effecting a signaling pathway that both inactivates cell cycle arrest and encourages the largely post-translational activation of the meiotic cell cycle machinery, and (3) induce substantial changes in the molecular composition and structure of the oocyte cortex as a result of physical interactions with the oolemma and the ends of transzonal projections. Only with a deeper understanding of the balance between oocyte intrinsic and extrinsic forces will it be possible to tailor human IVM for the various pre-conditions that fertility preservation strategies demand.