行政院衛生署九十七年度補(捐)助研究計
畫
成果報告
計畫名稱:以物理治療骨質疏鬆症所引起老年人骨折
之效用
執行機構:中國醫藥大學
主 持 人:湯智昕
研究人員:湯智昕, 劉軒誌
執行期間: 97 年 1 月 1 日至 97 年 12 月 31 日
**本研究報告僅供參考,不代表本署意見
目錄
目錄
目錄
目錄
頁 碼
目錄
2
摘要
3
英文摘要
4
前言
5
材料與方法
7
結果
10
討論
26
結論與建議
27
參考文獻
28
附錄
30
摘要
摘要
摘要
摘要
骨折癒合是一個複雜的生理作用,其中包含了多種細胞參與在其中。在 骨折癒合的過程之中需要血液的灌流及血管新生,最終促進造骨細胞合成新生 的骨質而促進骨癒合。在文獻的收集中我們發現超音波是一個具有低能量且可 以加速骨癒合之儀器,在臨床的研究及動物實驗當中都可以觀察到超音波可以 促進骨合成。而美國 FDA (美國食品及藥物管理局) 也證明超音波可以加速骨 折之癒合,但是美中不足的地方在於超音波經由那些機轉或訊息路徑促進骨癒 合是未知的,因此了解超音波治療之下造骨細胞(osteoblasts)產生那些反應或 引發那些路徑是很重要的課題。 細胞中的細胞間質可提供一個細胞平台使細胞生長,另外也參與在多種 細胞功能當中,而造骨細胞在骨癒合及骨再塑之過程之中通常需要多種細胞間 質來參與。細胞間質水解酵素 (Matrix metalloproteinases; MMP) 其主要的作用 是清除 collagen 及細胞間質,當 MMP 將不良的細胞間質清除之後造骨細胞才 可以分泌新生之細胞間質以促進骨合成及新生。 數種 MMPs 參與在骨合成之 中,其中人類造骨細胞可表現 MMP-1, -9 及 MMP-13,而老鼠造骨細胞只會表 現 MMP-9, -13 但不會表現 MMP-1。在之前有報告:機械性刺激可以增加造骨 細胞分泌 MMP-13,進而參與骨再塑作用,然而超音波刺激對於造骨細胞分泌 MMP-9 的作用並不明朗。我們的結果發現超音波可以增加人類造骨細胞分泌 MMP-9 的表現,另外超音波也可以增加轉錄因子 c-Fos 及 c-Jun 的表現,因 此本計劃將探討超音波刺激對 MMP-9 表現其及上下游的訊息傳遞路徑。由我 們的結果也發現超音波也可以增加細胞表面β3 integrin,另外超音波可以活化 ERK 的磷酸化。因此,我們的結果提供了一個新的路徑為:超音波增加 MMP-9 的表現是經由活化細胞上β3 integrin 再活化下游 ERK 路徑而產生。 這些結果將可以幫助我們了解臨床上利用超音波治療骨折時其作用機轉 及相關作用,由這些分子機轉的了解將可以幫助藥物的開發及超音波治療時合 併藥物的選擇,另外也可提供發展更有效治療超音波的基礎。我們的結果將可 以提供醫療儀器廠商發展更有效及更具治療效果超音波的基石,以增進國民的 健康。英文摘要
英文摘要
英文摘要
英文摘要
Fracture healing is a complex physiologic process that involves the coordinated participation of several types of cells. Among all the means to influence fracture healing, ultrasound (US) distinguishes itself by being non-invasive and easy to apply. Low-intensity levels US are used to accelerate fracture healing and are considered neither thermal nor destructive. It has been shown that low-intensity US accelerates fracture healing in animal model and clinical studies. In particular, US on osteoblasts
in vitro has been demonstrated to increase prostaglandin release, alter collagen synthesis, stimulate collagenase activity and promote bone remodeling.
The extracellular matrix (ECM) provides positional and environmental information that is essential for tissue function. The ECMs produced by osteoblasts are complex and consist of several different classes of molecules that may regulate the modeling and remodeling of bone. Although osteoblasts have been implicated only in bone formation, they may cease synthesis of collagen and start secreting neutral proteinases such as collagenase, in response to resorption stimulators. These collagenases may degrade the unmineralized osteoid layer covering bone surfaces, leading to the exposure of the mineralized matrix to osteoclasts. On the other hand, collagenases enable osteoblasts to initiate bone resorption by generating collagen fragments, which in turn activate osteoclasts and start the bone remodeling cycles.
Collagenases are matrix metalloproteinases (MMPs) that can initiate the cleavage of collagen fibrils at neutral pH. Consequently, they are considered central to the process of collagen degradation and matrix breakdown. Three collagenases have been described; human osteoblasts express collagenase (MMP-1) and collagenase 3 (MMP-13), whereas rat osteoblasts secrete MMP-13, but do not express MMP-1 (Rajakumar and Quinn, 1996; Varghese et al., 1996). MMP-9 has been proposed to participate actively in situations where rapid and effective remodeling of collagenous ECM is required. In this research plan, we found that US stimulation increased MMP-9 expression. In addition, US also enhanced the expression of β3 integrin. US stimulation increased the phosphorylation of ERK but not Akt. Therefore, our study
provided the results that US increased MMP-9 expression through β3 integrin and
前言
前言
前言
前言
台灣目前已慢慢進入老年化社會,而骨質疏鬆症是一個在老年社會常常出現 的病變,目前台灣在治療及預防骨質疏鬆症的費用是非常的龐大,而骨質疏鬆症 經常合併骨折的產生,因此國家花費在治療骨質疏鬆症所造成的骨折所需要的費 用是相當可觀的數目,所以如何來治療骨質疏鬆症所造成的骨折或增加骨折的癒 合速度是一個很重要的課程。 骨折癒合是一個複雜的生理作用,其中包含了多種細胞參與在其中。在骨折 癒合的過程之中需要血液的灌流及血管新生,最終促進造骨細胞合成新生的骨質 而促進骨癒合。在文獻的收集中我們發現超音波是一個具有低能量且可以加速骨 癒合之儀器,在臨床的研究及動物實驗當中都可以觀察到超音波可以促進骨合 成。而美國 FDA (美國食品及藥物管理局) 也證明超音波可以加速骨折之癒合 (Heckman et al., 1994),但是美中不足的地方在於超音波經由那些機轉或訊息路 徑促進骨癒合是很少被研究的課題,在目前的基礎的研究當中只有少數的資料報 告超音波可以促進造骨細胞的造骨作用,因此了解超音波治療之下造骨細胞產生 那些反應或引發那些路徑是很重要的課題,由了解超音波的治療的訊息或路徑將 可以提供發展更有效的超音波治療儀器及合併藥物治療的可能性。 骨折是一個在老年或停經後婦女所產生骨質疏鬆症病患中常見的現象,骨折 的老年人或婦女往往要接受手術的治療,這些過程常常會影響家人的作息及增加 社會的負擔,因此如何治療骨折及增加骨折癒合是一個相當重要的課題。骨折癒 合是一個相當複雜的機轉,其中通常包含了多種細胞參與這個作用當中。其中一 個相當重要的過程就是血液的供給,當血液的供給不足時會抑制骨折的癒合,因 此在骨折癒合的過程當中血管新生也扮演了重要的角色,由這些資料我們可以了 解當急診病人合併缺血的之情況時其骨折的癒合往往不易。而低能量超音波 (Ultrasound) 具有低能量、產熱低及非侵入性的好處,可以用在增加骨折之後的 癒合作用。而超音波也被報告在動物模式具有促進骨癒合作用(Duarte, 1983; Wang et al., 1994) 及在臨床數據上也具有相當良好的作用 (Heckman et al., 1994; Cook et al., 1997)。在資料的檢閱中發現只有少數資訊顯示超音波可促進造骨細 胞分泌 prostaglandin 產生(Tang et al., 2006) 及 collagen 之合成(Yang et al., 2005) [prostaglandin 及 collagen 都是造骨細胞在造骨過程中重要的物質],也可以增加 collagenase 活性及促進骨再塑作用 (Hayton et al., 2005)。因為超音波的研究資訊 非常少,所以一直以來市面上只有單一廠商生產超音波儀器 (Smith & Nephew Co., Exogen),也只有一個強度在使用 (1.5 MHz),因此了解超音波的作用效果 及訊息傳遞作用,將可以提供臨床依據以開發展更好更具治療效果的超音波進而 增進國人之健康。細胞間質具有提供支持細胞生長及提供細胞環境的作用,而造骨細胞在骨塑 造及再塑的過程之中可以分泌多種的細胞間質參與此作用 (Erebacher et al., 1995;Table 1 表示造)。雖然造骨細胞可以分泌 collagen 而參與骨再塑作用,另 一 方 面 造 骨 細 胞 也 可 以 分 泌 細 胞 間 質 水 解 酵 素 (Matrix metalloproteinases; MMP),將不良的細胞間質分解,進而促進骨再塑作用 (Chamber and Fuller, 1985)。 這些 MMPs 可以將不完整及礦化不良的細胞間質分解,進而促使造骨 細胞分泌 collagen,而進行骨合成之作用(Holliday et al., 1997)。MMPs 主要的作 用是在分解 collagen 及將間質分解。在骨再塑之中 MMP-1, -9 及 MMP-13 被報 告具有重要的作用,人類造骨細胞可表現 MMP-1, -9, -13,老鼠造骨細胞則只能 表現 MMP-9, -13 (Rajakumar and Quinn, 1996),在結構上人類 MMP-9 與老鼠的 MMP-9 具有 98 %的相似度 (Freije et al., 1994)。
而在訊息傳遞之上其主要是經由 mitogen-activated protein kinases (MAPKs) 為主,通常可以活化 p38 及 ERK 訊息傳遞路徑。MMP-9 在生理當中與骨再塑中 相當的重要 (Gack et al., 1995),在之前的報告指出機械性的刺激可以增加老鼠造 骨細胞分泌 MMP-13 (Yang et al., 2004)。然而對於超音波刺激所產生 MMP-9 進而增加骨再塑的作用並不明朗,因此本計劃將研究是否超音波刺激可以增加 MMP-9 表現及其活性。 由之前的研究結果發現超音波增加造骨細胞的作用是經由 integrin 而來,而 由我們也想了解是否超音波刺激可以增加 β3 integrin 基因及蛋白的表現。而 MAPK 參與細胞的增生及活化,由之前的研究也發現超音波可以促進 MPAK 的 活化,因此是否 MAPK 參與在超音波所促進 MMP-9 的表現也是一個重要的課 題。本計劃將研究 US 刺激增加何種訊息傳遞路徑而增加 MMP-9 的表現,其中 是否包含 MAPK,而下流的訊息傳遞因子為何,是否與一些機械性刺激有關的 轉錄因子如:c-Fos, c-Jun 及這些轉錄因子的基因結合位置 activator protein-1
材料與方法
材料與方法
材料與方法
材料與方法
Cell cultures
Osteoblastic cells were prepared by the method as previously described (Tang et al., 2003). The cells were grown on the plastic cell culture dishes in 95 % air-5 %
CO2 with α-MEM (Gibco, Grand Island, NY) which was supplemented with 20 mM
HEPES and 10 % heat-inactivated FBS, 2 mM-glutamine, penicillin (100 U/ml) and
streptomycin (100 µg/ml) (pH adjusted to 7.6). The characteristics of osteoblasts
were confirmed by morphology and the expression of alkaline phosphatase.
Ultrasound treatment
Cells (3 × 105 cells/well, six-well plates) were cultured for 24 hr and subjected
to US treatment. A UV-sterilized transducer (Exogene 2000; Smith &Nephew Inc.,
Memphis, TN, USA) that generated 1.5 MHz US in a pulsed-wave mode (200 µs
pulse burst width with repetitive frequency of 1 kHz at the intensity of 30 mW/cm2)
was immersed vertically into each culture well and placed to just contact the surface of the medium. The distance between the transducer and the cells was approximately 5-6 mm. Exposure time was 20 min for all cultures. Control samples were prepared in the same manner without US exposure. Cells were harvested at 5, 15, 30, 60, 120, 180 and 240 min after US stimulation (Tang et al., 2006).
Zymographic analysis
MMP secreted into the culture medium were collected after US exposure.
Moreover, the anti-MMP-9 mAb was used to immunoprecipitate the MMP-9
molecule in the culture medium. The fractions of immunoprecipitant were further
subjected to zymographic analysis. Briefly, aliquots of the fractions from
immunoprecipitant were electrophoresed on a 10% SDS-polyacrylamide gel
containing 1.25% gelatin. Afterward, the gel was washed with 2.5% Triton X-100 to
remove SDS, rinsedwith 50 mM Tris-HCl, pH 7.5, and then incubated overnight at
room temperature with the developing buffer (50 mM Tris-HCl, pH 7.5, 5 mM
CaCl2, 1 µM ZnCl2, 0.02% thimerosal, 1% Triton X-100). The zymographic
activities were revealed by stainingwith 1% Coomassie Blue.
Total RNA was extracted from osteoblasts using a TRIzol kit (MDBio Inc.,
Taipei, Taiwan). The reverse transcription reaction was performed using 2 µg of
total RNA that was reversely transcribed into cDNA using oligo(dT) primer, then amplified for 33 cycles using two oligonucleotide primers:
MMP-9 [324 base pairs (bp)] GCCCTGAATGGGTATGACAT and GCATGACTCTCACAATGCGA
c-Fos (463 bp) CATCGGCAGAAGGGGCAAAGTAGAG and TGCCGGAAACAAGAAGTCATCAAAG
c-Jun (565 bp) CAACATGCTCAGGGAACAGGTG and GGAGTTCATCCGCAATCTAGCC
GAPDH (452 bp) ACCACAGTCCATGCCATCAC and TCCACCACCCTGTTGCTGTA.
Each PCR cycle was carried out for 30 s at 94°C, 30 s at 55°C, and 1 min at 68°C. PCR products were then separated electrophoretically in a 2% agarose DNA gel and stained with ethidium bromide.
Western Blot Analysis
The cellular lysates were prepared as described previously (Tang et al., 2005). Proteins were resolved on SDS-PAGE and transferred to Immobilon polyvinyldifluoride (PVDF) membranes. The blots were blocked with 4% BSA for 1 hr at room temperature and then probed with rabbit anti-rat antibodies against p-ERK, p-p38 or p-JNK (1:1000) for 1 hr at room temperature. After three washes,
the blots were subsequently incubated with a donkey anti-rabbit
peroxidase-conjugated secondary antibody (1:1000) for 1 hr at room temperature. The blots were visualized by enhanced chemiluminescence using Kodak X-OMAT LS film (Eastman Kodak, Rochester, NY). Quantitative data were obtained using a computing densitometer and ImageQuant software (Molecular Dynamics, Sunnyvale, CA).
Transfection and reporter gene assay
Osteoblasts were cotransfected with 1 µg AP-1 promoter plasmid and 1 µg
β-galactosidase expression vector. Osteoblasts were grown to 70% confluent in
6-well plates and were transfected on the following day by Lipofectamin 2000
respectively, for 5 min. The mixture was then incubated for 25 min at room
temperature and added to each well. After 24 hr incubation, transfection was
complete, and cells were incubated with the indicated agents. The media were
removed 24 hr after US stimulation, and cells were washed once with cold PBS. To
preparelysates, 100 µl of reporter lysis buffer (Promega, Madison,WI) was added
to each well, and cells were scraped from dishes.The supernatant was collected
after centrifugation at 13,000 rpm for 30 sec. Aliquots of cell lysates (10 µl)
containingequal amounts of protein (10–20 µg) were placedinto wells of an opaque
black 96-well microplate. An equal volumeof luciferase substrate was added to all
samples,and luminescence was measured in a microplate luminometer. The value of
luciferase activity was normalized to transfection efficiency monitored by the
co-transfected β-galactosidase expressionvector.
DNA affinity protein-binding assay (DAPA)
Binding of transcription factors to the MMP-9 promoter DNA sequences was assayed, as described (Huang and Chen, 2005). Following expose with US, nuclear
extracts were prepared. Biotin-labelled double-stranded oligonucleotides (2 µg)
synthesized based on the rat MMP-9 promoter sequence, were mixed at room
temperature for 1 h with shaking with 200 µg nuclear extract proteins, and 20 µl
streptavidin agarose beads in a 70% slurry. Beads were pelleted and washed three times with cold PBS, then the bound proteins separated by SDS-PAGE, followed by Western blot analysis with specific antibodies.
Chromatin immunoprecipitation assay
Chromatin immunoprecipitation (ChIP) analysis was performed as described previously (Huang and Chen, 2005). DNA immunoprecipitated by anti-c-Fos or anti-c-Jun antibody was purified. The DNA was then extracted with phenol-chloroform. The purified DNA pellet was subjected to PCR. PCR products were then resolved by 1.5% agarose gel electrophoresis and visualized by UV. The primers: 5’-TCCATTTCCCTCAGGTTCTG-3’
and 5’-AGCAGTGCCTGGAGTCTCTC-3’ were utilized to amplify across the MMP-9 promoter region .
結果
結果
結果
結果
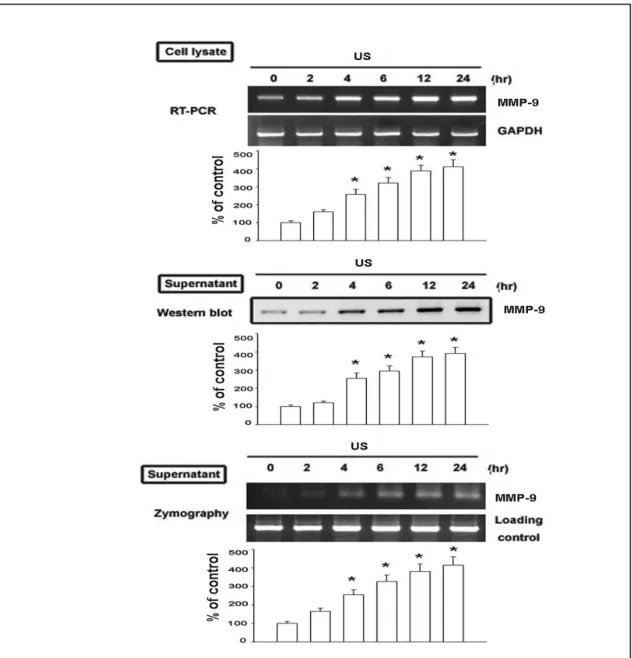
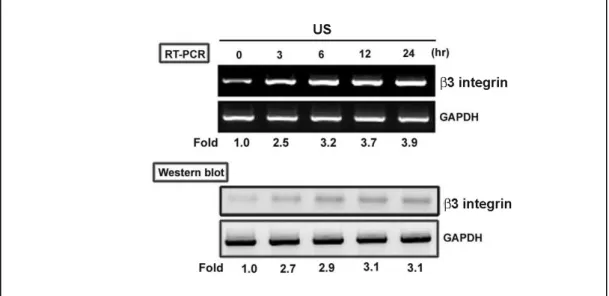
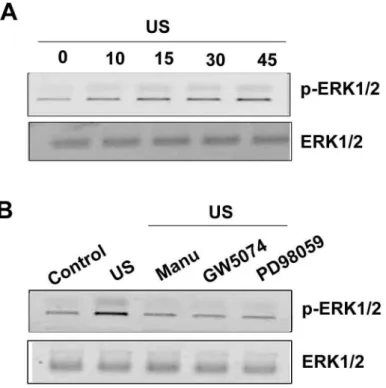
Fig. 2. 超音波刺激增加造骨超音波刺激增加造骨超音波刺激增加造骨超音波刺激增加造骨細胞細胞細胞細胞 ββββ3 integrin 的表現的表現的表現的表現 Fig. 3. 超音波刺激增加造骨細胞超音波刺激增加造骨細胞超音波刺激增加造骨細胞超音波刺激增加造骨細胞 ERK 的活化的活化的活化的活化 在訊息傳遞之上超音波主要是經由 MAPKs 為主,通常可以活化 p38 及 ERK 訊息傳遞路徑。MMP-9 在生理當中與骨再塑中相當的重要 (Gack et al., 1995), 在之前的報告指出機械性的刺激可以增加老鼠造骨細胞分泌 MMP-13 (Yang et al., 2004)。然而對於超音波刺激所產生 MMP-9 進而增加骨再塑的作用並不明 朗。我們的結果 (Fig. 1) 發現超音波可以增加 MMP-9 基因的表現,另外收集細 胞內的蛋白質,發現超音波也可以增加細胞內 MMP-9 的含量,再使用 zymography 的方式也可以看到超音波增加了 MMP-9 的 enzyme activity。因此,由我們的結 果發現超音波可以增加細胞內 MMP-9 蛋白質、mRNA 及 enzyme activity。
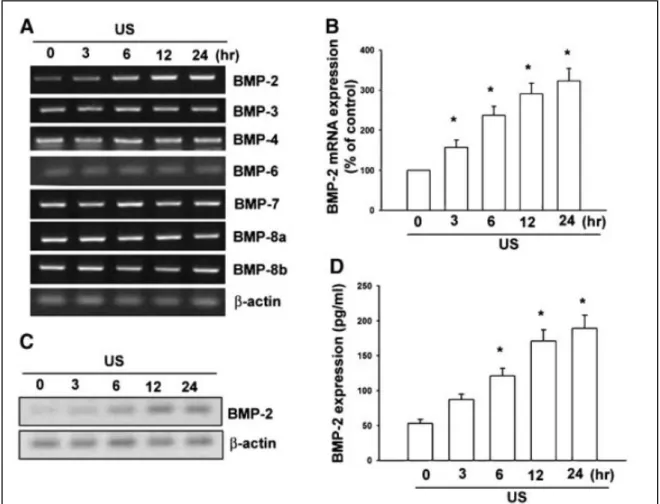
由之前的研究結果發現超音波增加造骨細胞的作用是經由 integrin 而來,而 由我們的結果也發現超音波刺激可以增加 β3 integrin 基因及蛋白的表現 (Fig. 2)。因此超音波增加細胞之內的 MMP-9 表現是經由增加細胞 integrin 的表現而 來。由之前的研究報告也指出,MAPK 參與細胞的增生及活化,由先前的研究 也發現超音波可以促進 MPAK 的活化,因此是否 MAPK 參與在超音波所促進 MMP-9 的表現也是一個重要的課題,由我們的結果也發現超音波可以促進 ERK 的活化,但對 Akt 的活化並没有影響 (Fig. 3)。因此,由我們的結果也發現了, 超音波可以增加細胞內 MMP-9 的表現是經由 integrin/ERK 的訊息傳遞路徑而來。 除了原本所預計完成的結果,我們在研究當中也發現了其他有可能為超音波 可以治療的作用(超音波會增加 BMP-2 而促進骨合成),接下來介紹此部份的結 果。由圖四的結果我們發現超音波會增加 BMP-2 的表現(利用 RT-PCR, Western blot 及 ELISA),但對 BMP-3, -4, -6, -7, -8a 及-8b 並沒有影響。
Fig. 4. US stimulation induces BMP-2 expression in osteoblasts. Osteoblasts were exposed to US for 20 min. mRNA levels of BMPs were analyzed by RT-PCR at various time intervals after US stimulation (A). Osteoblasts were exposed to US for 20 min. mRNA levels of BMP-2 were analyzed by qPCR at various time intervals after US stimulation (B). Osteoblasts were exposed to US for 20 min. Protein levels of BMP-2 were analyzed by Western blot (C) and ELISA (D) at various time intervals after US stimulation. Data are presented as mean_ SE. _P
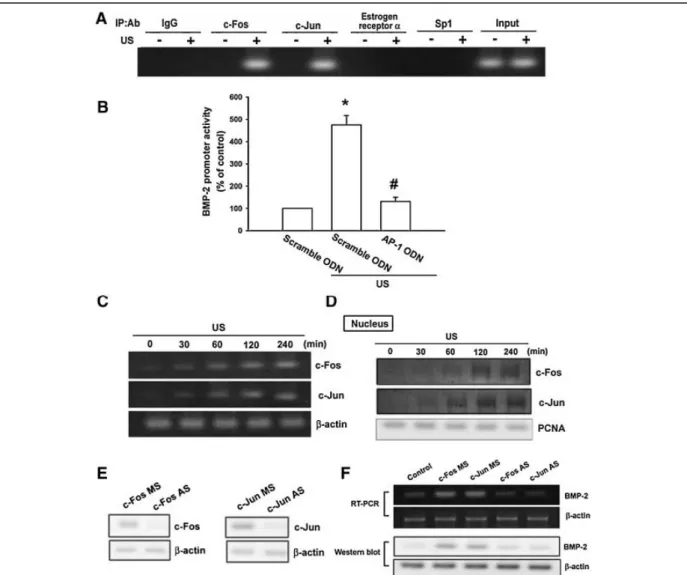
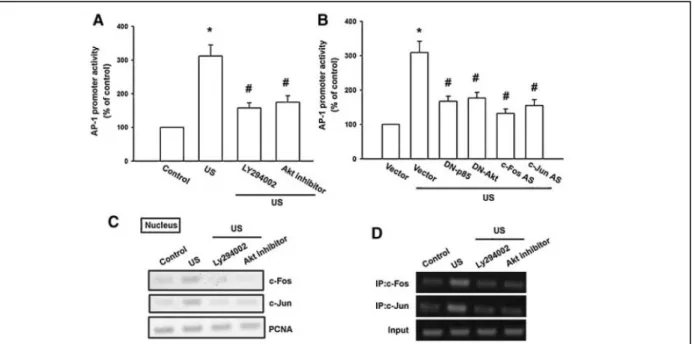
<
Fig. 5. AP-1 is involved in US-induced BMP-2 production. A: Osteoblasts were treated with US for the 20 min, and ChIP assay was then performed 120 min following US stimulation. Chromatin was immunoprecipitated with c-Fos, c-Jun, estrogen receptor a, SP1
antibodies or IgG control. One percent of the precipitated chromatin was assayed to verify equal loading (Input). B: Cells were cotransfected with BMP-2-Luc and the AP-1 or scramble ODN for 24 h then treated for 20 min with US. Luciferase activity was measured 24 h after US stimulation, and the results were normalized to the b
-galactosidase activity and expressed as the mean_SE
for three independent experiments performed in triplicate. _P
<
0.05 as compared with control. #P
<
0.05 compared with US-treated group. C: Osteoblasts were exposed to US for 20 min. mRNA levels of c-Fos and c-Jun were analyzed by RT-PCR at various time intervals after US stimulation. D: Osteoblasts were exposed to US for 20 min. The level of nuclear c-Fos and c-Jun was determined by immunoblotting with c-Fos and c-Jun-specific antibodies at various time intervals after US stimulation. E: Cells were transfected with c-Fos or c-Jun antisenseoligonucleotides (AS) or misense-oligonucleotides (MS) for 24 h, and the protein level of c-For or c-Jun was determined by using Western blot analysis. F: Cells were transfected with c-Fos or c-Jun (AS) and (MS) for 24 h followed by stimulation with US, and the mRNA and protein level of BMP-2 were determined by RT-PCR and Western blot analysis 24 h following US stimulation.
而 AP-1 會參與在超音波所增加的 BMP-2 作用中(但 ER 及 Sp-1 並無此作用),另 外超音波會促進 AP-1 之分子 c-Fos 及 c-Jun 轉錄及結合至 AP-1 位置(圖 5)。而在 圖 6 中也發超音波會增加 p85 之作用,而圖 7 中也發現超音波所增加 BMP-2 及 c-Fos, c-Jun 之轉錄作用需要 p85 之參與,因此我們也推出超音波增加 BMP-2 之 訊息傳遞路徑(圖 8)。
Fig. 6. Involvement of PI3K signaling pathway in response to US stimulation in osteoblast. A: Osteoblasts were stimulated with US for 20 min, and cell lysates were immunoprecipitated (IP) with an antibody specific for p85. Immunoprecipitated proteins were separated by SDS–PAGE and immunoblotted (WB) with anti-phosphotyrosine (PY) at various time intervals after US stimulation. B: Osteoblasts were pretreated with PI3K inhibitor Ly294002 (10 m M) for 30 min or transfected with p85 mutant for 24 h followed by stimulation with
US for 20 min, and BMP-2 expression was determined by Western blot (B), qPCR (C) and ELISA (D) 24 h after US. Data are presented as mean_ SE. _P
<
0.05 as compared with control. #P<
0.05 compared with US-treated group.
Fig. 7. PI3K and Akt pathway is mediated US-induced AP-1 activity. A: The AP-1 promoter activity was evaluated by transfection with the AP-1-Luc luciferase expression vector. Osteoblasts were pretreated with Ly294002 (10 m M) or Akt inhibitor (10 m M) for 30 min
antisense-oligonucleotides (AS) of c-Fos and c-Jun then treated for 20 min with US. Luciferase activity was measured 24 h after US stimulation, and the results were normalized to the b
-galactosidase activity and expressed as the mean_SE for three independent
experiments performed in triplicate. _P
<
0.05 as compared with control. #P<
0.05 compared with US-treated group. C: Osteoblasts were pretreated with Ly294002 (10 m M) or Akt inhibitor (10 m M) for 30 min followed by exposure to US for 20 min, and the accumulation of
c-Fos and c-Jun in nucleus was then determined 240 min after US stimulation. D: Osteoblasts were pretreated with Ly294002 (10 m M)
or Akt inhibitor (10 m M) for 30 min followed by exposure to US for 20 min, and ChIP assay was then determined 240 min after US
stimulation.
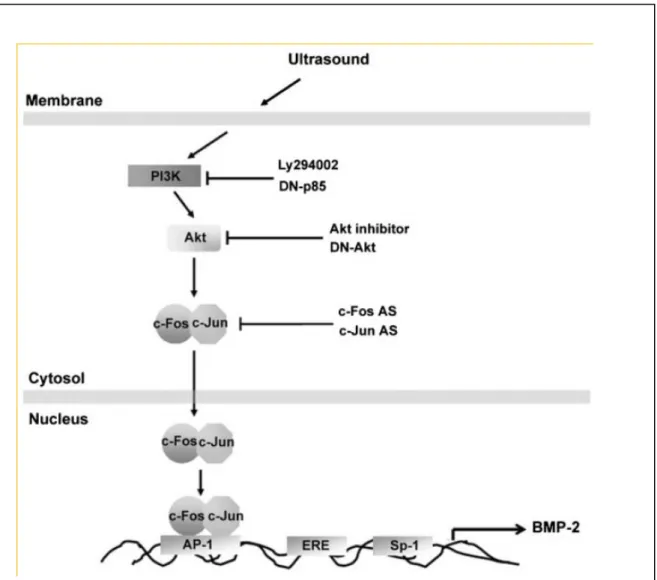
Fig. 8. Schematic diagram of the signaling pathways involved in US-induced BMP-2 expression in osteoblasts. Osteoblasts increases BMP-2 expression by activation of PI3K, Akt, which enhances binding of c-Fos and c-Jun to the AP-1 site, resulting in the
transactivation of BMP-2 expression.
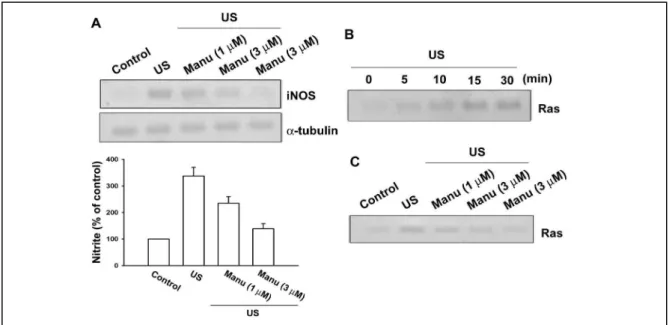
此外,我們也研究超音波增加 iNOS 之調控作用。由我們的結果發現了超音波增 加 iNOS 的表現是經由 Ras/Raf-1/MEK/ERK/IKKαβ and NF-κB 而來。
Ras is involved in US-induced iNOS expression
It has been demonstrated that pulse application of low-intensity US increased
NOrelease via up-regulation of iNOS, which is important for mechanically induced
bone formation. To explore whether Ras might mediate US-induced iNOS expression, manumycin A, a Ras inhibitor was used. As shown in Fig. 9A, pretreatment of
osteoblasts with manumycin A (1 and 3 µM)inhibited US-induced iNOS expression
in a concentration-dependent manner. In addition, manumycin A also reduced
US-induced NO production by using Griess reaction (Fig 9A, lower panel). Next, we
directly measured the Ras activityin response to US. Fig. 9B shows that exposure of
osteoblasts to US for 20 min induced an increase in Ras activity in a time-dependent
manner, as assessed by immunoblotting samples for Ras immunoprecipitatedfrom
lysates using Raf-1 RBD. Maximal activation was detected after 5-15 min of
stimulation (Fig. 9B). The US-induced increase in Ras activity was markedly
inhibited by pretreatment of cellsfor 30 min with manumycin A (1 and 3 µM) in a
concentration-dependentmanner (Fig. 9C). Taken together, these results imply that
Ras activation is involved in US-induced iNOS expression.
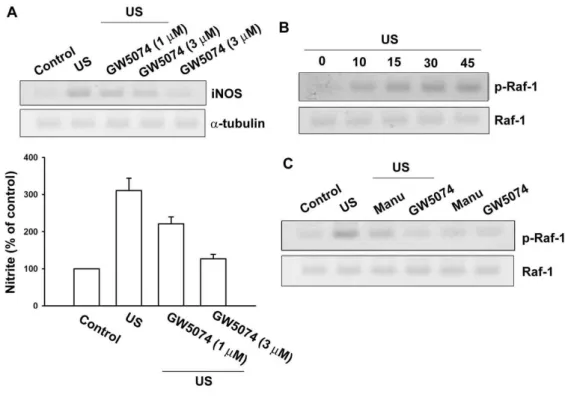
US promotes Raf-1 activation in osteoblasts
To examine whether Raf-1, a target protein for Ras, might playa crucial role in
US-induced iNOS expression, the Raf-1 inhibitor,GW 5074, was used. As shown in
Fig. 10A, pretreatment of osteoblast cells with GW5074 (1 and 3 nM) concentration-dependently inhibited US-induced iNOS expression and NO production.
Raf-1 is associated with Ras-GTP, and then by additional modifications such as
phosphorylation at Ser338, becomes the active form.The activated Raf-1 then triggers
sequential activation of downstreammolecules. Thus, phosphorylation of Raf-1 at
Ser338 is a criticalstep in Raf-1 activation. Next, we further examined Raf-1 Ser338
phosphorylation by US stimulation in osteoblasts using the anti-phospho-Raf-1Ab at
Ser338. When cells were expoused to US for 20 min, Raf-1 Ser338 phosphorylation
increased at 10min and peaked at 10-45 min (Fig. 10B). In addition,US-induced
Raf-1 Ser338 phosphorylation was inhibited by treatment with manumycin A and
GW5074 (Fig. 10C). Theresults indicate that Raf-1 is a downstream molecule of Ras
and is involved in US-mediated iNOS expression and NO production.
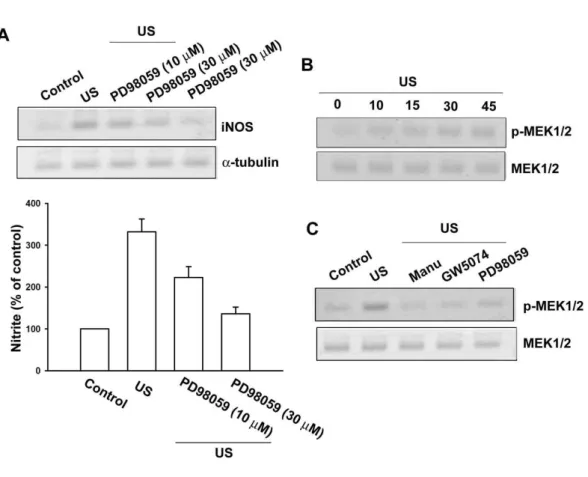
The signaling pathways of MEK and ERK are involved in the potentiating action of US stimulation
We next wished to determine whether US is able to activate MEK,a critical
downstream target of Raf-1, which has been shownto induce gene expression. We
tested the role of MEK inUS-induced iNOS expression by using the specific MEK
inhibitor, PD98059. As shown in Fig. 11A, US-induced iNOS expression was
markedly attenuated by pretreatment of cells with PD98059 (10 and 30 µM) in a
concentration-dependent manner. Pretreatmentof cells with PD98059 also inhibited
US-induced NO production (Fig. 11A, lower panel). To directly confirm thecrucial
role of MEK in iNOS expression, we determined MEK1/2 phosphorylation in
response to US. As shown in Fig. 3B, exposuret to US for 20 min induced MEK1/2
phosphorylation in a time-dependentmanner. The protein level of MEK1/2 was not
affected by US stimulation (Fig. 11B). Pretreatment of cells with manumycin A, GW5074 and PD98059 inhibited US-induced MEK1/2 activation (Fig. 11C). Next, we examine whether ERK activation is involved in the elevation of iNOS expression
caused by US stimulation. After exposure to US for 20 min ERK phosphorylation increased at 10 min, reaching maximum between 15-45 min (Fig. 12A). US-induced ERK phosphorylation was markedly inhibited by the pretreatment of cells for 30 min with manumycin A, GW5074 and PD98059. Taken together, these results indicated that the Ras/Raf-1/MEK/ERK pathway is involved in US-induced iNOS expression.
Involvement of NF-
κ
κ
κ
κ
B in US-induced iNOS expressionNF-κB activation has been reported to be necessary for US-induced COX-2
expression in human chondrocytes. To examinewhether NF-κB activation is involved
in the signal transduction pathway leading to iNOS expression caused by US, the
NF-κBinhibitor pyrrolidine dithiocarbamate (PDTC) was used. Fig. 13A shows that
PDTC (30 and 60 µM) inhibited the enhancement of iNOS expression induced by US. Furthermore, pretreatment of osteoblasts with an IκB protease inhibitor
[L-1-tosylamido-2-phenylenylethyl chloromethyl ketone (TPCK, 1 and 3 µM)] also
antagonized the potentiating action of US (Fig. 13A). Treatment of cells with PDTC
and TPCK also redced the US-induced NO production (Fig. 13A, lower panel).It has
been reported that the NF-κB element was important for the activation of the iNOS gene. NF-κB activation was further evaluated by analyzing the translocation of NF-κB from cytosol to the nucleus, as well as by chromatin immunoprecipitation (ChIP) assay. Stimulation of cells with US resulted in a marked translocation of p65 and p50 NF-κB from the cytosol to the nucleus (Fig. 13B). The in vivo recruitment of p65 and p50 to the iNOS promoter was assessed by ChIP assay. In vivo binding of p65 and p50 to the NF-κB element of iNOS promoter occurred as early as 10 min and sustained to 60 min after US stimulation (Fig. 13C). The binding of p65 and p50 to NF-κB element by US stimulation was attenuated by manumycin A, GW5074 and PD98059 (Fig. 13D). To further confirm the NF-κB element involved in the action
of US-induced iNOS expression, transient transfection was performed using the κB
promoter-luciferase constructs. Osteoblasts exposed with US led to a 3.1-fold increase
in κB promoter activity. The increase of κB activity by US was antagonized by
manumycin A, GW5074 and PD98059 (Fig. 13E). Theseresults suggest that NF-κB
activation is necessary for US-induced iNOS expression in osteoblasts.
US causes an increase in IKK
α/β
α/β
α/β
α/β
phosphorylation, Iκ
κ
κ
κ
Bα
α
α
α
phosphorylation and p65phsophorylation
We further examined the upstream molecules involved in US-induced NF-κB activation. Stimulation of cells with US induced IKKα/β phosphorylation in a time-dependent manner (Fig. 14A). Transfection with IKKα or IKKβ mutant markedly inhibited the US-induced NO production (Fig. 14B). These data suggest that IKKα/β activation is involved in US-induced iNOS expression in osteoblasts. Stimulation of osteoblasts with US also caused IκBα phosphorylation and p65
phosphorylation at Ser276 in a time-dependent manner (Fig. 14C). Pretreatment of
cells with manumycin A, GW5074 and PD98059 attenuated US-induced IKKα/β, IκBα and p65 phosphorylation (Fig. 14D).
Increase of iNOS promoter activity by US stimulation
To further study the pathways involved in the action of US-induced iNOS expression, transient transfection was performed using the iNOS promoter-luciferase construct, piNOS-Luc, which contains the mouse iNOS gene between positions -1592 and +171 fused to the luciferase reporter gene. Exposure to US led to a 3.1-fold increase in iNOS promoter activity in osteoblasts. The increase of iNOS activity by US stimulation was antagonized by manumycin A, GW5074, PD98059, PDTC and TPCK (Fig. 15A). In cotransfection experiments, the increase of iNOS promoter activity by US was inhibited by the RasN17, ERK2, IKKα and IKKβ mutant (Fig. 15B). Taken together, these data suggest that the activation of the Ras/Raf-1/MEK/ERK/IKKαβ and NF-κB pathway is required for the US-induced increase of iNOS in osteoblasts.
Fig. 9 Involvement of Ras signaling pathway in response to US stimulation in osteoblasts.
Osteoblasts were pretreated with manumycin A (1 and 3 µM) for 30 min, followed by exposure to US for 20 min. iNOS protein level was determined by immunoblotting at 24 hr after US (A, upper panel). The cultured media were collected at 24 hr after US. The production of NO was evaluated by Griess reaction by measuring the level of nitrite (A, lower panel). (B) Osteoblasts were stimulated with US for 20 min, and then cell lysates were immunoprecipitated with an antibody specific for Raf-1 RBD. The Ras activity assay is described in Material and Methods. (C) Cells were pretreated with manumycin A (1 and 3 µM) for 30 min, and the stimulated with US for another 20 min. Cells were then lysed for the Ras activity assay 15 min after US stimulation. Results are expressed as the mean ± S.E. *, p<0.05 as compared with control. #, p < 0.05 as compared with US-stimulation group.
Fig. 10 Raf-1 is involved in the US-mediated an increase of iNOS expression.
Osteoblasts were pretreated with GW5074 (1 and 3 nM) for 30 min followed by stimulation with US for 20 min, and the iNOS expression was determined by immuoblotting at 24 hr following US (A, upper panel). The cultured media were collected at 24 hr after US. The production of NO was evaluated by Griess reaction by measuring the level of nitrite (A, lower panel). Osteoblasts were stimulated with US for 20 min, and then Raf-1 phosphorylation was determined at various time intervals
after US stimulation (B). Cells were pretreated with manumycin A (3 µM) and
GW5074 (3 nM) for 30 min, and the stimulated with US for another 20 min. The Raf-1 phosphorylation was determined by using Western blot analysis 30 min after US stimulation. Results are expressed as the mean ± S.E. *, p<0.05 as compared with control. #, p < 0.05 as compared with US-stimulation group.
Fig. 11 MEK pathway is involved in the US-mediated an increase of iNOS expression.
Osteoblasts were pretreated with PD98059 (10 and 30 µM) for 30 min followed by
stimulation with US for 20 min, and the iNOS expression was determined by immuoblotting at 24 hr following US (A, upper panel). The cultured media were collected at 24 hr after US. The production of NO was evaluated by Griess reaction by measuring the level of nitrite (A, lower panel). Osteoblasts were stimulated with US for 20 min, and then MEK1/2 phosphorylation was determined at various time intervals after US stimulation (B). Cells were pretreated with manumycin A (3 µM), GW5074 (3 nM) and PD98059 (30 µM) for 30 min, and the stimulated with US for another 20 min. The MEK1/2 phosphorylation was determined by using Western blot analysis 30 min after US stimulation. Results are expressed as the mean ± S.E. *, p<0.05 as compared with control. #, p < 0.05 as compared with US-stimulation group.
Fig. 12 ERK pathway is involved in the US-mediated an increase of iNOS expression. Osteoblasts were stimulated with US for 20 min, and then ERK1/2 phosphorylation was determined at various time intervals after US stimulation (A). Cells were pretreated with manumycin A (3 µM), GW5074 (3 nM) and PD98059 (30 µM) for 30 min, and the stimulated with US for another 20 min. The ERK1/2 phosphorylation was determined by using Western blot analysis 30 min after US stimulation (B)
Fig. 13 NF-κB is involved in the potentiation of iNOS expression by US stimulation.
Cells were pretreated for 30 min with PDTC (30 and 60 µM) and TPCK (1 and 3 µM) followed by stimulation with US for 20 min, and iNOS expression was determined by immuoblotting 24 hr following US (A; upper panel). The cultured media were collected at 24 hr after US. The production of NO was evaluated by Griess reaction by measuring the level of nitrite (A, lower panel). Cells were stimulated with US for 20 min, and the levels of cytosolic and nuclear p65 or p50 were determined at various time intervals after US stimulation by immunoblotting with p65 or p50 specific antibodies, respectively (B). Cells were exposed to US for 20 min, and cells were collected after indicated time intervals (C) or pretreated manumycin A (3 µM), GW5074 (3 nM) and PD98059 (30 µM) before stimulation with US (D), and ChIP assay was then performed. Chromatin was immunoprecipitated with anti-p65 or anti-p50 antibody. One percent of the precipitated chromatin was assayed to verify equal loading (Input). (E) NF-κB promoter activity was evaluated by transfection with the NF-κB luciferase expression vector. Osteoblasts were pretreated with manumycin A (3 µM), GW5074 (3 nM) and PD98059 (30 µM) before stimulation with US for 30 min. Luciferase activity was measured 24 hr after US stimulation, and the results were normalized to the β-galactosidase activity and expressed as the mean ± SE. for three independent experiments performed in triplicate. *, p<0.05 as compared with control. #, p < 0.05 as compared with US-treated group
Fig. 14 US induces IKK α/β phoshorylation, IκBα phosphorylation and p65 Ser276 phosphorylation in osteoblasts.
(A) Cells were exposed to US for 20 min, and cells were collected after indicated time
intervals and IKK α/β phosphorylation was determined by immunoblotting using
phospho-IKK α/β specific antibody. (B) Osteoblasts were transfected with IKKα,
IKKβ or vector for 24 h. The production of NO was evaluated by Griess reation by measuring the level of nitrite followed by stimulation with US for 20 min. (C)
Osteoblasts were exposed to US for 20 min, and IκBα phosphorylation and p65 Ser276
phosphorylation were determined at indicted time interval after US stimulation. (D) Cells were pretreated with manumycin A (3 µM), GW5074 (3 nM) and PD98059 (30 µM) for 30 min, and the stimulated with US for another 20 min. The IKK, IκBα and p65 phosphorylation was determined by using Western blot analysis 30 min after US stimulation. Results are expressed as the mean ± S.E. *, p<0.05 as compared with control. #, p < 0.05 as compared with US-stimulation group
Fig. 15 The signaling pathways involved in the increase of iNOS promoter activity by US stimulation.
(A) The iNOS promoter activity was evaluated by transfection with the piNOS-Luc luciferase expression vector. Osteoblasts were pretreated with manumycin A (3 µM), GW5074 (3 nM), PD98059 (30 µM), PDTC (60 µM) and TPCK (3 µM) for 30 min before stimulation with US. (B) Cells were cotransfected with piNOS-Luc and dominant-negative (DN) mutant of RasN17, ERK2, IKKα, IKKβ or vector and then treated for 20 min with US. Luciferase activity was measured 24 h after US
stimulation, and the results were normalized to the β-galactosidase activity and
expressed as the mean ± S.E. for three independent experiments performed in triplicate. *, p<0.05 as compared with control. #, p < 0.05 as compared with US-treated group
利用此計畫經費投稿審查中及接受的文章 利用此計畫經費投稿審查中及接受的文章 利用此計畫經費投稿審查中及接受的文章 利用此計畫經費投稿審查中及接受的文章 ( ( (我們在 (我們在我們在我們在 Acknowledgments 感謝衛生感謝衛生感謝衛生感謝衛生 署及 署及 署及 署及此計畫編號此計畫編號此計畫編號此計畫編號 DOH97-PA1001)
Hou CH, Hou SM, Tang CH*. Ultrasound increased BMP-2 expression via PI3K, Akt, c-Fos/c-Jun, and AP-1 pathways in cultured osteoblasts. J Cell Biochem. (In Press) Hou CH, Hsiao YC, Fong YC, Tang CH*. Bone morphogenetic protein-2 enhances the motility of chondrosarcoma cells via activation of matrix metalloproteinase-13.
Bone (In Press)
Hou CH, Lin J, Huang SC, Hou SM, Tang CH*. Ultrasound stimulates NF-κB activation and iNOS expression via the Ras/Raf/MEK/ERK signaling pathway in cultured preosteoblasts. J. Cellular Physiology (In revise)
討論
討論
討論
討論
本計劃主要是針對造骨細胞給予超音波刺激治療之下對 MMP-9 分泌所造成 的影響,而其相關的訊息傳遞路徑有那些會參與,因為由我們的結果發現超音 波可以增加 MMP-9 表現,另外也可以增加 β3 integrin 的表現,而 MPAK 之中 的 ERK 也會受到超音波的刺激而增加,但是超音波並不會增加 Akt 的作用, 因此 US 是經由 integrin/ERK 訊息傳遞路徑而調控 MMP-9,另外也發現了 c-Fos 及 c-Jun 的表現也會增加,所以最後超音波是經由 c-Fos/c-Jun 至 AP-1 上而轉錄 出 MMP-9。由這些結果讓我們可以了解超音波治療的訊息傳遞路徑,將可以提 供臨床上超音波治療的選擇性及科學的證據,另外也將了解是否可以合併目前 治療的藥物以降低藥品的使用及藥物之副作用。這些基礎的訊息研究也將可提 供其他研究者治療的基礎以發展更好的治療藥物或更有治療效果的超音波,這 些結果將提供大量基礎研究資料在物理性刺激在骨質疏鬆症治療之下以改善 國民之健康。 另外,本計畫發現超音波可以增加 BMP-2 的產生但對於其他的 BMPs 沒有 影響,因此本計畫是第一個利用細胞實驗的技術觀察到超音波可以增加 BMP 合成的實驗室。由我們的結果發現在骨合成的過程之中如果能利用超音波治 療,超音波可能是利用此作用而增加了骨合成之作用,因此如果能開發增加超 音波的加強作用劑,應能幫助骨折的修補。在 iNOS 的合成部份,因為 iNOS 為發炎介質,但一般而言 iNOS 可以增加 NO 的產生,而 NO 為小分子的傳遞物質可以增加骨折的修補,因此本計畫也 第一個發現 Ras 是增加 iNOS 的實驗室,也可以為治療骨折的指標。
另外此計劃也培養了研究骨質疏鬆、骨質治療及物理性刺激治療的研究人 才,這些人才的訓練相信在往後必可以提供更多的研究資料之增進國民之健 康。
結論與建議
結論與建議
結論與建議
結論與建議
本人非常榮幸可以獲得衛生署的補助而完成此計畫,此計畫在進行時都一直依 照我們的計畫書內容在執行,因此,在執行及過程都沒有發生一些問題。 非常感謝衛生署提供一些研究經費讓我們這些基礎的研究人員可以從事基礎 的研究,這些經費讓我們更了解超音波的訊息機轉,因此也提供了往後治療骨質 疏鬆症及骨折的新方式。我們也利用此計畫的經費完成了三篇文章,二篇已經投 稿接受,另外一篇在 revised 當中,希望衛生署在往後還能繼續提供研究經費來 協助基礎研究人員以進行基礎研究,改善國民健康。參考文獻
參考文獻
參考文獻
參考文獻
Chambers TJ and Fuller K (1985) Bone cells predispose bone surfaces to resorption by exposure of mineral to osteoclastic contact. J Cell Sci 76:155-165.
Cook SD, Ryaby JP, McCabe J, Frey JJ, Heckman JD and Kristiansen TK (1997) Acceleration of tibia and distal radius fracture healing in patients who smoke.
Clin Orthop Relat Res 337:198-207.
Duarte LR (1983) The stimulation of bone growth by ultrasound. Arch Orthop
Trauma Surg 101:153-159.
Erlebacher A, Filvaroff EH, Gitelman SE and Derynck R (1995) Toward a molecular understanding of skeletal development. Cell 80:371-378.
Freije JM, Diez-Itza I, Balbin M, Sanchez LM, Blasco R, Tolivia J and Lopez-Otin C (1994) Molecular cloning and expression of collagenase-3, a novel human matrix metalloproteinase produced by breast carcinomas. J Biol Chem
269:16766-16773.
Gack S, Vallon R, Schmidt J, Grigoriadis A, Tuckermann J, Schenkel J, Weiher H, Wagner EF and Angel P (1995) Expression of interstitial collagenase during skeletal development of the mouse is restricted to osteoblast-like cells and hypertrophic chondrocytes. Cell Growth Differ 6:759-767.
Hayton MJ, Dillon JP, Glynn D, Curran JM, Gallagher JA, Buckley KA (2005) Involvement of adenosine 5'-triphosphate in ultrasound-induced fracture repair.
Ultrasound Med Biol 31:1131-1138.
Heckman JD, Ryaby JP, McCabe J, Freym JJ and Kilcoyne RF (1994) Acceleration of tibial fracture-healing by non-invasive, low-intensity pulsed ultrasound. J
Bone Joint Surg 76:26-34.
Holliday LS, Welgus HG, Fliszar CJ, Veith GM, Jeffrey JJ and Gluck SL (1997) Initiation of osteoclast bone resorption by interstitial collagenase. J Biol Chem
272:22053-22058.
Huang WC and Chen CC (2005) Akt phosphorylation of p300 at Ser-1834 is essential for its histone acetyltransferase and transcriptional activity. Mol Cell
Biol 25:6592-6602.
Rajakumar RA and Quinn CO (1996) Parathyroid hormone induction of rat interstitial collagenase mRNA in osteosarcoma cells is mediated through an AP-1-binding site. Mol Endocrinol 10:867-878.
expression through EP1 receptor, phospholipase C, protein kinase Calpha, and c-Src pathway in primary cultured rat osteoblasts. J Biol Chem 280: 22907-22916.
Tang CH, Yang RS, Huang TH, Lu DY, Chuang WJ, Huang TF and Fu WM (2006) Ultrasound stimulates cyclooxygenase-2 expression and increases bone formation through integrin, focal adhesion kinase, phosphatidylinositol 3-kinase, and Akt pathway in osteoblasts. Mol Pharmacol 69:2047-2057.
Tang CH, Yang RS, Liou HC and Fu WM (2003) Enhancement of fibronectin synthesis and fibrillogenesis by BMP-4 in cultured rat osteoblast. J Bone Miner
Res 18:502-511.
Wang SJ, Lewallen DG, Bolander ME, Chao EY, Ilstrup DM and Greenleaf JF (1994) Low intensity ultrasound treatment increases strength in a rat femoral fracture model. J Orthop Res 12:40-47.
Yang CM, Chien CS, Yao CC, Hsiao LD, Huang YC and Wu CB (2004) Mechanical strain induces collagenase-3 (MMP-13) expression in MC3T3-E1 osteoblastic cells. J Biol Chem 279:22158-22165.