Scintigraphic assessment of salivary function after intensity-modulated
radiotherapy for head and neck cancer: Correlations with parotid dose
and quality of life
Wen-Cheng Chen
a,b, Chia-Hsuan Lai
a, Tsair-Fwu Lee
c, Chao-Hsiung Hung
a, Kuo-Chi Liu
a,
Ming-Fong Tsai
d, Wen-Hung Wang
e, Hungcheng Chen
f, Fu-Ming Fang
g, Miao-Fen Chen
a,b,h,⇑ aDepartment of Radiation Oncology, Chang Gung Memorial Hospital, Chiayi, Taiwanb
Chang Gung University, College of Medicine, Tao-yuan, Taiwan
c
Medical Physics & Informatics Lab., Department of Electronics Engineering, National Kaohsiung University of Applied Sciences, Kaohsiung, Taiwan
d
Department of Nuclear Medicine, Chang Gung Memorial Hospital, Chiayi, Taiwan
e
Department of Otolaryngology-Head and Neck Surgery, Chang Gung Memorial Hospital, Chiayi, Taiwan
f
Department of Radiation Oncology, Helen F. Graham Cancer Center, Newark, DE, United States
gDepartment of Radiation Oncology, Chang Gung Memorial Hospital-Kaohsiung, Taiwan
hGraduate Institute of Clinical Medicine, College of Medicine, Chang Gung University, Taoyuan, Taiwan
a r t i c l e
i n f o
Article history: Received 27 April 2012
Received in revised form 5 July 2012 Accepted 6 July 2012
Available online 31 July 2012 Keywords:
Head and neck cancer Xerostomia Parotid gland function
Intensity-modulated radiotherapy Quality of life
s u m m a r y
Objective: We investigated salivary function using quantitative scintigraphy and sought to identify func-tional correlations between parotid dose and quality of life (QoL) for head and neck cancer (HNC) patients receiving intensity-modulated radiotherapy (IMRT).
Materials and methods: Between August, 2007 and June, 2008, 31 patients treated IMRT for HNC were enrolled in this prospective study. Salivary excretion function (SEF) was previously measured by salivary scintigraphy at annual intervals for 2 years after IMRT. A dose-volume histogram of each parotid gland was calculated, and the normal tissue complication probability (NTCP) was used to determine the toler-ance dose. QoL was longitudinally assessed by the EORTC QLQ-C30 and H&N35 questionnaires prior to RT, and at one, three, 12 and 24 months after RT.
Results: A significant correlation was found between the reduction of SEF and the mean parotid dose measured at 1 year (correlation coefficient, R2= 0.651) and 2 years (R2= 0.310) after IMRT (p < 0.001).
The TD50of the parotid gland at 1 year after IMRT is 43.6 Gy, comparable to results from western
coun-tries. We further found that contralateral parotid and submandibular gland function preservation was correlated with reduced sticky saliva and a better QoL compared to the functional preservation of both parotid glands, as determined by the EORTC QLQ-H&N35 questionnaire.
Conclusion: A significant correlation was found between the reduction of SEF and the mean parotid dose. Preservation of contralateral parotid and submandibular gland function predicts a better QoL compared to preservation of the function of both parotid glands.
Ó 2012 Elsevier Ltd. All rights reserved.
Introduction
Xerostomia is a common side-effect that occurs after radiother-apy (RT) in patients with head and neck cancer (HNC). Modern RT techniques, such as three-dimensional conformal RT, or intensity-modulated RT (IMRT), can spare salivary glands, thus preserving salivary flow rates and improving observer-accessed xerostomia compared to conventional RT.1–3The parotid gland dose-volume
response following RT has been investigated in several large pro-spective studies.4–8A wide range (26–43 Gy) of threshold mean
dose (TD50), the dose resulting in a 50% probability of a complica-tion for the whole organ irradiated uniformly, for parotid gland-stimulated salivary flow, has been reported. The mean dose reported to preserve parotid gland function in Asian studies varies from 31 to 43.9 Gy9–11; yet, none reported the TD50.
Although the apparent improvement in objective salivary func-tion and observed xerostomia was achieved by 3-D conformal or IMRT, the result does not necessarily improve the patient-reported xerostomia.12Since xerostomia is mainly a quality of life issue, a
patient-reported quality of life questionnaire is more useful in assessing salivary function. Therefore, in the present prospective
1368-8375/$ - see front matter Ó 2012 Elsevier Ltd. All rights reserved.
http://dx.doi.org/10.1016/j.oraloncology.2012.07.004
⇑Corresponding author. Address: Department of Radiation Oncology, Chang Gung Memorial Hospital, Chia-Yi, #6, Chia-Pu Rd., Putz City, Chia-Yi, Hsien, Taiwan. Tel.: +886 5 362 1000x2011; fax: +886 5 362 1000x2067.
E-mail address:miaofen@adm.cgmh.org.tw(M.-F. Chen).
Oral Oncology 49 (2013) 42–48
Contents lists available atSciVerse ScienceDirect
Oral Oncology
study, we longitudinally recorded the recovery of parotid gland function using salivary scintigraphy in patients receiving IMRT. The NTCP model was used to determine the TD50 of the parotid gland. The patient-reported quality of life (QoL) questionnaire, EORTC QLQ-C30, and the Head and Neck module (H&N35), were gi-ven to patients prior to, and periodically following, RT to assess perceived salivary function over time. Patient-, tumor-, and ther-apy-related factors were simultaneously assessed as predictors of parotid gland function and QoL.
Materials and methods Patient and disease characteristics
The study population consisted of patients with HNC treated with IMRT at our hospital. All patients provided written informed consent approved by the institutional review board. Patients who suffer from Sjögren’s syndrome or any other medical cause of xero-stomia were excluded. Patient use of any medications known to af-fect salivary gland function was prohibited. During the period from August, 2007 to June, 2008, a total of 31 patients with primary (n = 15) or post-operative RT (n = 16) for HNC were entered into this prospective study. Patient and tumor characteristics are listed inTable 1. IMRT was delivered by the computer-controlled auto-sequencing segmented or dynamic multileaf collimator of a Varian linear accelerator (Linac 21 EX), according to methods described previously.13IMRT delivery directed at sparing the parotid glands
(predominantly contralateral side), while treating the primary tar-gets and lymph nodes at risk was conducted. The prescribed doses were 67.4–70.8 Gy (mean, 69.8 Gy) to the macroscopic tumor plan-ning target volume, 54.8–70.8 Gy (mean, 62.0 Gy) to the surgical tumor bed planning target volume, and 46.8 Gy to the subclinical disease planning target volume, at 1.8–2 Gy per fraction. Nineteen
patients received concurrent chemotherapy. Of these, five patients received additional adjuvant chemotherapy. Nineteen patients re-ceived concurrent chemotherapy with weekly CDDP (40 mg/m2) for 5–7 courses (n = 18) or PF regimen (CDDP 80 mg/m2 on day 1 + 5-FU 800 mg/m2, days 1–5, every 21 days) for two courses (n = 1). Of these, five patients received additional adjuvant chemo-therapy with PF regimen for 2–3 courses (n = 4) or TEF regimen (Taxol 60 mg/m2 on day 1 + CDDP 20 mg/m2 on day 1 + 5-FU 800 mg/m2on days 1–2) for one course (n = 1).
Salivary scintigraphy
All patients received salivary scintigraphy prior to IMRT deliv-ery. Patients fasted for 4 h prior to beginning the study. The study commenced with patients receiving 10 mCi 99mTc pertechnetate intravenously. Sequential imaging of 1 frame/min was acquired for 30 min over the left- and right-anterior views of the head and neck. Salivary gland function was represented by saliva excretion following sialogogue stimulation with acidic material (lemon juice or 200 mg ascorbic acid tablet on the dorsal tongue). Salivary excretion factor (SEF) was quantified by determining the maximal excretion activity per gland as a function of maximal uptake, as de-scribed previously by Roesink et al.6The salivary scintigraphies are
scheduled prior to IMRT and then 1 and 2 years after its comple-tion. The excretion response was analyzed per patient and subse-quently per individual gland. All patients received scintigraphies 1 year after RT, whereas only 25 patients (25/31, 81%) received examinations 2 years following RT. The six patients who did not re-ceive scintigraphies were due to tumor recurrence (n = 2) or fol-low-up refusal (n = 4).
Normal tissue complication probability model
The data were applied to the NTCP model proposed by Lyman.14
The NTCP model quantitatively assesses the effects of both the radiation dose and the volume of the gland irradiated on the prob-ability of radiation-induced changes in parotid gland function. Three parameters are presented in the sigmoid dose–response curve: n, m, and TD50, where n accounts for the volume effect of an organ, m describes the slope of the dose–response curve and the TD50is the dose resulting in a 50% probability of a complication for uniform irradiation of the whole partial volume.
EORTC quality of life questionnaires (QLQ-C30 & H&N35)
Subjective salivary function was evaluated by the EORTC QLQ-C30 and H&N35 questionnaires. Patients were asked to answer the questions prior to receiving IMRT, and then 1, 3, 12, and 24 months later. The Traditional Chinese versions of the EORTC QLQ-C30 and H&N35 questionnaires were obtained from the Qual-ity of Life Unit, EORTC Data Center in Brussels, Belgium.15All scales
pertaining to the EORTC QLQ-C30 and H&N35 range from 0 to 100. A high score for a functional or global QoL scale represents a rela-tively high/healthy level of functioning or global quality of life, whereas a high score for a symptom scale represents the presence of a symptom or problem.
Statistical methods
A logistic regression statistical method was used to study the dose–response relationship and volume effects in parotid glands. Spearman’s correlation was used to evaluate the factors associated with recovery of parotid gland function. Multivariate analysis was performed using a multiple regression model. The level of signifi-cance was set at p < 0.05. A paired sample t-test was used to com-pare the mean scores at each time point, with significance
Table 1
Patients and tumor characteristics of 31 patients.
Characteristic Value Age (y) Mean 53 Range 28–78 Gender (n) Female 1 (3) Male 30 (97) Tumor site NPC 11 (35) Oral cavity 14 (45) Oropharynx 4 (13) Larynx 1 (3) Parotid 1 (3)
Stage (TNM staging system)
T1 3 (10) T2 12 (39) T3 6 (19) T4 7 (22) Not applicable/recurrent 3 (10) N0 16 (52) N1 5 (16) N2 7 (22) N3 0 (0) Not applicable/Recurrent 3 (10) Surgery before RT Yes 16 (52) No 15 (48) Chemotherapy Yes 19 (61) No 12 (39)
Data in parentheses are percentage.
patient groups were inferior to those reported by patients receiv-ing IMRT in the study of Pow et al.1, but are comparable to those of Graff et al.3This may be because of the different patient cohorts
(100% NPC patients in the study by Pow et al. vs. 16% NPC in Graff et al.). In our study, 45% of patients were treated with IMRT for their oral cavity cancer. Leung et al. confirmed that oral cancer sur-vivors have significantly worse QoL scales (social eating, social con-tact, open mouth, appetite loss, pain, swallowing, speech, social eating and social contact) compared to NPC survivors.19This could
be attributed to that most of the oral cancer patients also received radical surgery as part of their initial treatment.
In the H&N35 module, swallowing, mouth opening, dry mouth and sticky saliva were reported as the four worst symptoms. In a subgroup analysis, we found that surgery, which might cause se-vere trismus (interincisor distance (IID)<2.5 cm), is an important contributor to poor QoL scores. In the current study, seven of eight patients (87.5%) with IID < 2.5 cm received surgery as part of their initial treatment. Another important factor that may affect patient-reported xerostomia is submandibular gland function. Although only 20–30% of stimulated saliva flow is produced by the subman-dibular glands, submansubman-dibular saliva is responsible for the major-ity of saliva production in the non-stimulated state. Further, its mucin component could serve as the mucosal lubricant that tributes to a patient’s subjective sense of moisture. In a study con-ducted by Eisbruch et al.20, the major salivary gland flow rates were found to have only a weak correlation with xerostomia scores. Factors found to be independently associated with xerosto-mia scores included the pre-RT baseline scores, the time since RT, and the mean doses delivered to the major salivary glands (notably to the submandibular glands) and to the oral cavity. These findings are comparable to those of the present study. We found that pres-ervation of one submandibular and one parotid gland, rather than of both parotid glands, produced better xerostomia-related QoL scores.
In conclusion, recovery of parotid gland excretion was strongly correlated with the mean parotid gland dose, as determined by scintigraphy analysis. The TD50 of the parotid gland (43.6 Gy) inferred in our study is comparable to reports from western countries. Preservation of the function of at least one parotid and
one submandibular gland predicts a better QoL and minimal sticky saliva, as determined by self-reporting on the EORTC QLQ-H&N35 questionnaire.
Funding sources
The study was supported by the National Science Council, Taiwan (Grant 96-2321-B-182A-004) and Chang Gung Memorial Hospital (Grant CMRPG670371-2).
Conflict of interest statement None declared.
References
1. Pow EH, Kwong DL, McMillan AS, Wong MC, Sham JS, Leung LH, et al. Xerostomia and quality of life after intensity-modulated radiotherapy vs. conventional radiotherapy for early-stage nasopharyngeal carcinoma: initial report on a randomized controlled clinical trial. Int J Radiat Oncol Biol Phys 2006;66(4):981–91.
2. Jabbari S, Kim HM, Feng M, Lin A, Tsien C, Elshaikh M, et al. Matched case-control study of quality of life and xerostomia after intensity-modulated radiotherapy or standard radiotherapy for head-and-neck cancer: initial report. Int J Radiat Oncol Biol Phys 2005;63(3):725–31.
3. Graff P, Lapeyre M, Desandes E, Ortholan C, Bensadoun RJ, Alfonsi M, et al. Impact of intensity-modulated radiotherapy on health-related quality of life for head and neck cancer patients: matched-pair comparison with conventional radiotherapy. Int J Radiat Oncol Biol Phys 2007;67(5):1309–17.
4. Eisbruch A, Ten Haken RK, Kim HM, Marsh LH, Ship JA. Dose, volume, and function relationships in parotid salivary glands following conformal and intensity-modulated irradiation of head and neck cancer. Int J Radiat Oncol Biol Phys 1999;45(3):577–87.
5. Roesink JM, Moerland MA, Battermann JJ, Hordijk GJ, Terhaard CH. Quantitative dose-volume response analysis of changes in parotid gland function after radiotherapy in the head-and-neck region. Int J Radiat Oncol Biol Phys 2001;51(4):938–46.
6. Roesink JM, Moerland MA, Hoekstra A, Van Rijk PP, Terhaard CH. Scintigraphic assessment of early and late parotid gland function after radiotherapy for head-and-neck cancer: a prospective study of dose-volume response relationships. Int J Radiat Oncol Biol Phys 2004;58(5):1451–60.
7. Saarilahti K, Kouri M, Collan J, Hamalainen T, Atula T, Joensuu H, et al. Intensity modulated radiotherapy for head and neck cancer: evidence for preserved salivary gland function. Radiother Oncol 2005;74(3):251–8.
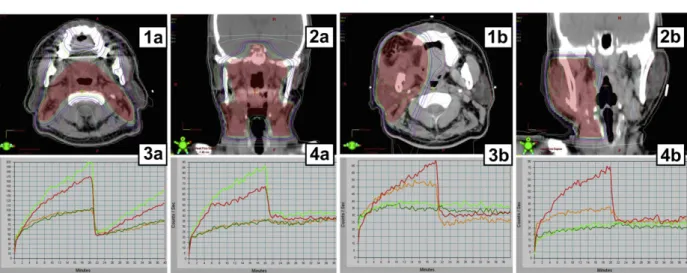
Figure 3 The axial (1a, 1b) and coronal (2a, 2b) isodose and time activity curves on quantitative salivary scintigraphy of the four major salivary glands prior to intensity-modulated radiotherapy (IMRT) (3a, 3b) and 12 months post-IMRT (4a, 4b) of two patients (A and B). The recovery rates of R’t parotid (green line), L’t parotid (red line), R’t submandibular (dark green line) and L’t submandibular (orange line) gland function for patient A were 72, 64, 0 and 0% respectively. For patient B, only the L’t parotid and L’t submandibular gland functions were preserved (recovery rates, 115 and 59%, respectively). In the patient-reported quality of life questionnaire, the dry mouth score was 33.3 for both patients; however, the sticky saliva scores were 66.7 and 0, respectively, indicating a better quality of life in patient B. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
8. Blanco AI, Chao KS, El Naqa I, Franklin GE, Zakarian K, Vicic M, et al. Dose-volume modeling of salivary function in patients with head-and-neck cancer receiving radiotherapy. Int J Radiat Oncol Biol Phys 2005;62(4):1055–69. 9. Kam MK, Teo PM, Chau RM, Cheung KY, Choi PH, Kwan WH, et al. Treatment of
nasopharyngeal carcinoma with intensity-modulated radiotherapy: the Hong Kong experience. Int J Radiat Oncol Biol Phys 2004;60(5):1440–50.
10. Kwong DL, Pow EH, Sham JS, McMillan AS, Leung LH, Leung WK, et al. Intensity-modulated radiotherapy for early-stage nasopharyngeal carcinoma: a prospective study on disease control and preservation of salivary function. Cancer 2004;101(7):1584–93.
11. Hsiung CY, Ting HM, Huang HY, Lee CH, Huang EY, Hsu HC. Parotid-sparing intensity-modulated radiotherapy (IMRT) for nasopharyngeal carcinoma: preserved parotid function after IMRT on quantitative salivary scintigraphy, and comparison with historical data after conventional radiotherapy. Int J Radiat Oncol Biol Phys 2006;66(2):454–61.
12. Kam MK, Leung SF, Zee B, Chau RM, Suen JJ, Mo F, et al. Prospective randomized study of intensity-modulated radiotherapy on salivary gland function in early-stage nasopharyngeal carcinoma patients. J Clin Oncol 2007;25(31):4873–9. 13. Chen WC, Hwang TZ, Wang WH, Lu CH, Chen CC, Chen CM, et al. Comparison
between conventional and intensity-modulated post-operative radiotherapy for stage III and IV oral cavity cancer in terms of treatment results and toxicity. Oral Oncol 2009;45(6):505–10.
14. Lyman JT. Complication probability as assessed from dose-volume histograms. Radiat Res Suppl 1985;8:S13–9.
15. Bjordal K, Hammerlid E, Ahlner-Elmqvist M, de Graeff A, Boysen M, Evensen JF, et al. Quality of life in head and neck cancer patients: validation of the European Organization for Research and Treatment of Cancer Quality of Life Questionnaire-H&N35. J Clin Oncol 1999;17(3):1008–19.
16. Dijkema T, Raaijmakers CP, Ten Haken RK, Roesink JM, Braam PM, Houweling AC, et al. Parotid gland function after radiotherapy: the combined michigan and utrecht experience. Int J Radiat Oncol Biol Phys 2010;78(2):449–53.
17. Jensen SB, Mouridsen HT, Reibel J, Brunner N, Nauntofte B. Adjuvant chemotherapy in breast cancer patients induces temporary salivary gland hypofunction. Oral Oncol 2008;44(2):162–73.
18. Marzi S, Iaccarino G, Pasciuti K, Soriani A, Benassi M, Arcangeli G, et al. Analysis of salivary flow and dose-volume modeling of complication incidence in patients with head-and-neck cancer receiving intensity-modulated radiotherapy. Int J Radiat Oncol Biol Phys 2009;73(4):1252–9.
19. Wan Leung S, Lee TF, Chien CY, Chao PJ, Tsai WL, Fang FM. Health-related quality of life in 640 head and neck cancer survivors after radiotherapy using EORTC QLQ-C30 and QLQ-H&N35 questionnaires. BMC Cancer 2011;11:128. 20. Eisbruch A, Kim HM, Terrell JE, Marsh LH, Dawson LA, Ship JA. Xerostomia and
its predictors following parotid-sparing irradiation of head-and-neck cancer. Int J Radiat Oncol Biol Phys 2001;50(3):695–704.