Factors related to poor glycemic control in type 2 diabetic outpatients in a medical center
全文
(2) Ching-Chu Chen, et al.. status; it is estimated that the number of cases will approach 300 million by 2025 [2]. DM presents a substantial socioeconomic and quality-of-life burden, mainly as a result of its chronic complications. The Diabetes Control and Complications Trial [3] and the U.K. Prospective Diabetes Study (UKPDS) [4] demonstrated that microvascular complications in type 1 and type 2 DM can be reduced by improving glycemic control. Diet control, weight reduction, and adequate self-management remain the cornerstones of diabetic treatment. Because DM is a disease that requires a high level of selfmanagement, successful patient education is an important component of glycemic control. Overall, 90% to 95% of diabetic patients have type 2 DM. However, previous evaluations of factors related to glycemic control have mainly been conducted in individuals with type 1 DM [5-9]. To our knowledge, only one group has investigated the influential factors on glycemic control among insulin-using adults with type 2 DM [10]. Thus, little is known about the characteristics related to poor glycemic control in patients with type 2 DM. We believe that knowledge of the demographic, socioeconomic and diabetes-related characteristics will enable health-care providers to better select patients for compensatory intervention. The purpose of this study was to explore the clinical characteristics related to glycemic control in patients with type 2 DM. PATIENTS AND METHODS. Patients with type 2 DM needed to be over 30 years of age and were required to undergo a minimum follow-up period of 6 months at our out-patient clinic. Type 2 DM was diagnosed based on the criteria proposed in the 1997 Report of the Expert Committee of the American Diabetes Association [11]. The questionnaire was given to consecutive patients at the out-patient. 91. clinic. An out-patient clinic nurse read and explained the meaning of the questions in Taiwanese to help illiterate patients complete the questionnaire. Data were collected from selfreported questionnaires administered from April to May 2001. Items in the questionnaire explored basic data, duration of DM, education level, monthly household income, therapeutic modality, status of exercise, self-monitoring of blood glucose, and diabetic education. Exercise was defined as a minimum of 30 minutes of aerobic exercise (e.g. walking, jogging, bicycling) performed long enough to sweat at least twice a week. Self-monitoring of blood glucose was defined as monitoring blood glucose at least once per week; alternative self-monitoring methods such as urinalysis were excluded. Diabetic education was defined as attending at least one individual diabetic education meeting with a diabetic educator or dietitian. The HbA1c level was used as an index of glycemic control. A single HbA 1c value obtained from 2 months preceding the study visit to 1 month after was used as measurement of short-term glycemic control (HbA1c-S) for the past 2 to 3 months. The mean HbA1c value, and at least one other HbA1c value obtained 3 months from the HbA1c-S in the year preceding the visit, was used as measurement of long-term glycemic control (HbA1c-L) for the past 6 to 12 months. HbA1c level was measured by ion-exchange highperformance liquid chromatography (HPLC) (HLC-723 GHbV; Tosoh, Tokyo, Japan). Statistical analysis. All data are presented as mean standard deviation (SD). The differences between sexes were compared by either the Student t test or the χ 2 test . The associations between continuous variables, HbA1c-S and HbA1c-L, were assessed by Pearson's correlation. The within-characteristic differences of HbA 1c -S or HbA 1c -L were.
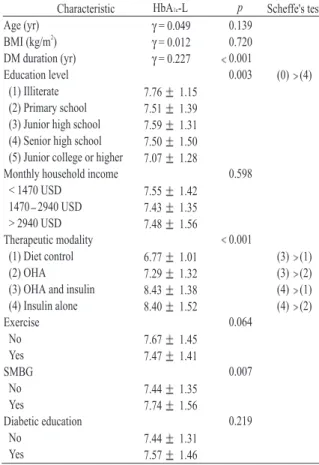
(3) 92. Factors Related to Poor Glycemic Control in Subjects with Type 2 DM. Table 1. Clinical characteristics of the patients. All (n = 945) 61.03 10.50 25.12 3.59 9.27 6.61. Men (n = 403) 60.21 10.83 24.82 3.07 9.00 6.62. Women (n = 542) 61.63 10.21 25.35 3.93 9.47 6.60. p. 0.390 Age (yr) 2 0.020 BMI (kg/m ) 0.278 DM duration (yr) 0.001 Education level, No. (%) 209 (22.1) 177 (32.7) 32 (7.9) Illiterate/uneducated 380 (40.2) 233 (43.0) 147 (36.5) Primary school 59 (14.6) 111 (11.7) 52 (9.6) Junior high school 86 (21.3) 141 (14.9) 55 (10.1) Senior high school 79 (19.6) 104 (11.0) 25 (4.6) Junior college or higher 0.001 Monthly household income, No. (%) 442 (81.5) 274 (68) 716 (75.8) < 1470 USD 82 (20.3) 145 (15.3) 63 (11.6) 1470 2940 USD 37 (6.8) 47 (11.7) 84 (8.9) > 2940 USD 0.126 Therapeutic modality, No. (%) 5 (0.9) 11 (2.7) 16 (1.7) Diet control 416 (76.8) 308 (76.4) 724 (76.6) OHA 60 (14.9) 154 (16.3) 94 (17.3) OHA and insulin 27 (5.0) 24 (6.0) 51 (5.4) Insulin alone 0.035 Exercise, No. (%) 164 (30.3) 97 (24.1) 261 (27.6) No 378 (69.7) 306 (75.9) 684 (28.9) Yes 0.004 SMBG, No. (%) 672 (71.1) No 405 (74.7) 267 (66.3) 273 (28.9) Yes 137 (25.3) 136 (33.7) 0.703 Diabetic education, No. (%) 290 (30.7) No 169 (31.2) 121 (30.0) 655 (69.3) Yes 373 (68.8) 282 (70.0) 0.120 7.75 1.55 7.66 1.58 7.82 1.53 HbA1c-S 0.417 7.53 1.42 7.48 1.45 7.56 1.40 HbA1c-L Values are presented as mean SD. BMI = body mass index; DM = diabetes mellitus; OHA = oral hypoglycemic agent; SMBG = self-monitoring of blood glucose.. evaluated by an analysis of variance (ANOVA) and the Scheffe's test. The significant factors related to poor glycemic control were identified by multiple linear regression analysis. A p value of less than 0.05 was considered statistically significant. RESULTS. In total, 1081 questionnaires were collected. Of these, 136 were eliminated because of incomplete data, resulting in a final study population of 945. Table 1 shows the clinical characteristics of the patients. The mean age was 10.50 years. There was no significant 61.03 difference in age between the men and women. (p = 0.390). The mean body mass index (BMI) 3.59 kg/m2, and the mean duration was 25.12 6.61 years. Female patients of DM was 9.27 had a higher BMI than male patients (p = 0.020); however, DM duration was similar between men and women (p = 0.278). Education level, monthly household income, exercise and self-monitoring of blood glucose status were significantly different between men and women; however, the therapeutic modality and diabetic education status were not significantly different. Of the 945 patients, 1.7% were treated with diet alone, 76.6% were treated with oral hypoglycemic agents (OHAs), 16.3% were treated with insulin and OHAs, and 5.4% were treated with insulin alone..
(4) Ching-Chu Chen, et al.. 93. Table 2. Association between clinical characteristics and HbA1c-S and the within-characteristic difference of HbA1c-S p Characteristic HbA1c-S Scheffe's test 0.093 γ = 0.055 Age (yr) 2 0.872 γ = 0.055 BMI (kg/m ) 0.001 γ = 0.202 DM duration (yr) 0.004 (0) (4) Education level 7.99 1.67 (0) Illiterate 7.73 1.51 (1) Primary school 7.91 1.62 (2) Junior high school 7.64 1.50 (3) Senior high school (4) Junior college or higher 7.31 1.35 0.396 Monthly household income 7.76 1.53 < 1470 USD 7.61 1.44 1470 2940 USD 7.89 1.91 > 2940 USD 0.001 Therapeutic modality (3) (1) 6.96 1.65 (1) Diet control (3) (2) 7.52 1.46 (2) OHA (4) (1) 8.66 1.52 (3) OHA and insulin (4) (2) 8.50 1.50 (4) Insulin alone 0.332 Exercise 7.83 1.58 No 7.72 1.54 Yes 0.004 SMBG 7.65 1.46 No 8.00 1.74 Yes 0.477 Diabetic education 7.70 1.48 No 7.78 1.58 Yes Values are presented as mean SD. BMI = body mass index; DM = diabetes mellitus; OHA = oral hypoglycemic agent; SMBG = self-monitoring of blood glucose.. Table 3. Association between clinical characteristics and HbA1c-L and the within-characteristic difference of HbA1c-L p HbA1c-L Scheffe's test Characteristic Age (yr) 0.139 γ = 0.049 2 BMI (kg/m ) 0.720 γ = 0.012 0.001 DM duration (yr) γ = 0.227 0.003 Education level (0) (4) (1) Illiterate 7.76 1.15 (2) Primary school 7.51 1.39 (3) Junior high school 7.59 1.31 (4) Senior high school 7.50 1.50 (5) Junior college or higher 7.07 1.28 0.598 Monthly household income < 1470 USD 7.55 1.42 1470 2940 USD 7.43 1.35 > 2940 USD 7.48 1.56 0.001 Therapeutic modality (1) Diet control (3) (1) 6.77 1.01 (2) OHA (3) (2) 7.29 1.32 (3) OHA and insulin (4) (1) 8.43 1.38 (4) Insulin alone (4) (2) 8.40 1.52 0.064 Exercise No 7.67 1.45 Yes 7.47 1.41 0.007 SMBG No 7.44 1.35 Yes 7.74 1.56 0.219 Diabetic education No 7.44 1.31 Yes 7.57 1.46 Values are the mean SD. BMI = body mass index; DM = diabetes mellitus; OHA = oral hypoglycemic agent; SMBG = self-monitoring of blood glucose.. The mean HbA1c-S and HbA1c-L values were 7.75 1.55% and 7.53 1.42%, respectively; values were not significantly different between men and women (HbA1c-S, p = 0.120; HbA1c-L, p = 0.417). Tables 2 and 3 show that age and BMI were not associated with HbA1c-S and HbA1c-L. DM duration positively correlated with HbA1c-S and HbA1c-L (γ = 0.202, p < 0.001; γ = 0.227, p < 0.001). Education level had a significant influence on glycemic control (HbA 1c-S, p = 0.004; HbA1c-L, p = 0.003). The glycemic control of illiterate patients was worse than that of patients with at least a junior-college level of education. Monthly household income was not a significant factor related to poor glycemic control. (HbA1c-S, p = 0.396; HbA1c-L, p = 0.598). The therapeutic modality was a significant factor (HbA 1c -S, p < 0.001; HbA 1c -L, p < 0.001). Therapy consisting of insulin, alone or in combination with OHAs, indicated worse glycemic control than diet control or OHAs alone. Exercise and diabetic education status were not significant factors, but exercise was marginally significant in long-term glycemic control (p = 0.064) (Table 3). Patients who self monitored their blood glucose had poorer glycemic control than those who never monitored their blood 1.46% vs 8.00 glucose (HbA 1c -S 7.65 1.74%, p = 0.004; HbA1c-L 7.44 1.35% vs 7.74 1.5%, p = 0.007)..
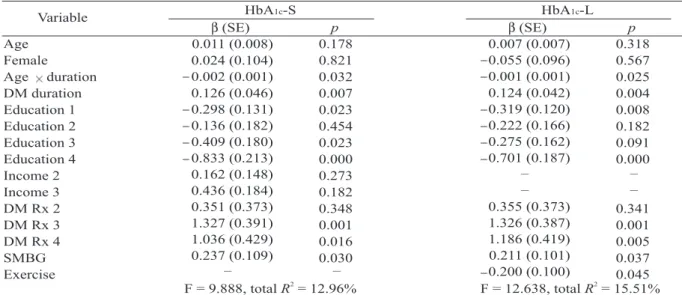
(5) 94. Factors Related to Poor Glycemic Control in Subjects with Type 2 DM. Table 4. Multiple linear regression analysis of the predictors of glycemic control HbA1c-S HbA1c-L Variable p p β (SE) β (SE) Age 0.011 (0.008) 0.318 0.178 0.007 (0.007) 0.055 (0.096) Female 0.024 (0.104) 0.567 0.821 0.001 (0.001) 0.002 (0.001) Age duration 0.025 0.032 0.124 (0.042) 0.126 (0.046) DM duration 0.004 0.007 0.319 (0.120) 0.298 (0.131) Education 1 0.008 0.023 0.222 (0.166) 0.136 (0.182) Education 2 0.182 0.454 0.275 (0.162) 0.409 (0.180) Education 3 0.091 0.023 0.701 (0.187) 0.833 (0.213) Education 4 0.000 0.000 – – 0.162 (0.148) Income 2 0.273 – – 0.436 (0.184) Income 3 0.182 0.355 (0.373) 0.351 (0.373) DM Rx 2 0.341 0.348 1.326 (0.387) 1.327 (0.391) DM Rx 3 0.001 0.001 1.186 (0.419) 1.036 (0.429) DM Rx 4 0.005 0.016 0.211 (0.101) 0.237 (0.109) SMBG 0.037 0.030 – – 0.200 (0.100) Exercise 0.045 2 2 F = 12.638, total R = 15.51% F = 9.888, total R = 12.96% EC = estimated coefficient; SE = standard error; Education 1 = primary school; Education 2 = junior high school; Education 3 = senior high school; Education 4 = junior college or higher; Income 2 = household income 1470 – 2940 USD; Income 3 = household income > 2940 USD; DM Rx 2 = OHA; DM Rx 3 = OHA and insulin; DM Rx 4 = insulin alone; SMBG = self-monitoring of blood glucose.. Multiple linear regression analysis revealed that a long duration of DM, illiteracy, therapy consisting of insulin, alone or in combination with OHAs, and self-monitoring of blood glucose were significant factors related to poor short-term glycemic control (Table 4). These four predictors 2 accounted for 12.96% of the variance (R = 0.1296) (Table 4). The highest regression coefficients were for the therapeutic modality of OHAs combined with insulin (β = 1.327), followed by the therapeutic modality of insulin alone (β = 1.036), an education level of junior college or higher (β = 0.823), self-monitoring of blood glucose (β = 0.237), and duration of DM (β = 0.126). In addition to these four predictors, a lack of exercise was also a significant factor related to poor long-term glycemic control. The estimated coefficient for exercise was 0.200 (p = 0.045). These five predictors accounted for 2 15.51% of the variance (R = 0.1551) (Table 4). DISCUSSION. Type 2 DM is a progressive disease in. which β cells deteriorate with DM duration; most patients require a multi-pharmaceutical approach to control their plasma glucose level. The UKPDS revealed that the HbA1c level in both conventional and intensive groups decreased in the first study year and then subsequently increased with each following year. The median HbA1c level in the intensive group was 6.6% in the first 5-year follow-up period but progressively increased to 8.1% in the third 5-year follow-up period [4]. Our result was consistent with the UKPDS which reported that the longer a patient has DM the poorer glycemic control will be. However, Nichols and co-workers demonstrated that a shorter duration of DM was a factor related to poor glycemic control [10]. This discrepancy may have been due to the different inclusion criteria. In our study, we excluded patients in whom DM had recently been diagnosed and those with a follow-up period of less than 6 months because the initial HbA1c levels do not reflect real shortterm glycemic control. In addition, glycemic control in those individuals was unstable during.
(6) Ching-Chu Chen, et al.. the first few months. Considering the benefits and risks of intensive glycemic control and the lifespan of patients, controling blood glucose levels is less important in elderly patients than in young patients. Thus, we would expect that age is a factor related to poor glycemic control, as shown by Nichols and co-workers [10]. However, age was not a factor related to glycemic control in our study. They also found that lower BMI was the strongest and most consistent factor related to poor glycemic control. Their explanation was that improved glycemic control causes weight gain, a finding consistent with the UKPDS in which the intensive group gained 2- to 5-kg compared with the conventional group. Individuals in the intensive group gained weight, but their glycemic control worsened over time. In our study, BMI was not a significant factor related to glycemic control. Harris and co-workers also found that BMI was not related to glycemic control [12]. Socioeconomic status is often an important factor in morbidity among nondiabetic patients. One study [7] revealed that education level and income were related to glycemic control in patients with type 1 DM; a low education level, a low monthly income, and a low socioeconomic status indicated poor glycemic control. However, Harris and co-workers [12] showed that education level and income were not factors related to glycemic control in patients with type 2 DM. In our study, we found that illiteracy but not income was a factor related to poor glycemic control. The finding that illiteracy is related to poor glycemic control may be explained by the fact that a lack of understanding of DM, OHAs and insulin, leads to poorer compliance and more patients switching to alternative medications. Most patients with type 2 DM achieve good glycemic control when they are initially treated with OHAs, but only approximately 50% continue to have satisfactory glycemic control. 95. after 10 years [13]. This clinical phenomenon is referred to as secondary failure of OHAs. The annual rate of secondary failure is about 0.7% to 2.7% per year [14]. These individuals are then often treated with insulin alone or OHAs combined with insulin. Our study revealed that therapy consisting of insulin, alone or in combination with OHAs, was a factor related to poor glycemic control. This finding was consistent with that reported by Harris and coworkers [12,15]. Poor glycemic control is most common among insulin-treated patients because the majority of them are patients with secondary failure of OHAs or patients presenting with chronic complications of DM. In fact, glycemic control is more difficult to achieve in those individuals than in individuals in whom DM responds to OHAs alone. This study was performed before the introduction of thiazolidinedions; therefore, whether glycemic control with three OHAs (sulfonylurea plus biguanide plus thiazolidinedions) in patients with secondary failure (maximal dose of sulfonylurea plus biguanide) is better than control with two OHAs (maximal dose of sulfonylurea plus biguanide) combined with bedtime insulin or insulin alone needs further investigation. Education about diabetes, including knowledge about the disease and nutritional restrictions, remains one of the cornerstones of diabetic management. A model education program for patients with type 2 DM revealed 2.0% that the HbA1c level improved from 9.0 1.6% after 1 year of participation in a to 7.8 structured education program [16]. Our study showed that the HbA 1c level did not differ significantly between patients who were educated by a diabetic educator or dietitian and patients who were not; therefore, one education session seems to be insufficient to make a difference. In our study, self-monitoring of blood glucose was a factor related to poor glycemic.
(7) 96. Factors Related to Poor Glycemic Control in Subjects with Type 2 DM. control. This finding was consistent with that of Harris et al [12,15] but inconsistent with that of Nichols and et al [10]. We found that individuals who monitored their blood glucose had higher HbA1c-S values; this observation indicates that patients with poor control are more motivated to monitor their blood glucose and that selfmonitoring of blood glucose does not contribute to poor glycemic control. Our observation may also indicate that medical personnel are to blame for failing to educate patients to adjust their dosage based on the results of self-monitoring blood glucose levels. Regular exercise is another cornerstone of diabetic management because it produces beneficial effects on this metabolic syndrome. A meta-analysis of the effects of exercise on glycemic control in patients with type 2 DM who were not being treated with drug co-interventions revealed that exercise reduced HbA 1c by an amount (0.66%) that should decrease the risk of diabetic complications [17]. Our study showed that exercise has a beneficial effect on long-term glycemic control. This finding supports our practice of advising our patients to exercise regularly. In summary, the significant factors related to poor glycemic control in patients with type 2 DM were longer DM duration, illiteracy, therapeutic modality consisting of insulin, selfmonitoring of blood glucose, and lack of exercise. We recommend that health-care providers pay more attention to type 2 diabetic patients with longer duration and/or who are uneducated. If there is no contraindication, OHAs should have priority over insulin. Whether glycemic control with triple oral therapy (sulfonylurea plus biguanide plus thiazolidinedions) is better than the maximal dose of sulfonylurea plus biguanide combined with bedtime insulin or insulin alone in secondary failure patients needs to be investigated further.. REFERENCES. 1. World Health Organization. Prevention of Diabetes Mellitus: Technical Report Series No. 844, Geneva, 1994. 2. King H, Aubert RE, Herman WH. Global burden of diabetes, 1995-2025: prevalence, numerical estimates, and projections. Diabetes Care 1998;21: 1414-31. 3. The Diabetes Control and Complications Trial Research Group. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med 1993;329:977-86. 4. UK Prospective Diabetes Study (UKPDS) Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet 1998;352:837-53. 5. Anderson BJ, Miller JP, Auslander WF, et al. Family characteristics of diabetic adolescents: relationship to metabolic control. Diabetes Care 1981;4:586-94. 6. Kovacs M, Kass RE, Schnell TM, et al. Family functioning and metabolic control of school-aged children with IDDM. Diabetes Care 1989;12:409-14. 7. Bott U, Jorgens V, Grusser M, et al. Predictors of glycemic control in type 1 diabetic patients after participation in an intensified treatment and teaching program. Diabetic Med 1994;11:362-71. 8. Rosilio M, Cotton JB, Wieliczko MC, et al. Factors associated with glycemic control. A cross-sectional nationwide study in 2,579 French children with type 1 diabetes. The French Pediatric Diabetes Group. Diabetes Care 1998;21:1146-53. 9. Ramchandani N, Cantey-Kiser JM, Alter CA, et al. Self-reported factors that affect glycemic control in college students with type 1 diabetes. Diabetes Educ 2000;26:656-66. 10. Nichols GA, Hillier TA, Javor K, et al. Predictors of glycemic control in insulin-using adults with type 2 diabetes. Diabetes Care 2000;23:273-7. 11. Report of the Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Diabetes Care 1997;20:1183-97. 12. Harris MI, Eastman RC, Cowie CC, et al. Racial and ethnic differences in glycemic control of adults with type 2 diabetes. Diabetes Care 1999;22:403-8. 13. Gerich JE. Oral hypoglycemic agents. [Review] N Engl J Med 1989;321:1231-45..
(8) Ching-Chu Chen, et al.. 14. Pontiroli AE, Calderara A, Pozza G. Secondary failure of oral hypoglycemic agents: frequency, possible causes, and management. [Review] Diabetes Metab Rev 1994;10:31-43. 15. 2 1999;12:39-50. 16. Gagliardino JJ, Etchegoyen G, PEDNID-LA Research. 97. Group. A model educational program for people with type 2 diabetes: a cooperative Latin American implementation study (PEDNID-LA). Diabetes Care 2001;24:1001-7. 17. Boule NG, Haddad E, Kenny GP, et al. Effects of exercise on glycemic control and body mass in type 2 diabetes mellitus: a meta-analysis of controlled clinic trials. [Review] JAMA 2001;286:1218-27..
(9) 98. 2 1 1. 2 2 2001 30. 4. 5. 6. 2 (HbA1c-S). HbA1c-S. 3. 1 HbA1c-S. 1. HbA1c-S. (HbA1c-L) 1081. 136. 945 (. ). (p < 0.05. R2 = (R 2 =. 12.96%) 15.51%) 2 2005;10:90-8. 2. 404. 2. 2004. 8. 11. 2005. 3. 30. 2005. 3. 17.
(10)
數據
相關文件
used a technique with a 1.5–2 cm linear incision of mucosa parallel to the vermillion border and lateral to midline, and the incidence of long-term paraesthesia in 75 patients
Although the clinical and radiologic features of our patient ’s lesion suggested a more con fined intraosseous process, we could not entirely exclude the possibility of in
We present a case of a 15- year-old male who presented with multiple papulo-nodular lesions in the central face and a family history of a similar type of lesions from his
In this section we introduce a type of derivative, called a directional derivative, that enables us to find the rate of change of a function of two or more variables in any
We solve the three-in-a-tree problem on
A Phase 2/3 Multicenter, Open-label, 3-arm, 2-stage Randomized Study of ASP2215 (Gilteritinib), Combination of ASP2215 Plus Azacitidine and Azacitidine Alone in the Treatment
6 《中論·觀因緣品》,《佛藏要籍選刊》第 9 冊,上海古籍出版社 1994 年版,第 1
Breu and Kirk- patrick [35] (see [4]) improved this by giving O(nm 2 )-time algorithms for the domination and the total domination problems and an O(n 2.376 )-time algorithm for