SYSTEMATICS
Phylogeny of the Genera of Eubrianacinae and Descriptions of
Additional Members of Eubrianax (Coleoptera: Psephenidae)
C.-F. LEE, P.-S. YANG,1ANDM. SATOˆ2
Department of Entomology, the Ohio Sate University, Museum of Biological Diversity, 1315 Kinnear Road, Columbus, OH 43212
Ann. Entomol. Soc. Am. 94(3): 347Ð362 (2001)
ABSTRACT A cladistic analysis of Eubriancinae was performed, using 21 characters from external
morphology (eight from larvae, four from pupae, and nine from adults), and considering Psephe-ninae as the outgroup. The analysis yielded nine equally parsimonious cladograms (47 steps, CI ⫽ 56, RI ⫽ 68), and a strict consensus cladogram was calculated (51 steps, CI ⫽ 56, RI ⫽ 61). The result suggests that the phylogenetic relationships between genera within the subfamily are monophyletic, despite their ecological diversity. The strict consensus cladogram indicates that the genus Heibrianax is paraphyletic to Eubrianax. The monophyly of Heibrianax ⫹ Eubrianax is supported by three synapomorphic characters. Therefore, Heibrianax is regarded as a junior synonym of Eubrianax. The current article also deals with some members of Eubrianax, although they display signiÞcant difference from other representatives of the genus. The evolution of characters in the immature stages is discussed. Eubrianax pellucidus species group and E. serratus species group are treated. Eubrianax manakikikuse amamiensisSatoˆ, 1965 is raised to speciÞc rank. A new subspecies, Eubrianax manakikikuse kimuraissp. nov., and a new species, Eubrianax serratus sp. nov., are described. Keys to genera of the subfamily, based on adults, larvae, pupae, and a checklist of the species are provided.
KEY WORDS Psephenidae, Eubrianacinae, systematics, phylogenetics
THEEUBRIANACINAE ISa monogeneric subfamily of the family Psephenidae and is composed of about 50 spe-cies (Lee and Ja¨ch 1995). The larvae live in the lotic environment and pupate above the water. The imma-ture stages are well described (Blackwelder 1930, Ja¨ch 1984); but no attempt has been made to use them in phylogenetic studies due to their disassociation with adults. Recent studies support the suprageneric clas-siÞcation of Eubrianacinae by associating adults with immatures. Lee et al. (1999a) redeÞned the type genus
Eubrianaxin a strict sense and described a new genus,
Heibrianax,although larvae were not distinguishable. Lee et al. (1999b) also described the new genus,
Mu-brianaxbased on larval morphology and ecology.
Jin-brianax(Lee et al. 1999c) was described and possesses some pleisomorphic characters not found in other known genera. The new genus Odontanax (Lee et al. 2000a) was described based on unexposed pupae, and the new genus Jaechanax (Lee et al. 2000b) was de-scribed based on the immature stages containing char-acters shared with Odontanax and Mubrianax.
In addition, we have found some species close to
Eubrianaxbut that display signiÞcant differences in adults and larvae. Our main objective was to use ev-idence from the adult and immature stages to conduct a phylogenetic analysis of genera within the
Eu-brianacinae, and to test monophyly of Eubrianax and
Heibrianaxby adding those problematic species. We have also tested some evolutionary hypotheses pro-posed by Lee et al. (1999b, 1999c, 2000a).
Materials and Methods
Measurements for specimens are given in millime-ters (mm). In descriptions of the species of Eubrianax, measurements of antennomeres, mouth parts, and body proportions are given as ratios (LP ⫽ pronotal length, WP ⫽ pronotal width, LE ⫽ elytral length, WE ⫽ elytral width).
Specimens examined are deposited in the following institutions (letter codes according to Arnett et al. (1993), except CNJ and NWU): BPBM, Bernice P. Bishop Museum, Honolulu; CNJ, Collection of Na-kane, Japan (now in NSMT); NHMB, Naturhisto-risches Museum, Basel; NHMW, Naturhistroisches Musem, Wien; NSMT, National Science Museum, To-kyo; NTUC, National Taiwan University, Taipei; NWU, Nagoya WomenÕs University, Nagoya. Check-list of the Species and their Distributions of Eu-brianacinae.
The species examined are shown in the Checklist of
the Species and Their Distributions of Eubrianacinae
section. The immature stages of one species for each genus are available for study (except Eubrianax):
Jin-brianax jaechi Lee et al. 1999c, Odontanax dohertyi (Pic 1915), Mubrianax robustior (Pic 1928), Jaechanax
dentatusLee et al. 2000b, and Heibrianax secretus Lee
1Department of Entomology, National Taiwan University, Taipei, 106 Taiwan.
2Laboratory of Nature Conservation, Graduate School of Nagoya WomenÕs University, Mizuho-ku, Nagoya, 467Ð8610 Japan.
et al. 1999a. Mataeopsephus taiwanicus Lee et al. 1990 was examined and selected as an outgroup. In addi-tion, we used some larvae that were not associated with adults.
Eubrianacinae and its Phylogenetic Relations within Psephenidae. Although subfamilies within the
Psephenidae are well deÞned, only some characters in the immature stages reveal phylogenetic relationships. The respiratory system of larval Eubrianacinae is sim-ilar to that of the Psepheninae (Hinton 1955). The tracheal system is amphineustic in all instars, and the functional spiracles are located on the mesothorax and the eighth abdominal segment, but those on the eighth abdominal segment are different in structure between both subfamilies. Although ventral gills are found in both subfamilies, their structure, position, and number differ. Thus, the mesothoracic spiracle is considered synapomorphic for the Psepheninae and Eubrianaci-nae; spiracles of the eighth abdominal segment and ventral gills are autapomorphic for the Psepheninae and Eubrianacinae. The two additional synapomor-phic characters for Psepheninae and Eubrianacinae
are found herein: the presence of posterior plates on all three thoracic segments, and the presence of costal lines. Thus, the subfamily Psepheninae is a probable sister group of the Eubrinacinae.
Larvae of Mubrianax and Jaechanax (Fig. 5) are adapted for a xylophagous lifestyle. The body is oblong to adhere tightly to slender twigs. Compared with other members of the Eubrianacinae, the mandibles are thicker and have apical teeth for feeding on wood (Fig. 3 C and D).
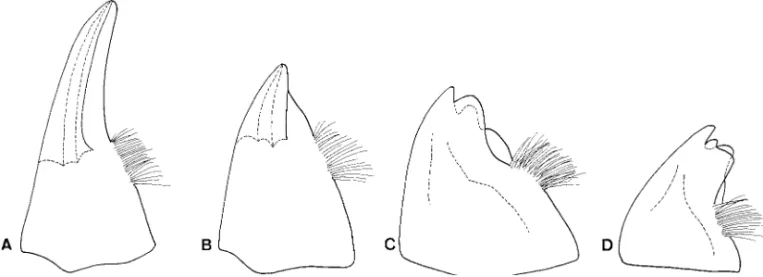
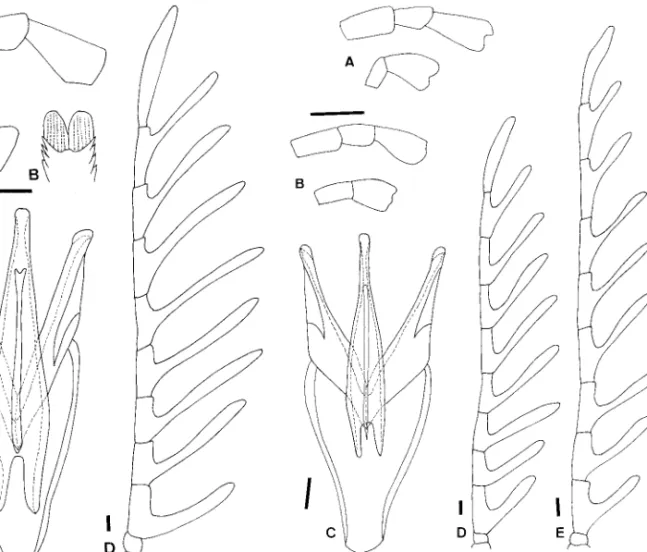
Pupae of Eubrianacinae are unique and apomorphic because they are the only known insect pupae with a metapneustic respiratory system (Hinton 1966). Hin-ton (1955) stated the following: “the Eubrianax retain the last larval cuticle over the pupa, although the last three segments of the larval cuticle are shed. The shed segments of the larval cuticle are replaced by heavily sclerotized pupal structures which, from a dorsal view, so closely resemble the missing part of the larval cu-ticle that the latter appears to be complete.” The pupae have previously been thought to be apneusitic (Hinton 1955) because no ordinary spiracular opening Fig. 1. Tarsal claws. (A) Jaechanax insignis (Fairmaire). (B) Mubrianax robustior (Pic). (C) Eubrianax tarokoensis Lee
& Yang. (D) Eubrianax granicollis Lewis. Scale bar ⫽ 0. 1 mm.
Fig. 2. Aedeagi. (A) Jinbrianax apicalis (Pic). (B) Odontanax dohertyi (Pic). (C) Mubrianax robustior (Pic). (D) Jaechanax insignis(Fairmaire). (E) Eubrianax secretus Lee et al.
was observed. In fact, the trachea connect to many small openings scattered widely across small tubercles. This condition does not resemble that other of beetle pupae nor any other known insect (Hinton 1966).
Hinton (1966) examined pupae of Asian, American, and African species and found there were no signiÞ-cant differences between them. Lee et al. (2000a, 2000b) found that Odontanax and Jaechanax have un-exposed pupae, rather different from un-exposed pupae (Hinton 1966). In mature larvae of Odontanax and
Jaechanax(Figs. 4C and 5A), the pleurites of abdom-inal segments 6 and 7 are not tightly connected; slen-der crevices are present between the posterior mar-gins of abdominal pleurites 6 and the anterior marmar-gins of abdominal pleurites 7. During pupation, the last three abdominal terga of the larva are not shed and the whole pupa is covered by this larval skin. The openings of the spiracles on the pupal abdominal tergum 7 are reduced except those on the outward margins (Fig. 7 D and E), which project from the crevices in the larval skin. All three apical abdominal terga are sclerotized
slightly, except the outward margins of the abdominal tergum 7.
Terminal Taxa. The cladistic analysis involves 11
terminal taxa, including an outgroup. Species within genera are invariant for characters listed here except for those of Eubrianax, which are listed separately here.
Characters and Polarity. Twenty-one
morphologi-cal characters were selected: eight from adult, nine from larvae, four from pupae. Character states were polarized by the outgroup comparison method (Watrous and Wheeler 1981). Psepheninae
(Matae-opsephus) was selected as the outgroup. However, because all pupal characters are autapomorphic, po-larization was based on a hypothetical outgroup. Transformation series were coded in discrete binary and multistate characters. When multistate characters could be ordered by morphological continuity, which implied a logical linear sequence of intermediate states, they were treated as additive; otherwise, they were treated as nonadditive (unordered), which re-Fig. 3. Larval mandibles. (A) Eubrianax tarokoensis Lee & Yang. (B) Eubrianax pellucidus Lewis. (C) Jaechanax sp. (D) Mubrianax robustior(Pic). Scale bar ⫽ 0. 1 mm.
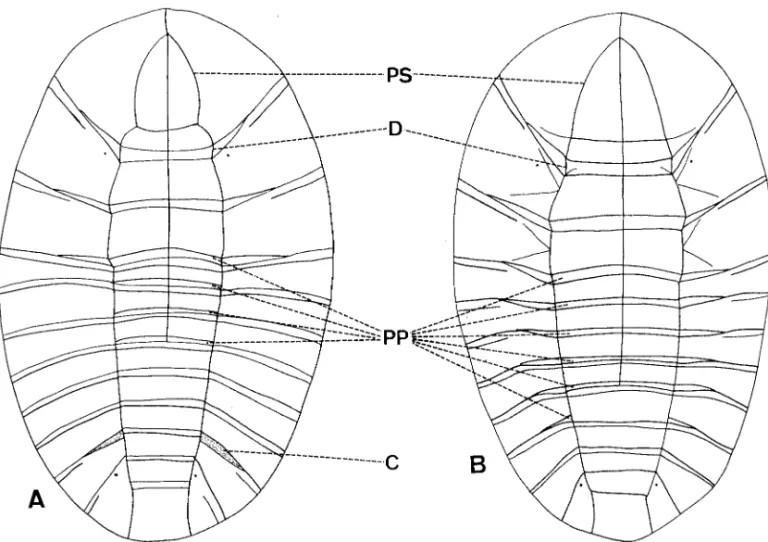
Fig. 4. Larvae. (A) Jinbrianax jaechi Lee et al. (B) Eubrianax tarokoensis Lee & Yang. (C) Odontanax dohertyi (Pic). C,
crevice between abdominal segments 6 and 7; CL, costal lines; MLS, midpronotal longitudinal sulcus; MPP, middorsal pronotal plate; PP, posterior plate; PS, periocullar sulcus.
sults in the equal cost of only one step for the trans-formation between any of its states.
A missing data code, “?,” is used when the character state was not applicable (autapomorphic or missing) or when the terminal taxon was polymorphic for the character.
Cladistic Analysis. Parsimony analysis was
per-formed using Hennig86 (Farris 1988). Tree length and consistency and retention indices were calculated ex-cluding autapomorphies of Eubrianacinae to avoid artiÞcially increasing these indices. When the analysis yielded more than one cladogram, a strict consensus tree was calculated with the Nelsen option of Hen-nig86. Clados (Nixon 1992) was used for tree manip-ulations and tracing character evolution.
We selected eight characters from adults (1Ð8), nine from larvae (9Ð17), and four from pupae (18Ð 21); the data matrix is shown as Appendix 1.
1. Male Antennae (CI ⫽ 40, RI ⫽ 25). (0) Serrate
(Fig. 16C); (1) pectinate, antennal rami originating from apices on segments 5 to 10 (Lee et al. 1999a; Figs. 7 and 11); (2) pecinate, antennal rami originating from bases or middles on segments 5 to 7 (Figs. 10C, 12D, 13E, and 15C). Serrate antennae are only found in
Eubrianax serratus. The intermediate apomorphic condition occurs in Jinbrianax, Mubrianax, Eubrianax
tarokoensis,and Heibrianax. The character was coded as polymorphic for Odontanax. This multistate char-acter was treated as nonadditive.
Fig. 5. Larvae. (A) Jaechanax dentatus Lee, Sato, and Yang. (B) Mubrianax robustior (Pic). C, crevice between abdominal
segments 6 and 7; D, dividing sulcus at posterior plates on pronothorax; PP, posterior plate; PS, periocullar sulcus.
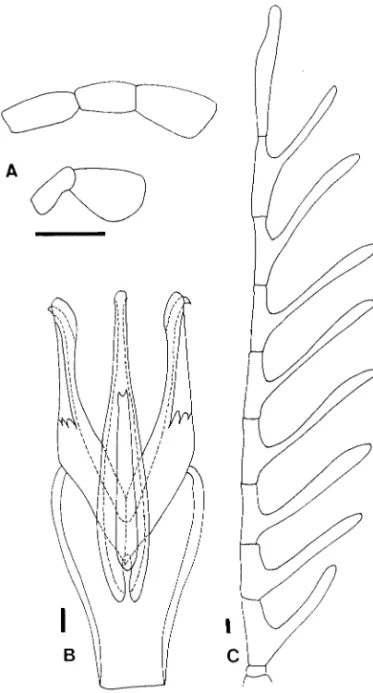
Fig. 6. Basal pieces of marginal peg setae. (A) Jinbrianax jaechiLee et al. (B) Mubrianax robustior (Pic). (C) Jae-chanaxsp. Scale bar ⫽ 0. 1 mm.
2. Apex of Prosternal Process (CI ⫽ 33, RI ⫽ 0). (0)
Dilated; (1) acute. The apex of the proternal process is dilated in Odontanax and Heibrianax. The character was coded as polymorphic for Mubrianax, and inap-plicable for Eubrianax serratus, because the apex of its posternal process is truncate.
3. Number of Apical Spurs on Pro-, Meso- and Metatibiae (CI and RI ⫽ 100). (0) 2-2Ð2; (1) 2-1Ð1.
The number of apical spurs on meso- and metatibiae is reduced to one in all genera of Eubrinacinae except
Jinbrianax.
4. Pulvilli at Tarsal Claws (CI and RI ⫽ 100). (0)
Absent; (1) membranous, (2) sclerotized and well developed. Membranous pulvilli are found only in
Jaechanax.They are well developed in all species of
Eubrianaxand Heibrianax. This character was treated as additive and multistate.
5. Tarsal Claws (CI and RI ⫽ 100). (1) Long and
slightly curved (Fig. 1 A and B); (1) short, moderately or strongly curved (Fig. 1 C and D). The apomorphic condition appears in all species of Eubrianax and
Heib-rianax.
6. Mesal Margins of Parameres (CI ⫽ 50, RI ⫽ 0).
(0) Smooth (Fig. 2 CÐE); (1) with one pair of pro-cesses (Fig. 2 A and B). The paired propro-cesses on the mesal margins of the parameres occur in Jinbrianax and Odontanax.
7. Mesal Margins of Parameres (CI and RI ⫽ 100).
(0) Moderately sclerotized (Fig. 2 AÐC); (1) strongly sclerotized (Figs. 2 D and E; 10B, 12C, 13C, 15B, and 16B). The slender darkened bands along the mesal margins of parameres appear in Jaechanax, Heibrianax, and all species of Eubrianax.
8. Apices of Parameres (CI and RI ⫽ 100). (0)
Without sclerites covered (Fig. 2 AÐD); (1) with rounded sclerites covered (Figs. 2E, 10B, 12C, 13C, 15B, and 16B). The apomorphic condition occurs in all species of Eubrianax and Heibrianax.
9. Mandibles (CI ⫽ 50, RI ⫽ 0). (0) Algae-feeding
(Fig. 3 A and B); (1) xylophagous (Fig. 3 C and D). All but Mubrianax and Jaechanax have ßattened man-dibles adapted for feeding on algae. Larvae of
Mu-brianaxand Jaechanax have wide and apically toothed mandibles suitable for feeding on wood.
10. Middorsal Pronotal Plate (CI ⫽ 33, RI ⫽ 20). (0)
Absent (Figs. 4C and 11); (1) incomplete (Figs. 5 A and B, 14); (2) well developed (Fig. 4 A and B, 17). The middorsal pronotal plate is found in Jinbrianax,
Eubrianax tarokoensis, E. serratus,and Heibrianax. It is incomplete in Eubrianax pellucidus, E. amamiensis (Fig. 9 BÐD); sometimes combined with periocullar sulci in Mubrianax and Jaechanax (Fig. 5 A and B). This multistate character was coded as nonadditive.
11. Mid-Pronotal Longitudinal Sulci (CI and RI ⫽
100). (0) Present (Fig. 4A); (1) absent (Fig. 4 B and C). Mid-pronotal longitudinal sulci are only found in
Jinbrianax.zzz.zzz.
12. Dividing Sulci at Posterior Plates on Prothorax
(CI ⫽ 33, RI ⫽ 33). (0) Absent (Figs. 4 A and B, 11, 17); (1) present (Figs. 4C, 5 A and B, 14). The pros-terior plates on the prothorax are divided by the basal margins of the pleurites in Odontanax, Mubrianax,
Jaechanax,and Eubrianax amamiensis.
13. Posterior Plates on Abdominal Terga (CI ⫽ 40,
RI ⫽ 0). (0) Absent (Fig. 4 A and B); (1) present on abdominal segments 1 to 4 (Figs. 4C, 5B, and 11); (2) Fig. 7. Pupal spiracles. (A) Jinbrianax jaechi Lee, Sato, and Yang. (B) Mubrianax robustior (Pic). (C) Eubrianax serratus
sp. n. (D) Odontanax dohertyi (Pic). (E) Jaechanax dentatus Lee, Sato, and Yang.
present on main plates of abdominal segments 1 to 3 (Figs. 5A and 17). The posterior plates can be found on abdominal terga 1Ð4 in Odontanax, Mubrianax, and
Eubrianax manakikikuse;on main plates of the abdom-inal segments 1 to 3 in Jaechanax and Eubrianax
ser-ratus.This multistate character was treated as nonad-ditive.
14. Crevices between Abdominal Segments 6 and 7
(CI ⫽ 25, RI ⫽ 0). (0) Absent (Figs. 4 A and B, 5B, 11, 14, 17). (1) Present (Figs. 4C and 5A). Crevices be-tween abdominal segments 6 and 7 are found in
Odon-tannaxand Jaechanax. They are also found in some
species of Eubrianax, although they are not as prom-inent as those of Odontannax and Jaechanax.
15. Body Form (CI ⫽ 50, RI ⫽ 0). (0) Oval (Figs.
4, 11, and 17); (1) oblong (Fig. 5). The oblong body form is found in Mubrianax and Jaechanax.zzz.zzz.
16. Sides of Basal Pieces of Marginal Peg Setae (ci
and ri ⫽ 100). (0) Smooth; (1) with one side toothed (Fig. 6A); (2) with both sides toothed (Fig. 6 B and C). Sides of basal pieces of marginal peg setae are smooth in the outgroup. The intermediate apomorphic con-dition is found only in Jinbrianax. This multistate char-acter was treated as additive.
Fig. 8. Strict consensus tree of the nine most parsimonious cladograms. E. ⫽ Eubrianax.
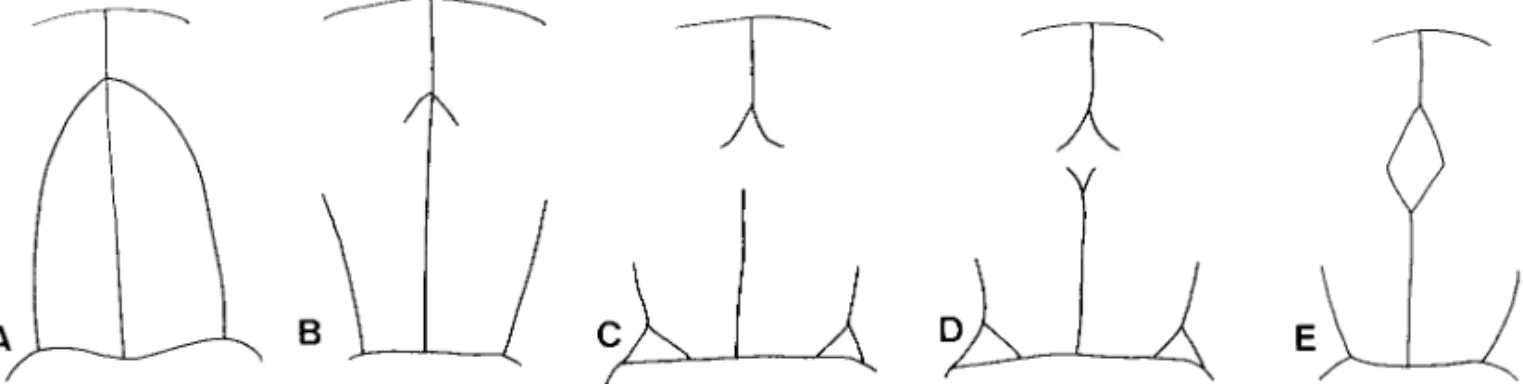
Fig. 9. Evolutionary trend from the apically conjoined periocullar sulci to the complete middorsal pronotal plate. (A) Mubrianax robustior (Pic). (B) Eubrianax pellucidus Lewis. (C) Eubrianax amamiensis kimura ssp. n. (D) Eubrianax amamiensis amamiensisSatoˆ. (E) Eubrianax tarokoensis Lee & Yang.
17. Apical Setae at Basal Pieces of Marginal Peg Setae (CI ⫽ 66, RI ⫽ 75). (0) Absent; (1) paired, oval
or lanceolate (Fig. 12B); (2) paired, apically comb-shaped (Lee et al. 1999c; Fig. 5). The outgroup does not have this character. The apomorphic condition appears in Eubrianax flavus, E. amamiensis, E.
man-akikikuse, E. serratus,and Heibrianax. This multistate character was treated as additive.
18. Outward Margins of Spiracles (CI and RI ⫽
100). (0) Without openings (Fig. 7A); (1) with rows of openings (Fig. 7 AÐE). The pleisomorphic condi-tion appears in only Jinbrianax.
19. Distribution Pattern of Openings of Spiracles
(CI ⫽ 66, RI ⫽ 50). (0) ConÞned to vermiculations and surrounding spiracles (Fig. 7A); (1) reduced ex-cept those on outward margins of spiracles (Fig. 7 D and E); (2) scattered or random (Fig. 7 B and C). In unexposed pupae of Odontanax and Jaechanax, the spiracles and openings are reduced except the out-ward margins. The openings of spiracles are scatterd or random on the spiracles in Mubrianax, Heibrianax, and all species of Eubrianax. This multistate character was treated as nonadditive.
20. Outward Margin of Each Spiracle (CI and RI ⫽
100). (0) Without processes (Fig. 7 A and D); (1) with one process near base (Fig. 7B); (2) emarginate
be-hind the process (Fig. 7 C and E). The pleisomorphic condition appears in Jinbrianax and Odontanax; the apomorphic condition appears in Jaechanax,
Heib-rianax,and all species of Eubrianax. This multistate character was treated as additive.
21. Posterior Margins of Last Three Abdominal Terga (CI and RI ⫽ 100). (0) With true setae (Lee et
al. 1999c; Fig. 15); (1) marginal extension (Lee et al. 1999b; Fig. 4; Lee et al. 1999c; Fig. 14). The pleiso-morphic condition appears in only Jinbrianax.
Phylogenetic Results. Although the phylogenetic
analysis yielded nine most parsimonious cladograms, all changes were restricted to relations between
Heib-rianaxand members of Eubrianax. Thus, when the strict consensus cladogram was preformed, there would be one unresolved clade (Fig. 8).
Position of Jinbrianax. The cladogram (Fig. 8) is
consistent with the hypothesis that Jinbrianax is the most primitive genus within Eubrianacinae, indicated by three primitive characters (excluding pupal stag-es): the number of the apical spurs on the pro-, meso-, and metatibiae; 2-2-2 (3.0) in adults; presence of the mid-pronotal longitudinal sulci (11.0) and only one side of the basal pieces of the marginal peg setae with teeth (17.1) in larvae.
Position of Odontanax. Monophyly of
“Odon-tanax ⫹ Mubrianax ⫹ Jaechanax ⫹ Heibrianax ⫹ Eu-brianax” is supported by Þve synapomorphic
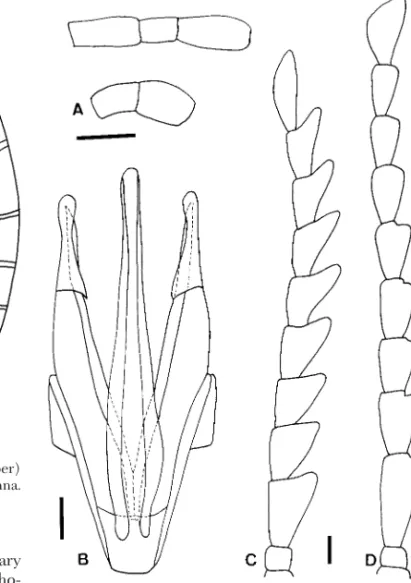
charac-ters in all stages: the number of the apical spurs on the pro-, meso-, and metatibiae; 2-1-1 (3.1) in adults; ab-sence of the mid-pronotal longitudinal sulci (11.1), and both sides of the basal pieces of the marginal peg Fig. 10. Eubrianax manakikikuse Satoˆ. (A) Maxillary
(up-per) and labial (lower) palpus. (B) Aedeagus. (C) Male antenna. Scale bar ⫽ 0. 1 mm.
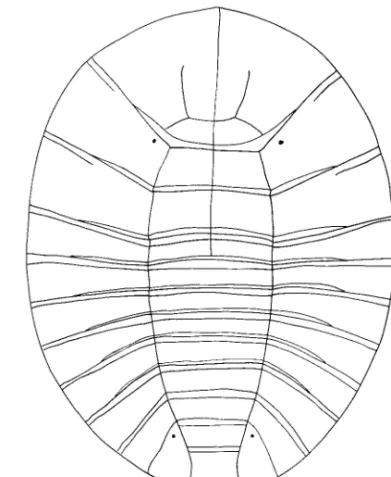
Fig. 11. Eubrianax manakikikuse Satoˆ, larva.
setae with teeth (17.2) in larvae; the outward margins of spiracles with rows of openings (18.1); and poste-rior margins of last three abdominal terga without setae (21.1). The latter two characters may result from the appearance the unexposed pupae. Lee et al. (2000a) supposed that an unexposed pupa had a se-lective advantage by recycling the cuticles of the last three abdominal terga.
Position of Mubrianax. Monophyly of “Mubrianax ⫹
Jaechanax ⫹ Eubrianax ⫹ Heibrianax” is supported by
the presence of one process near the base of the outward margin of each spiracle in pupae (16.1). This synapomorphic character may be caused by recovery of the exposed pupae based the following explanation. Compared with the pupae of Jinbrianax and
Mu-brianax,the latter lacks some complicated structures as in Jinbrianax, e.g., areas occupied by openings are raised and extend outside so that the entire spiracles are larger than the larval skin. The synapomorphic character appears to prevent the three apical pupal terga from pushing inside the larval skin when
Mu-brianaxhas evolved as exposed pupae. In addition, pupae of Mubrianax have the most and smallest open-ings within the Eubrinacinae, about nine times those of
Eubrianax edwardsiicompared with similar body size
in males. This suggests that larvae of Mubrianax can pupate under water.
Position of Jaechanax. Immature stages of Jaechanax
share some characters with Odontanax and Mubrianax. The larva is oblong (15.1) and has the periocellar sulci conjoined apically (10.1) as in Mubrianax. The unex-posed pupa and the presence of crevices between abdominal terga 6 and 7 in larvae (14.1) are also found in Odontanax. These characters make it difÞcult to determine which genus is the sister group of
Jae-chanax.However, comparing the unexposed pupae of
Odontanaxand Jaechanax, the latter differs by having one process near the base of each spiracle (20.1) that is functional for exposed pupae. This indicates there must be at least one evolutionary event producing exposed pupae in both genera. In addition, the distri-bution patterns of openings on exposed pupae are distinct among Jinbrianax, Mubrianax, and Eubrianax. This implies that the evolutionary event causing un-exposed pupae has happened more than once because the presence of unexposed pupae involves reducing openings of the spiracles, except those at the outward margins. Both characters are consistent with the present phylogenetic hypothesis that Mubrianax is the sister taxon of Jaechanax.
The oblong body shape (15.1) and the xylophagous mandibles (9.1) in larvae are alternative evolutionary Fig. 12. Eubrianax pellucidus Lewis. (A) Maxillary
(up-per) and labial (lower) palpus. (B) Apex of basal piece of marginal peg seta, larva. (C) Aedeagus. (D) Male antenna. Scale bar ⫽ 0. 1 mm.
Fig. 13. Eubrianax amamiensis amamiensis Satoˆ (B, C, D)
and Eubrianax amamiensis kimura ssp. n. (A, E). (A, B) Maxillary (upper) and labial (lower) palpus. (C) Aedeagus. (D, E) Male antenna. Scale bar ⫽ 0. 1 mm.
events because both Jaechanax and Mubrianax are in a sister relationship. It takes the same number of steps either way: evolves parallel in both genera or is syna-pomorphic for both genera and reverse in Eubrianax and Heibrianax.
Monophyly of Jaechanax ⫹ Eubrianax ⫹ Heibrianax is supported by (1) the presence of pulvilli at tarsal claws (4.1), (2) the strongly sclerotized mesal margins of the parameres (7.1) in adults, and (3) the outward margin of each spiracle being emarginate behind the process (20.2) in pupae. This latter synapomorphic character in pupae is interesting because it may be caused by the recurrence of unexposed pupae. The openings of spiracles above the process can be pro-jected from the larvae skin; however, the ones behind the process would be reduced because they are not functional below the larval skin. This may be a possible cause for the emargination of the outward margin behind the process (20.2).
Monophyly of Heibrianax ⴙ Eubrianax. The
present cladogram (Fig. 8) does not support the hy-pothesis of monophyly of Heibrianax, but that it is paraphyletic. Monophyly of Heibrianax ⫹ Eubrianax is supported by (1) having well-developed pulvilli on tarsal claws (4.2), (2) short and moderately curved tarsal claws (5.1), and (3) apex of each paramere with one rounded sclerites covered (8.1) in adults. Thus, we regard Heibrianx as a new junior synonym of
Eu-brianax.
Although adults of Eubrianax pellucidus, E.
amaien-sis,and E. manakikikkuse are similar, their larvae have some characters inconsistent with the other Eubrianax larvae. The phylogenetic analysis suggests that all three species should remain in the genus to maintain
its monophyly. A similar situation happens with E.
serratus.Some evolutionary trends are revealed from the present cladogram: (1) The paired lanceolate api-cal setae at the basal pieces of margin peg setae tend to be multiÞdous apically (17.132), the primitive con-dition appears in E. pelludicus; (2) the dividing sulci of the posterior plates on the prothorax tend to be absent (12.130), the primitive condition appears in E.
ama-miensis; (3) the apically conjoined periocellar sulci tend to transform the middorsal pronotal plate (10.132), that the middorsal pronotal plate is incom-plete in E. pellucidus and E. amamiensis. Both species provide good evidence to hypothesize the last trend. First, the apically conjoined periocellar sulci break up. The anterior part becomes the anterior sucli of the middorsal pronotal plate (E. pellucidus, Fig. 9B). The middorsal line in which the middorsal pronotal plate is located disappears(E. amamiensis kimurai, Fig. 9C). The posterior sulci appear (E. amamiensis amamiensis, Fig. 9D). Finally, the middorsal prontonal is complete Fig. 14. Eubrianax amamiensis amamiensis Satoˆ, larva.
Fig. 15. Eubrianax insularis Nakane. (A) Maxillary
(up-per) and labial (lower) palpus. (B) Aedeagus. (C) Male antenna. Scale bar ⫽ 0. 1 mm.
(E. tarokoensis, Fig. 9E). However, this evolutionary trend cannot reßect on Jinbrianax because it is ho-moplastic for Jinbrianax and Eubrianax. Moreover this character is rather different morphologically between
Jinbrianaxand Eubrianax.
Comparing the pupae of Jaechanax and other gen-era, Jaechanax has the fewest spiracular openings lo-cated only at the outward margins. Such a phenom-enon contradicts HintonÕs (1966) idea: “The pupa, unlike the larva of many Psephenidae, has no means of keeping the spiracular openings free of silt or other debris. Many separate openings over a large area seems to be the next best solution to the problem of preventing the spiracles from becoming totally blocked by debris.” It provides a possible explanation why the pupa of Eubrianax is exposed. Because ex-posed pupae could use more openings than unexex-posed ones, such a evolutionary trend can beneÞt pupal survival.
Although the phylogenetic relationships between members of Eubrianax are not clear, they may be classiÞed into several species groups based on adults. The E. granicollis species group comprises all species of Heibrianax (Lee et al. 1999a). The E. ramicornis species group is composed of all species of Eubrianax that Lee et al. (1999a) described. Eubrianax
pelluci-dus, E. amamiensis,and E. manakikikuse are grouped as
E. pellucidusspecies group. Eubrianax serratus should be separated into a discrete species group because it has a number of autapomorphies.
Systematic Account
Eubrianax pellucidus species group
Adult Diagnosis. This species group differs from
Eubrianax ramicollisspecies group by the male an-tennal rami beginning basally on segments 3 to 6, from bases or middles on segments 7 to 10.
Larval Diagnosis. Differs from Eubrianax ramicollis
species group by either lacking or having incomplete middorsal pronotal plates.
Included Species. Eubrianax manakikikuse Satoˆ, E.
pellucidusLewis, E. amamiensis Satoˆ stat. nov., E.
ama-miensis kimuraissp. nov., E. insularis Nakane.
Eubrianax manakikikuse Satoˆ
(Figs. 10 and 11)
Eubrianax manakikikuseSatoˆ, 1964: 35; Ð Satoˆ 1965: 91; ÐChuˆjoˆ and Satoˆ 1970: 15.
Eubrianax wulaiensisLee and Yang, 1990: 80 (NTUC). syn. n.
Type Material. Eubrianax manakikikuse.
HOLO-TYPE: male, JAPAN: Ishigaki Is.: Mt. Banna-dake: 8-V-63, Y. Arita, NWU (examined).
Fig. 16. Eubrianax serratus sp. n. (A) Maxillary (upper)
and labial (lower) palpus. (B) Aedeagus. (C) Male antenna. (D) Female antenna. Scale bar ⫽ 0. 1 mm.
Fig. 17. Eubrianax serratus sp. n., larva.
Eubrianax wulaiensis. HOLOTYPE: male, TAI-WAN: Wulai: 10-III-90, Lee. PARATYPES: 54 males, 10 females, same, NTUC (examined).
Male. 3.7Ð5.3 mm long, 2.6Ð3.8 mm wide. Coloration
dark brown to black; but coxae and femora paler. Antennae with rami started from bases on segements 3Ð7 (Fig. 10C) from middles on segments 8 to 9, from near apex on segment 10; relative lengths of ramus to antennomere from segment 3 to 10 ⬇1.5: 3.0: 3.0: 3.2: 2.7: 2.7: 2.6: 2.4. Maxillary palpus (Fig. 10A) slender; terminal segment apically dilated, apex truncate; rel-ative lengths of segments 2 to 4 ⬇1.8: 1: 1.4. Labial palpus (Fig. 10A) small, ⬇0.5 times length of maxillary palpus; terminal segment similar to that of maxillary palpus; relative lengths of segment 2 to 3 ⬇1: 1.0. WP/LP ⫽ 2.2. LE/WE ⫽ 1.3. WP/WE ⫽ 0.7.
Aedeagus (Fig. 10C). Aedeagus is 2.3 times as long
as wide. Penis 0.7 times the length of aedeagus, apex slender, widened toward base, baso-lateral apophyses wide. Fibula slender, apex narrowed rounded. Parameres with ventral apophyses subtriangular.
Female. The female is 4.4Ð6.1 mm long, 2.6Ð3.8 mm
wide. Coloration blackish brown except pronotum yellowish brown, medially darkened. WP/LP ⫽ 2.1. LE/WE ⫽ 1.3. WP/WE ⫽ 0.6.
Larval Diagnosis (Fig. 11). Similar to Eubrianax
tarokoensis,but the middorsal pronotal plate is absent, the posterior plates on abdominal segments 1 to 5 appear, and the costal lines well develop on main plates of abdominal segments 1 and 9.
Material Examined. JAPAN: Iriomote Is.: 1 male,
10-IV-69, Chujo, NWU; Ishigaki Is.: Omotoyama: 4 males, 12Ð13-V-75, Yano, NWU; 2 males, 13Ð15-V-75, Notsu, NWU; 1 female, Yoshihara, 5-IV-86, Sawai, NWU; TAIWAN: Kaohsung: Maolin: 1 male, 4 females, 17-II.-24-III-93, Lee, NTUC; Keelung: Nuannuan: 4 males, 4 females, III-93, Lee, NTUC; Taipei: Yang-mingshan: 30 males, 8 males, 24-IV-91, Chang & Lee, NTUC; Taitung: Chipen: 3 males, 1 female, 28-II-11-III-93, Lee, NTUC; Taitung: Peiyu falls: 1 male, 2 fe-males, 23-II-11-III-93, Lee, NTUC.
Distribution. Taiwan, Japan (Ishigaki and Iriomote
islands).
Eubrianax pellucidus Lewis
(Fig. 12)
Eubrianax pellucidusLewis, 1895: 104. Ð Nakane 1948: 14. Ð Nakane: 1952: 41.
Eurianaxsp. EA Gose 1957: 20 (larva)
Male. Male is 3.8 mm long, 2.2 mm wide. Coloration
dark brown to black; but pronotum yellowish brwon with median longitudinal band; thoracic sterna and legs yellowish brown. Antennae (Fig. 12D) pectinate from segments 3 to 10; rami started from base on segments 3 to 6, from middle on segments 6 to 10; relative lengths of ramus to antennomere from seg-ment 3 to 10 ⬇1.5: 3.1: 3.0: 2.7: 2.6: 2.2: 2.3: 1.8. Maxillar palpus (Fig. 12A) slender; terminal apically dilated, apex rounded; relative lengths of segment 2 to 4 ⬇1.3: 1: 1.3. Labial palpus (Fig. 12A) small, ⬇0.5 times the
length of maxillary palpus; terminal segment apically dilated, apex rounded; relativel lengths of segment 2 to 3 ⬇1: 1.4. WP/LP ⫽ 2.1. LE/WE ⫽ 1.3. WP/WE ⫽ 0.7.
Aedeagus (Fig. 12C). Aedeagus 2.7 times as long as
wide. Penis 0.9 times length of aedeagus, apex slender, widened toward base, baso-lateral apophyses wide. Fibula slender, apex emarginate. Ventral apophyses of parameres hook-like
Female. 5.0 mm long, 3.0 mm wide. Similar to males
in color. WP/LP ⫽ 2.4. LE/WE ⫽ 1.4. WP/WE ⫽ 0.7.
Adult Diagnosis. Easily distinguished from others
by the bicolored pronotum and the yellowish brown legs.
Larval Diagnosis. Similar to Eubrianax
man-akikikuse,but posterior plates are absent on all ab-dominal segments, anterior half of the middorsal pronotal plate are present (Fig. 9B), and apical setae (Fig. 12B) are oval..
Material Examined. JAPAN: 1 female, Dando, Aichi
Pref., 20-VII-69, Sato, NWU; 1 female, Kurokawa, N-Echigo. 22-VII-72, Baba, NWU; 1 male, Kyushu, Hirado-Yasumandake, Nagasaki Pref., 31-VII-2-VIII-74, Notsu, NWU; 3 males, MT, Amagi Naka-Izu, Shi-zuoka-Pref., 14-VII-90, Tsuyuki, NTUC, NWU; 1 fe-male, MT, Ishizuchi, Ehime Pref., 3-VII-60, Ohbayashi, NWU; 1 male, Neo oppa, 7Ð10-VII-52, Ohira, NWU; 1 male. Tuzuro, Tokushima, 22-VII-65, Sakai, NWU; CHINA: Sichuan: 1 male, Guanxian, 600 m, 21Ð22-V-89, Boca´k, NHMB; 1 male, 700 m, 20-V-21Ð22-V-89, Koliba´c, NHMB.
Distribution. Japan (Honshu, Shikoku, Kyushu),
China (Sichuan).
Eubrianax amamiensis Satoˆ stat. nov.
(Figs. 13 and 14)
Eubrianax manakikikuse amamiensisSatoˆ, 1965: 92.
Male. The male is 3.2Ð3.8 mm long, 2.1Ð2.3 mm wide.
Coloration blackish brown. Antenna (Fig. 13D) with rami started from bases on segments 3 to 9, from middle on segment 10; relative lengths ramus versus antennomere from segemtns 3 to 10 ⬇1.3: 2.0: 2.0: 2.1: 2.0: 1.8: 1.6: 1.4. Maxillary palpus (Fig. 13B) slender; terminal apically dilated, apex irregular; relative lengths of segments 2 to 4 ⬇1.4: 1: 1.6. Labial palpus (Fig. 13B) small, 0.5 times the length of maxillary palpus; terminal segment similar to that of mxillary palpus; relative lengths of segments 2 to 3 ⬇1: 1.1. WP/LP ⫽ 1.8Ð1.9. LE/WE ⫽ 1.2. WP/WE ⫽ 0.7.
Aedeagus (Fig. 13C). Aedeagus 2.5 times as long as
wide. Penis 0.7 times length of aedeagus, apex very slender, widened toward base, baso-lateral apophyses slender. Fibula very slender, apex narrowed rounded. Parameres with ventral apophyses hook-like.
Adult Diagnosis. This species is close to Eubrianax
manakikikuse,but differs by the shorter antennal rami.
Larval Diagnosis (Fig. 14). Similar to Eubrianax
pelludicus, but the posterior half of the middorsal pronotal plate are present, not connected with ante-rior one; the middorsal line is absent between the anterior and posterior halves of middorsal pronotal
plate; one additional sulcus is present arising from anterior margin of main plate of prothorax and apically conjoined with each periocellar sulcus; each posterior plate on prothorax separated by basal margins of pleu-rites, one obliquely longitudinal sulcus on each pos-terior plate; some derived sulci recognized on thora-ces and abdomen; one arising from antero-lateral angles of main plates on prothorax, one obliquely transverse and arising from middle of basal margins of pleurites on mesothorax; on metathorax one paired V-shaped one arising from the similar position as me-sothrax; one transverse and arising from basal margins of pleurites between abdominal segments 8 and 9.
Material Examined. JAPAN: 1 male,
Amami-Os-hima, Hatsuno, 14-VI-62, Sato, NWU; 1 male, Mikyo, Is. Tokunoshima, 12-IV-68, Tomokuni, NWU.
Distribution. Japan (Amami-Oshima and
To-kunoshima islands).
Eubrianax amamiensis kimurai sspn. nov.
(Fig. 13)
Type Material. Holotype, male, JAPAN: Okinawa,
I-III-98, Lee, NHMW. PARATYPES: 2 males, 5 fe-males, NHMW, NTUC, same data; 1 male, Haneji-ohkawa, 26-III-97, Satoˆ, NWU;1 male, Matsuda Ginoza V., 8-III-95, Kimura, NTUC; 1 female, Nago, Nago-shi, 1-IV-96, Yoshitomi, NWU; 2 males, Ohkuni-rindo, 4-V-99, Utsunomiya, NWU; 1 male, Uehara, Ohgimi, 26-XII-98, Sugimoto, NWU; 1 male, Yona, 24Ð25-III-64, Yoshimoto and Harrell, BPBM; 1 male, Yona, Kunigami-mura, 2-IV-96, Yoshitomi, NWU.; Tarama-jima: 1 male, 6-VI-96, Kimura, NTUC.
Male. Male 3.1Ð4.9 mm long, 2.0Ð2.4 mm wide.
Col-oration blackish brown, but pronotum yellowish brown, medially darkened; pro-, meso, and metasterna brown; trochanters, coxae, and femora yellowish brown. Antennae (Fig. 13E) pectinate from segments 3 to 10; rami stretched from base on segments 3 to 5, from middle on segments 6 to 10; relative lengths of ramus to antennomere from segment 3 to 10 ⬇1.3: 1.7: 1.8: 1.7: 1.5: 1.4: 1.1: 1.0. Maxillary palpus (Fig. 13A) slender; terminal apically dilated, apex emarginate; relative lengths of segments 2 to 4 ⬇1.4: 1: 1.7. Labial palpus (Fig. 13A) small, 0.5 times the length of max-illary palpus; terminal segment similar to that of mx-illary palpus; relative lengths of segments 2 to 3 ⬇1: 1.8. WP/LP ⫽ 1.8Ð2.1. LE/WE ⫽ 1.3Ð1.4. WP/WE ⫽ 0.6Ð 0.7.
Female. Female 3.6Ð5.0 mm long, 2.1Ð3.1 mm wide.
Similar to males in color. WP/LP ⫽ 2.0Ð2.1. LE/WE ⫽ 2.3Ð2.4. WP/WE ⫽ 0.6.
Adult Diagnosis. This new subspecies is different
from the nominate one by the shorter antennal rami and the emarginate apices of the maxillary and labial palpi.
Laval Diagnosis. Similar to the nominate subspecies,
but differing by lacking the posterior sulci of the mid-dorsal pronotal plate (Fig. 9C).
Etymology. Named after the distinguished native
collector, Mr. M. Kimura.
Distribution. Japan (Okiwana island, Tarama
is-land).
Eubrianax insularis Nakane
Eubrianax insularis Nakane, 1952: 41(CNJ; now in NSMT). Ð Satoˆ 1965: 91.
Type Material. Types were examined: a couple
mounted together, labeled “HOLOTYPE ALLO-TYPE JAPAN Yakushima 10.IV.Õ12 G.2.1.110 756.
Eubrianax insularisNakane DET. T. NAKANE 72Ð 11.” However, Nakane (1952) didnÕt indicate either specimen as holotype or paratype in the original pa-per. According to ICZN Art. 74.1, we formally desig-nate the male as lectotype, and the female as paralec-totype.
Male. Male 3.6 mm long, 2.3 mm wide. Coloration
brown. Antennae (Fig. 15C) pectinate from segments 3 to 10; rami started from bases on segments 3 to 5, from middles on segments 6 to 10; relative lengths of ramus to antennomere from segment 3 to 10 ⬇1.5: 2.2: 2.3: 2.2: 2.1: 2.0: 1.8: 1.3. Maxillar palpus (Fig. 15A) slender; terminal apically dilated, apex truncate; rel-ative lengths of segment 2 to 4 ⬇1.2: 1: 1.3. Labial palpus (Fig. 15A) small, ⬇0.6 times the length of max-illary palpus; terminal segment apically dilated, apex rounded; relativel lengths of segment 2 to 3 ⬇1: 1.4. WP/LP ⫽ 1.8. LE/WE ⫽ 1.2. WP/WE ⫽ 0.6.
Aedeagus (Fig. 15B). Aedeagus -2.7 times as long as
wide. Penis 0.8 times length of aedeagus, apex very slender, widened toward base, baso-lateral apophyses wide. Fibula wide, apex emarginate. Basal margins of ventral apophyses of parameres strongly serrate.
Variation. Consequent specimens differ from types
by the blackish brown body, similar to Eubrianax
man-akikikuseand E. amamiensis.
Adult Diagnosis. Eubrianax insularis is similar to E.
pellucidus,but differs by the wider Þbula, the longer penis, and the blackish brown body.
Material Examined. JAPAN: Yaku Island: 1 male,
Shiratani-rindo, 14Ð16-VII-97, Yoshitomi, NHMW;1 male, Yodogawa-path, 9-VII-94, Tsuyuki, NWU.
Distribution. Japan (Yaku-shima Island).
Eubrianax serratus species group
Adult Diagnosis. Differs from the other two groups
by the male serrate antennae and the truncate apex of the prosternal process.
Larval Diagnosis. Differs from other two groups by
having the posterior plates on the main plates of the abdominal segments 1 to 3.
Pupal Diagnosis. Marginal extensions without
mi-cro-spined projections.
Included Species. Eubrianax serratus sp. nov.
Eubrianax serratus sp. nov.
(Figs. 16 and 17)
Type Material. HOLOTYPE: male, TAIWAN:
Kaohsiung, Changshan Rd. 20, 92 km,
93, Lee, NHMW. PARATYPES: 16 males, 11 females, same data, NTUC, NHMW; 8 males, 1 female, 100 km, 29-XII-92-2-II-93, Lee, NTUC; 4 males, 2 females, 108 km, 2Ð24-I-93, Lee, NTUC; Nantou: 5 males, 3 females, Luku, 13Ð22-I-93, Lee, NTUC.
Male. Male 4.2Ð4.4 mm long, 2.3Ð2.4 mm wide.
Col-oration blackish brown, but pronotum laterally yel-lowish brown and femora paler. Antenna (Fig. 16C) serrate from segments 3 to 10; segment 3 longest and less serrate than others; segments 4 to 10 similar in length. Maxillary palpus (Fig. 16A) short, apex round-ed; relative lengths of segments 2 to 3 ⬇1.7: 1: 1.7. Labial palpus (Fig. 16A) ⬇0.6 times as long as maxil-lary palpus; terminal segment enlarged; relative length of segments 2 to 3 ⬇1: 1.1. LE/WE ⫽ 1.4. WP/LP ⫽ 1.9Ð2.0. WP/WE ⫽ 0.6.
Aedeagus (Fig. 16B). Aedeagus 2.5 times as long as
wide. Penis 0.9 times the total length of genitalia; apically tapering from basal one-Þfth; apex rounded. Parameres 0.8 times the length of tegmen; ventral apophyses short..
Female. Female 5.3Ð6.2 mm long, 3.5Ð4.1 mm wide.
Similar to males in color, but pronotum yellowish brown with median longitudinal black band and ab-domen yellowish brown. Antenna (Fig. 16D) Þliform from segments 3 to 10, segment 3 longest, segments 4 to 10 similar in length. Pronotum more transverse. Prosternal process wider. Abdomen bigger than elytra, apex exposed. LE/WE ⫽ 1.2Ð1.3. WP/LP ⫽ 2.7Ð3.0. WP/WE ⫽ 0.6.
Adult Diagnosis. This new species is characterized
by the male serrate antennae and the truncate apex of the prosternal process..
Larval Diagnosis (Fig. 17). The posterior plates are present on abdominal segments 1 to 3, not including pleurites.
Pupal Diagnosis. Opening of spiracles are restricted
in lateral sides (Fig. 7C), similar to Eubrianax
ed-wardsi. Number of opening is variable because of sexual dimorphism; 29Ð33 in males (n ⫽ 5); 59Ð65 in females (n ⫽ 5). Marginal extensions have no micro-spined projections.
Biology. Eubrianax serratus is sympatric with E.
ni-gerLee & Yang. However, the emergence of adults of the former precede the latter by 1 mo. Such differ-entiation is enough to cause preproductive isolation between both species because adults are short-lived.
Etymology. Serratus from Latin (serrate),
indicat-ing the serrate male antennae.
Distribution. Some larvae collected from Tainan
County. Thus far, known from three counties in cen-tral and south Taiwan.
Key to the Genera of the Eubrianacinae Based on Larvae
1. Body form oblong; periocellar sulci conjoined apically (Fig. 5 A and B); mandibles thick and with apical teeth (Fig. 3 C and D) . . . 2 1⬘. Body form oval; periocellar sulci incomplete or
absent (Fig. 4 AÐC); mandibles ßattened and without teeth (Fig. 3 A and B) . . . 3
2. With crevices between abdominal terga 6 and 7 (Fig. 5A); teeth at sides of basal pieces of marginal peg setae reduced near bases (Fig. 6C); posterior plates on main areas of abdom-inal segments 1 to 3 (Fig. 5A) . . . Jaechanax 2⬘. Without crevices between abdominal terga 6
and 7 (Fig. 5B); teeth at sides of basal pieces of marginal peg setae developed well (Fig. 6B); posterior plates on abdominal segments 1 to 4 and pleurites on segment 5 (Fig. 5B). . . . Mubrianax
3. Costal lines absent; mid-pronotal longitudinal sulci present (Fig. 4A); only one side of apical pieces of marginal peg setae with teeth (Fig. 6A) . . . Jinbrianax 3⬘. Costal lines present; mid-pronotal longitudinal
sulci absent (Fig. 4 B and C); both sides of apical pieces of marginal peg setae with teeth (Fig. 6B) . . . 4 4. With crevices between abdominal terga 6 and 7;
dividing sulci of posterior plates on thoracic segments present (Fig. 4C); apical setae at apical pieces of marginal peg setae lanceolate. . . . Odontanax 4⬘. Without crevices between abdominal terga 6
and 7; dividing sulci of posterior plates on thoracic segments absent (Fig. 4B) (except E.
amamiensis); apical setae at apical pieces of marginal peg setae multifurcate (Lee et al. 1999c; Fig. 5) (except E. pellucidus) . . . .
Eubrianax
Key to the Genera of the Eubrianacinae Based on Pupae
1. Unexposed pupae; only spiracles sclerotized; all openings of spiracles concentrated at outward margins (Figs. 7 D and E) . . . 2 1⬘. Exposed pupae; last three abdominal terga
scle-rotized; at least some openings extending in-side spiracles (Fig. 7 AÐC) . . . 3 2. Outward margin of each spiracle with one row of
openings, with one process near base (Fig. 7E). . . . Jaechanax 2⬘. Outward margin of each spiracle with two rows
of openings, without processes near base (Fig. 7D) . . . Odontanax 3. Whole spiracles raised; openings conÞned to
ver-miculations and surrounding perimeter (Fig. 7A); posterior margins of abdominal terga with setae (Lee et al. 1999c; Fig. 15) . . Jinbrianax 3⬘. Spiracles ßat; at least one row of openings
con-Þned to outward margins of spiracles (Fig. 7 B and C); posterior margins of abdominal terga without setae . . . 4 4. Openings numerous and tiny, densely and
ran-domly distributed on spiracles, outward mar-gins not emarginate behind processes (Fig. 7B); marginal extensions further divided, tightly connected (Lee et al. 1999b; Fig. 4) . . . . Mubrianax
4⬘. Openings fewer and large, sparsely scattered on spiracles, outward margins emarginate be-hind processes (Fig. 7C); marginal extension with pointed processes (Lee et al. 1999c; Fig. 14) . . . Eubrianax
Key to the Genera of the Eubriancinae Based on Adults
1. Without pulvilli at tarsal claws . . . 2 1⬘. With pulvilli at tarsal claws . . . 4 2. Formula of tibial spurs 2-2-2; elytra shining
me-tallically. . . Jinbrianax 2⬘. Formula of tibial spurs 2-1-1; elytra not shining.
. . . 3 3. Tarsal claws with notches near bases (Fig. 1B);
mesal margins of parameres smooth (Fig. 2C); antennal rami originating apically on segments 4 to 7; prosternal process short and acute. . . . . . Mubrianax 3⬘. Tarsal claws wthout notches near bases (Fig.
1A); with processes at mesal margins of each paramere (Fig. 2B); antennal rami originating basally or medially on segments 4 to 7 (except
Odontanax maculicollis); prosternal process long and dilated . . . Odontanax 4. Pulvilli membranous; tarsal claws long and
slightly curved (Fig. 1A); apices of parameres without rounded sclerites covered (Fig. 2D). . . . Jaechanax
4⬘. Pulvilli well developed and pigmented; tarsal claws short and moderately or strongly curved (Fig. 4 C and D); apices of parameres with rounded sclerites covered (e.g., Figs. 2E and 10B) . . . (Eubrianax) 5 5. Male antennae serrate (Fig. 16C); apex of
pro-sternal process truncate . . . serratus group 5⬘. Male antennae pectinate (e.g., Fig. 10C); apex of
prosternal process acute or dilated . . . 6 6. Male antennal rami originating from bases or
middles on segments 5 to 7 (Fig. 10C, 12D, 13 D and E, 15C) . . . pellucidus group 6⬘. Male antennal rami originating from apices on
segments 5 to 10 (Lee et al. 1999a; Figs. 7 and 11) . . . 7 7. Apex of posternal process dilated; tarsal claws
strongly curved (Fig. 1D) . . . group 7⬘. Apex of posternal process acute; tarsal claws
moderately curved (Fig. 1C) . . . . . . . ramicollis group
Checklist of the Species and their Distributions of Eubrianacinae
Ja¨ch (1984) listed 38 species and three subspecies in
Eubrianax,excluding a fossil species E. vandeli Ber-trand and Laurentiaux, 1963. Of these E. flabellicornis Pic, 1922 was designated as the type species of
Mi-croeubrianaxby Pic (1954). This genus was synony-mized with Psephenoides in another subfamily Pse-phenoidinae (Lee and Ja¨ch 1995). And E. luteosignatus Pic, 1947 from Chile should be transferred to
subfam-ily Eubriinae. Three species, E. palawanus Pic (1926),
E. albomaculatus Pic (1926), and E. longipennis Pic (1915) are not in this list. Eubrianax longipennis is excluded from the present checklist because it is pos-sible that no members of the subfamily are distributed in South America, although the type specimens of E.
longipennishave not been found in the Paris museum. Although we are making attempts to revise and redescribe all species of this subfamily, most African species and E. albomaculatus are not covered because only type specimens are available. They are tempo-rarily treated as species incertae sedis.
Genus Jinbrianax Lee, Satoˆ, and Yang 1999c
1. Jinbrianax apicalis (Pic 1913) Indonesia
2. Jinbrianax incompositus Lee, Satoˆ, and Yang 1999c Nepal
3. Jinbrianax jaechi Lee, Satoˆ, and Yang 1999c E. Malaysia
4. Jinbrianax javanus (Pic 1913) Indonesia
5. Jinbrianax metallicus (Pic 1922) Vietnam, Thai-land, W. Malaysia ⫽ Eubrianax binhana Pic 1928 6. Jinbrianax rotundatus Lee, Satoˆ, and Yang 1999c
Nepal
7. Jinbrianax schillhammeri Lee, Satoˆ, and Yang 1999c Laos
8. Jinbrianax semiaenescens (Pic 1921) Philippines, E. Malaysia
9. Jinbrianax tenuis Lee, Satoˆ, and Yang 1999c China
Genus Odontanax Lee, Satoˆ, and Yang 2000a
10. Odontanax ceylonicus (Ja¨ch 1982) Sri Lanka 11. Odontanax chinensis Lee, Satoˆ, and Yang 2000a
China
12. Odontanax dohertyi (Pic 1915) E. Malaysia, Indo-nesia ⫽ Eubrianax dohertyi variety angustatus Pic 1930.
13. Odontanax dudgeoni Lee, Satoˆ, and Yang 2000a China
14. Odontanax flinti Lee, Satoˆ, and Yang 2000a Sri Lanka
15. Odontanax laosensis (Pic 1923) Laos, Vietnam, In-dia ⫽ Eubrianax tonkineus Pic 1935
16. Odontanax lioneli (Ja¨ch 1982) Sri Lanka 17. Odontanax maculicollis (Fairmaire 1888)
Viet-nam, Laos, Thailand
18. Odontanax minimus (Pic 1913) E. Malaysia, Phil-ippines
19. Odontanax obscuripes (Pic 1914a) India 20. Odontanax palawanus (Pic 1926) Philippines 21. Odontanax thai Lee, Satoˆ, and Yang 2000a
Thai-land
Genus Mubrianax Lee, Satoˆ, and Yang 1999b
22. Mubrianax atripennis (Pic 1931) Cameroon 23. Mubrianax basipennis (Pic 1913) Indonesia,
Phil-ippines ⫽ Eubrianax bicolor Pic 1955a.
24. Mubrianax robustior (Pic 1928) E. Malaysia Phil-ippines
Genus Jaechanax Lee, Satoˆ, and Yang 2000b
25. Jaechanax dentatus Lee, Satoˆ, and Yang 2000b In-donesia
26. Jaechanax elongatus Lee, Satoˆ, and Yang 2000b Philippines
27. Jaechanax illiesi (Satoˆ 1983) Philippines
28. Jaechanax insignis (Fairmaire 1904) Vietnam, Laos, Myanmar
29. Jaechanax major (Pic 1913) Thailand, Malaysia, Indonesia, Philippines ⫽ Eubrianax limbatithorax Pic 1923.
Genus Eubrianax Kiesenwetter 1874 ⴝ Placonycha
Horn 1880 ⫽ Heibrianax Lee, Satoˆ, and Yang 1999a syn.
n.
Ramicornis Species Group
30. Eubrianax edwardsii (LeConte 1874) United States
31. Eubrianax ihai Chuˆjoˆ and Satoˆ 1970 Japan 32. Eubrianax loochooensis Nakane 1952 Japan 33. Eubrianax niger Lee and Yang 1990 Taiwan ⫽
Eubrianax alishanensisLee and Yang 1990 34. Eubrianax nobuoi Satoˆ 1965 Japan
35. Eubrianax ramicornis Kiesenwetter 1874 Japan, Korea ⫽ Eubrianax ramicornis brunneicornis Na-kane 1952
36. Eubrianax meridianus Lee, Satoˆ, and Yang 1999a Taiwan
37. Eubrianax tarokoensis Lee and Yang 1990 Taiwan ⫽ Eubrianax flavus Lee and Yang 1990.
Granicollis Species Group
38. Eubrianax granicollis Lewis 1895 stat. rev. Japan 39. Eubrianax maai (Lee, Satoˆ & Yang 1999a) com. n.
from Heibrinax China
40. Eubrianax sichuanus (Lee, Satoˆ, and Yang 1999a) com. n. from Heibrinax China
41. Eubrianax secretus (Lee, Satoˆ, and Yang 1999a) com. n. from Heibrinax Taiwan, China
Pellucidus Species Group
42. Eubrianax manakikikuse Satoˆ 1964 Japan, Taiwan ⫽ Eubrianax wulaiensis Lee and Yang, 1990 syn. n. 43. Eubrianax pellucidus Lewis 1895 Japan, China 44. Eubrianax amamiensis Satoˆ 1965 stat. n. Japan ⫽
Eubrianax amamiensis kimuraissp. n. Japan. 45. Eubrianax insularis Nakane 1952 Japan
Serratus Species Group
46. Eubrianax serratus sp. n. Taiwan
Species Incertae Sedis
47. Eubrianax africanus Pic 1914b Angola 48. Eubrianax albomaculatus Pic 1926 China 49. Eubrianax brevis Pic 1955b Urundi
50. Eubrianax invittatus Pic 1951 Congo
51. Eubrianax mirei Bertrand & Villiers 1970 Cam-eroon
52. Eubrianax scotti Pic 1953 Ethiopia 53. Eubrianax vittaticollis Pic 1934 Congo
Acknowledgments
We deeply thank J. Wenzel and W. D. Shepard for reading the manuscript. We thank G. A. Samuelson, M. A. Ja¨ch, and the late T. Nakane for the loan of specimens. This research is supported by National Science Council NSC 89Ð2313-B-002Ð028.
References Cited
Arnett, Jr., R. H., G. A. Samuelson, and G. M. Nishida. 1993.
The insect and spider collections of the world. Sandhill Crane Press, Gainesville, FL.
Bertrand, H., and D. Laurentiaux. 1963. Une Larve
(Pse-phenoõ¨de) du Genre Eubrianax Kiesenw. (Cole´opte`res Eubrianacidae) dans lÕE´oce`ne Lacustre de lÕAude. Bull. Soc. His. Nat. Toulouse 98: 232Ð241.
Bertrand, H., and A. Villiers. 1970. Cole´opte`res Eubriidae
Re´colte´s au Cameroun par Ph. Bruneau de Mire´. Bull. I. F. A. N. 32: 442Ð448.
Blackwelder, R. E. 1930. The larva of Eubrianax edwardsii
(LeC.) (Coleoptera: Psephenidae). Pan-Pac. Entomol. 6: 139Ð141.
Chuˆjoˆ, M., and M. Satoˆ. 1970. Coleoptera of the Loo-Choo
Archipelago (II): 12. Family Psephenidae; 13:Family Ptilodactylidae;14. Family Chelonariidae. Mem. Fac. Educ. Kagawa Univ., (2), 192: 14Ð16.
Fairmaire, M. L. 1888. Descriptions de Col 233 opt 232 res de
l 146 Indo-Chine. Ann. Soc. Entomol. Fr.6: 333Ð378.
Fairmaire, M. L. 1904. Descriptions de Lamellicornes
Indo-chinois nouveaux ou peu connus. Mission Pavie In-dochine 1879Ð1895: 3: 86Ð90.
Farris, J. S. 1988. Hennig86, version 1.5. Published by the
author, Port Jefferson, NY.
Gose, K. 1957. On three larvae of the genus Eubrianax. Bull.
Kansai Nat. Sci. Soc. 10: 20Ð23 (in Japanese).
Hinton, H. E. 1955. On the respiratory adaptations, biology,
and taxonomy of the Psephenidae, with notes on some related families (Coleoptera). Proc. R. Entomol. Soc. Lond. 125: 543Ð68.
Hinton, H. E. 1966. Respiratory adaptations of the pupae of
beetles of the family Psephenidae. Philos. Trans. R. Soc. Lond. (B) 37: 211Ð245.
Horn, G. H. 1880. Synopsis of the Dascyllidae of the United
Sates. Trans. Am. Entomol. Soc. 8: 76Ð114.
Ja¨ch, M. 1982. Neue Dryopoidea und Hydraenidae aus
Cey-lon, Nepal, Neu Guinea und der Tu¨rkei (Col.). Koleopt. Rdsch. 56: 103Ð114.
Ja¨ch, M. 1984. Die Koleopterenfauna der Bergba¨che von
Su¨dwest-Ceylon. Arch. Hydrobiol. Suppl. 69: 228Ð332.
Kiesenwetter, H. 1874. Die malacodermen Japans nach dem
ergenisse der sammlungen des Herrn G. Lewis wa¨hrend der Jahre 1869Ð1871. Berl. Entomol. Z. 18: 241Ð288.
LeConte, J. L. 1874. Descriptions of new Coleoptera cheißy
from the PaciÞc slope of North America. Trans. Am. Entomol. Soc. 5: 43Ð72.
Lee, C.-F., and M. A. Ja¨ch. 1995. Psephenidae: 1. Check list
of the Psephenidae of China (Coleoptera), pp. 349Ð354. InM. A. Ja¨ch and L. Ji [eds.], Water beetles of China. Zoologisch-Botanische Gesellschaft in O¨sterreich and Wiener Coleopterologenverein, Wien.
Lee, C.-F., and P.-S. Yang. 1990. Five new species of the
genus Eubrianax from Taiwan (Coleoptera: Psepheni-dae). J. Taiwan Mus. 43: 79Ð88.
Lee, C.-F., M. Satoˆ, and P.-S. Yang. 1999a. Revision of
Eu-brianacinae (Coleoptera, Psephenidae) I. Eubrianax Kiesenwetter and Heibrianax gen. n. Jpn. J. Syst. Entomol. 5: 9Ð25.
Lee, C.-F., M. Satoˆ, and P.-S. Yang. 1999b. A revision of
Eubrianacinae (Coleoptera, Psephenidae) II. Mubrianax gen. nov. Elytra 27: 429Ð438.
Lee, C.-F., M. Satoˆ, and P.-S. Yang. 1999c. A revision of
Eubrianacinae (Coleoptera, Psephenidae) III. Jinbrianax gen. nov. Entomol. Rev. Jpn. 54: 169Ð187.
Lee, C.-F., M. Satoˆ, and P.-S. Yang. 2000a. A revision of
Eubrianacinae (Coleoptera, Psephenidae) IV. Odontanax gen. nov. Jpn. J. Syst. Entomol. 6: 151Ð170.
Lee, C.-F., M. Satoˆ, and P.-S. Yang. 2000b. A revision of
Eubrianacinae (Coleoptera, Psephenidae) V. Jaechanax gen. nov. Elytra 28: 119Ð129.
Lee, C.-F., P.-S. Yang, and H. P. Brown. 1990. Notes on the
genus Mataeopsephus (Coleoptera: Psephenidae) in Tai-wan with description of a new species. J. TaiTai-wan Mus. 43: 73Ð78.
Lewis, G. 1895. On the Dascillidae and Malacorderm
Co-leoptera of Japan. Ann. Mag. Nat. Hist. 6: 98Ð122.
Nakane, T. 1948. On the Japanese Dascillidae (Coleoptera).
Bull. Takarazuka Insect 45: 1Ð16 (in Japanese).
Nakane, T. 1952. New or little known Coleoptera from
Ja-pan and its adjacent regions. 7. Dascillidae. Sci. Rep. Saikyo Univ. (Nat. Liv. Sci.) 1: 35Ð41.
Nixon, K. C. 1992. Clados, version 1.2. Published by the
author, Ithaca, NY.
Pic, M. 1913. Diagnoses de Dascillides et Cyphonides
nou-veau. E´change 19: 171Ð173.
Pic, M. 1914a. Noveaux Cole´opte`res de diverses familles.
Mel. Exot. Entomol. 10: 7Ð20.
Pic, M. 1914b. Dascillidae, Helodidae, Scraptiidae et
Salpin-gidae, in: Voyage de Ch. Alluaud et R. Jeannel en Afrique Orientale (1911Ð1912). Result. Sci. Mem. Coleoptera 9: 299Ð326.
Pic, M. 1915. Descriptions agre´ge´es diverses. Mel. Exot.
En-tomol. 12: 3Ð20.
Pic, M. 1921. Diagnoses de Cole´opte`res exotiques. Echange
37: 15Ð16.
Pic, M. 1922. Nouveaute´s diverses. Mel. Exot. Entomol. 37:
1Ð32.
Pic, M. 1923. Nouveaute´s diverses. Mel. Exot. Entomol. 40:
3Ð32.
Pic, M. 1926. Nouveaute´s diverses. Mel. Exot. Entomol. 46:
1Ð32.
Pic, M. 1928. Cole´opte`res exotiques en partie nouveaux.
Echange 44: 7Ð8.
Pic, M. 1930. Dr. E. Mjo¨bergÕs Zoological Collections from
Sumatra. 10 Dascilidae et Malacodermata. Ark. Zool. 21A: 1Ð6.
Pic, M. 1931. Nouveaute´s diverses. Mel. Exot. Entomol. 57:
1Ð36.
Pic, M. 1934. XXXIII.- Nouveaux Cole´opte`res dÕAfrique.
Ann. Mag. Nat. Hist. 10: 386Ð392.
Pic, M. 1935. Cole´opte`res exotiques en partie nouveaux.
Echange 51: 15Ð16.
Pic, M. 1947. Diversit 233 s Entomologiques. Les
Imprim-eries Re´unies, Moulins 1: 1Ð16.
Pic, M. 1951. Un Eubrianax (Ksw.) nouveau du Congo. Rev.
Zool. Bot. Afr. 44: 156.
Pic, M. 1953. Expedition to the Gughe´ Highlands (Southern
Ethiopia), 1948Ð49: Coleoptera, Dascillidae: Une nou-velle espe`ce du genre Eubrianax. J. Linn. Soc. Lond. 42: 293Ð294.
Pic, M. 1954. Cole´opte`res nouveaux de Chine. Bull. Soc.
Entomol. Mulhouse 1954: 61Ð64.
Pic, M. 1955a. Nouveaux Cole´opte`res de la collection
Oberthur (1). Rev. Fr. Entomol. 22: 228Ð236.
Pic, M. 1955b. Contributions a lÕe´tude de la faune
ento-mologique du Ruande-Urundi (Mission P. Basilewsky 1953):Anna. Mus. R. Congo Bel. Tervuren (Ser.8) Sci. Zool. 36: 125Ð135.
Satoˆ, M. 1964. Descriptions of new Dryopoid-beetles from
the Ryukyus. Bull. Jpn. Entomol. Acad. 1: 31Ð37.
Satoˆ, M. 1965. Dryopoidea of the Ryukyu Archipelago, I. J.
Nagoya Wom. Coll. 11: 76Ð94.
Satoˆ, M. 1983. A new Eubrianax species (Col., Psephenidae)
from the Philippines. Aqua. Insects 5: 65Ð58.
Watrous, L. E., and Q. D. Wheeler. 1981. The outgroup
comparison method of character analysis. Syst. Zool. 30: 1Ð11.
Received for publication 5 October 2000; accepted 9 January 2001.
Appendix 1. Data matrix for phylogenetic analysis
Character 0 0 0 0 0 0 0 0 0 1 1 1 1 1 1 1 1 1 1 2 2 Species 1 2 3 4 5 6 7 8 9 0 1 2 3 4 5 6 7 8 9 0 1 Mataeopsephus 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 Jinbrianax 1 1 0 0 0 1 0 0 0 2 0 0 0 0 0 1 1 0 0 0 0 Odontanax ? 0 1 0 0 1 0 0 0 0 1 1 1 1 0 2 1 1 1 0 1 Mubrianax 1 ? 1 0 0 0 0 0 1 1 1 1 1 0 1 2 1 1 2 1 1 Jaechanax 2 1 1 1 0 0 1 0 1 1 1 1 2 1 1 2 1 1 1 2 1 Heibrianax 1 0 1 2 1 0 1 1 0 2 1 0 0 0 0 2 2 1 2 2 1 Eubrianax tarokoensis 1 1 1 2 1 0 1 1 0 2 1 0 0 1 0 2 2 1 2 2 1 Eubrianax pellucidus 2 1 1 2 1 0 1 1 0 1 1 0 0 0 0 2 1 1 2 2 1 Eubrianax amaniensis 2 1 1 2 1 0 1 1 0 1 1 1 0 0 0 2 2 1 2 2 1 Eubrianax manakikikuse 2 1 1 2 1 0 1 1 0 0 1 0 1 1 0 2 2 1 2 2 1 Eubrianax serratus 0 ? 1 2 1 0 1 1 0 2 1 0 2 0 0 2 2 1 2 2 1